Abstract
Camurati-Engelmann disease (CED) is a rare autosomal dominant bone disorder caused by a mutation in transforming growth factor β1 (TGFβ1). The present study aimed to identify a Chinese family with suspected CED based on the clinical symptoms, including pain in extremities, waddling gait, muscle weakness, cortical thickening of the diaphysis of the long bones, and sclerosis of the skull, facial bone, and pelvis. Molecular analysis revealed the presence of the p.Glu169Lys (E169K) mutation in exon 2 of TGFβ1 in patients when compared with the controls. Therefore, the Chinese family was diagnosed with CED due to the presence of the E169K mutation. The present study emphasized the importance of clinical and genetic evidence for the diagnosis of CED. The data presented in the present study are of significance to clinicians, as well as genetic counselors, in the prenatal screening of CED.
Keywords: Camurati-Engelmann disease, diagnosis, TGFβ1, mutation, genetic counseling
Introduction
Camurati-Engelmann disease (CED, OMIM: 131300), also termed Engelmann disease or progressive diaphyseal dysplasia (PDD), is a rare autosomal dominant bone disorder, with >300 reported cases since the disease was first characterized in the 1920's (1).
Patients with CED commonly present pain in extremities, waddling gait, easy fatigability, muscle weakness (2) and cortical thickening of the diaphysis of the long bones. Sclerotic changes at the base of the skull and pelvis are also frequently observed in the patients (3,4). The genetic cause of CED has been identified as a mutation in the transforming growth factor β1 (TGFβ1) gene (5,6). However, diagnosis of the disease is a challenge for clinicians due to its extensive phenotypic variation (7,8).
The present study aimed to identify a Chinese family with rare bone abnormalities. The proband and relatives in the family received comprehensive clinical evaluation and provided venous blood samples for molecular genetic analysis. The clinical and genetic data pointed to a positive diagnosis of CED for the present family. The present study may provide references for diagnosis of novel CED cases, as well as for prenatal screening.
Materials and methods
Study subjects
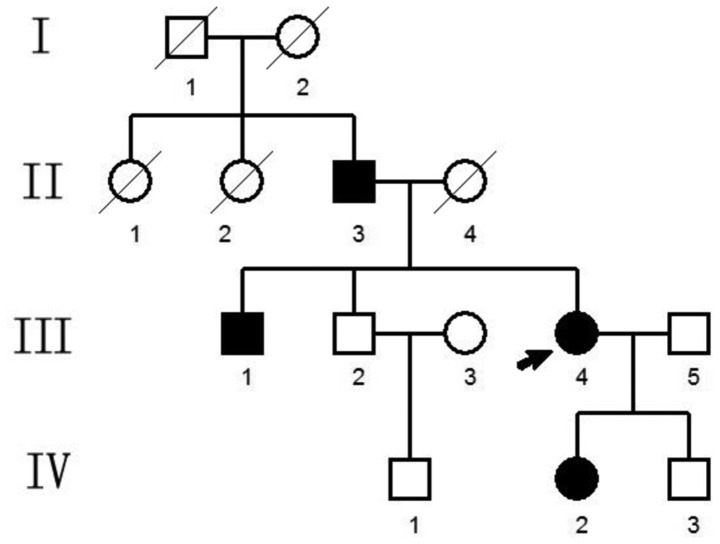
The present study included a Chinese family that was suspected of having CED (Fig. 1). The proband was a visitor in the outpatient department at The Third Xiangya Hospital Affiliated to Central South University (Changsha, China). Written informed consent was received from all individuals and the present study was approved by the Medical and Ethical Committee of The Third Xiangya Hospital.
Figure 1.
Pedigree of Chinese family with Camurati-Engelmann disease. White square, unaffected male; white circle, unaffected female; black square, affected male; black circle, affected female. The score-through indicates disease. The proband is denoted with a black arrow.
Clinic diagnosis
All individuals received a detailed clinical evaluation at The Third Xiangya Hospital. The patients underwent radiological examination, and serum biochemical tests to detect thyroxine (T4), parathyroid hormone (PTH), thyroid stimulating hormone (TSH) and procalcitonin (PCT) were performed in the clinical laboratory of the hospital.
Molecular analysis
A total of 5 ml peripheral vein blood was drawn from the proband and relatives. The genomic DNA was extracted using the phenol-chloroform method, quantified using a UV spectrometer (DU800; Beckman Coulter, Inc., Brea, CA, USA) and stored at −20°C until use. Specific primers (Sunny Biotech Co., Ltd., Shanghai, China) were designed using Primer3 software (version, 4.0.0; http://primer3.ut.ee) for the amplification of all exons and the exon/intron boundary sequences of TGFβ1 (Table I). PCR was run in a volume of 20 µl containing the PrimeSTAR Max DNA polymerase and PCR buffer (Takara Biotechnology Co., Ltd., Dalian, China) and specific primers for 30 cycles at 95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec on an ABI9700 thermal cycler (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) using the genomic DNA as a template. PCR products were purified by agarose electrophoresis, followed by forward and backward sequencing. The sequences were determined using the ABI 3730 DNA Analyzer (Applied Biosystems; Thermo Fisher Scientific, Inc.) and blasted with reference sequences from GenBank (National Center for Biotechnology Information, National Institutes of Health, Bethesda, MD, USA).
Table I.
Primer sequences for TGFβ1 gene exon amplification.
| TGFβ1 Exon | Sequence (5′-3′) | Amplicon size (bp) |
|---|---|---|
| Exon 1 | 427 | |
| Upstream | CTCCTACCTTTTGCCGGGA | |
| Downstream | CTCCTTGGCCTAGTAGTCGG | |
| Exon 2 | 243 | |
| Upstream | TCACCTCTGCCAAACCCCTG | |
| Downstream | ATGCCCTGACCTTCCTTCTG | |
| Exon 3 | 383 | |
| Upstream | TTTCTGGAAGCCTGTTTGGG | |
| Downstream | AATCACTCAGGTTTCCATGCC | |
| Exon 4 | 246 | |
| Upstream | TGAGCTGCACTCTCAGAGTG | |
| Downstream | AGTCAGGGGATAGGGGCAT | |
| Exon 5 | 272 | |
| Upstream | CCCTCTGAGAGTGTGTGTGT | |
| Downstream | AGCACCTGGTCAGCAGATA | |
| Exon 6 | 352 | |
| Upstream | AGGGAGACCCAGATGGAGATAG | |
| Downstream | CTTTCTCTCTCCTCTTCCTCCG | |
| Exon 7 | 398 | |
| Upstream | GGAGATGGGAAGAGGGGATG | |
| Downstream | GGCACGGGTGTCCTTAAATAC |
TGFβ1, transforming growth factor β1.
Results
Pedigree analysis
The pedigree covered four generations and 14 individuals in total (Fig. 1). A total of four family members exhibited bone abnormalities, namely the proband (III.4, female), and her father (II.3), brother (III.1) and daughter (IV.2). This suggested a pattern of autosomal dominant inheritance.
Clinic diagnosis
The clinical data from the patients are summarized in Table II. All patients, with the exception of IV.2, manifested pain in their extremities. All patients in the family presented symptoms of waddling gait, muscle weakness, skin tension over the affected bones and exophthalmos. No patient exhibited acute pain syndrome, hepatosplenomegaly, cranial nerve impairment, mental retardation, or a change to secondary sexual characteristics. The proband, and patients II.3 and III.1 exhibited various symptoms, including dizziness, vision change and hearing loss, which were not present in patient IV.2. The levels of serum alkaline phosphatase (ALP), thyroid hormone and calcitonin were in the normal range for all patients (ALP reference range for males and females >15 years, 40–150 U/l; T4, 66–181 nmol/l; TSH, 0.27–4.2 µIU/ml; and PCT, <0.5 ng/ml). However, all patients demonstrated a significant elevation in levels of serum PTH (reference range, 15–65 pg/ml).
Table II.
Overview of patient clinical data.
| Patient | ||||
|---|---|---|---|---|
| Characteristic | II3 | III1 | III4 | IV2 |
| Gender/Age (years) | M/78 | M/47 | F/42 | F/17 |
| Pain in extremities | + | + | + | − |
| Acute pain syndrome | − | − | − | − |
| Waddling gait | + | + | + | + |
| Muscle weakness | + | + | + | + |
| Skin tension over affected bones | + | + | + | + |
| Hepatosplenomegaly | − | − | − | − |
| Cranial nerve impairment | − | − | − | − |
| Metal retardation | − | − | − | − |
| Dizziness | + | + | + | − |
| Vision change | + | + | + | − |
| Hearing loss | + | + | + | − |
| Exophthalmos | + | + | + | + |
| Secondary sexual characteristics change | − | − | − | − |
| Serum alkaline phosphatase elevation | − | − | − | − |
| Parathyroid hormone elevation | + | + | + | + |
| Thyroid hormone elevation | − | − | − | − |
| Calcitonin elevation | − | − | − | − |
Radiological findings
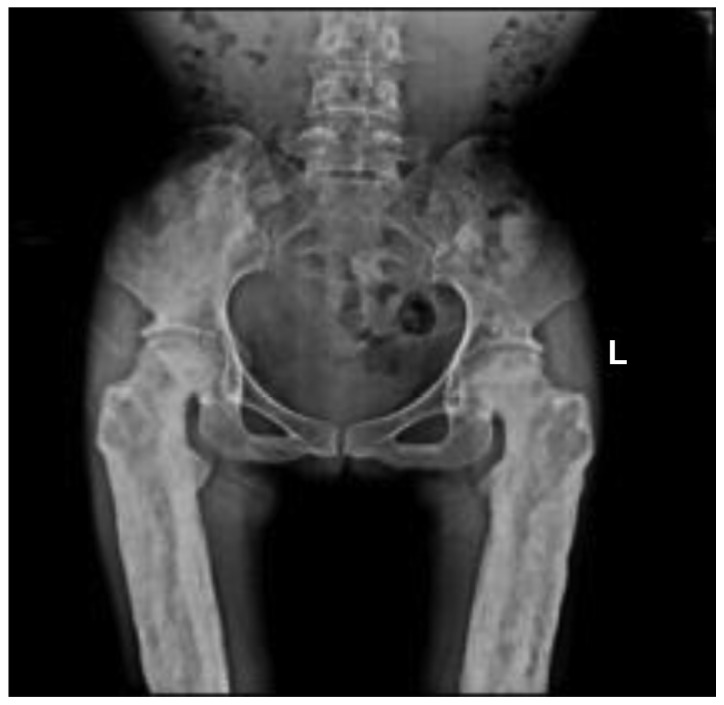
Radiographic images demonstrated cortical thickening of the diaphysis of long bones with irregular endosteal sclerosis. The bones involved included the humerus, radius, ulna, femur, tibia and fibula. The joint space between radius and ulna was narrowed in the patients, as well as the joint space of the tibia and fibula. The metaphysis and epiphysis of the long bones were unaffected (Figs. 2 and 3). Osteoproliferation and osteosclerosis of the ilium, acetabulum and ramus ossis ischii was observed (Fig. 4). The images also revealed periosteal thickening and sclerosis of the skull and facial bone. The diploe of skull disappeared, and the mastoid and sella turcica presented cortical sclerosis (Fig. 5).
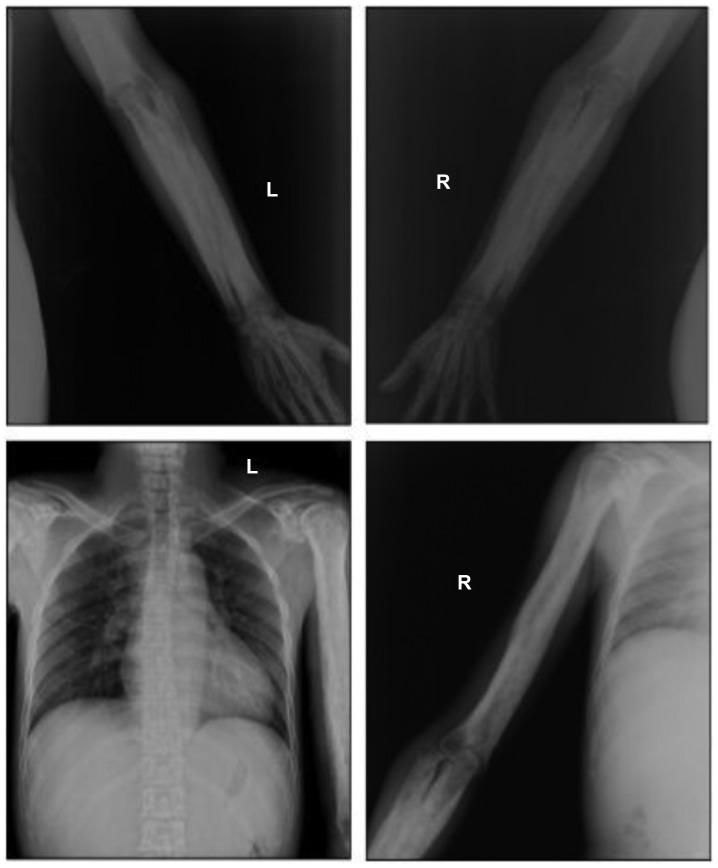
Figure 2.
Anteroposterior radiographs of the proband humerus, radius and ulna. Cortical thickening at the diaphysis of the long bones with irregular endosteal sclerosis was observed. The joint space between radius and ulna was narrowed. The metaphysis and epiphysis was unaffected. L, left; R, right.
Figure 3.
Anteroposterior radiograph of proband pelvis. The image indicated osteoproliferation and osteosclerosis of each ilium, acetabulum and ramus ossis ischia. L, left.
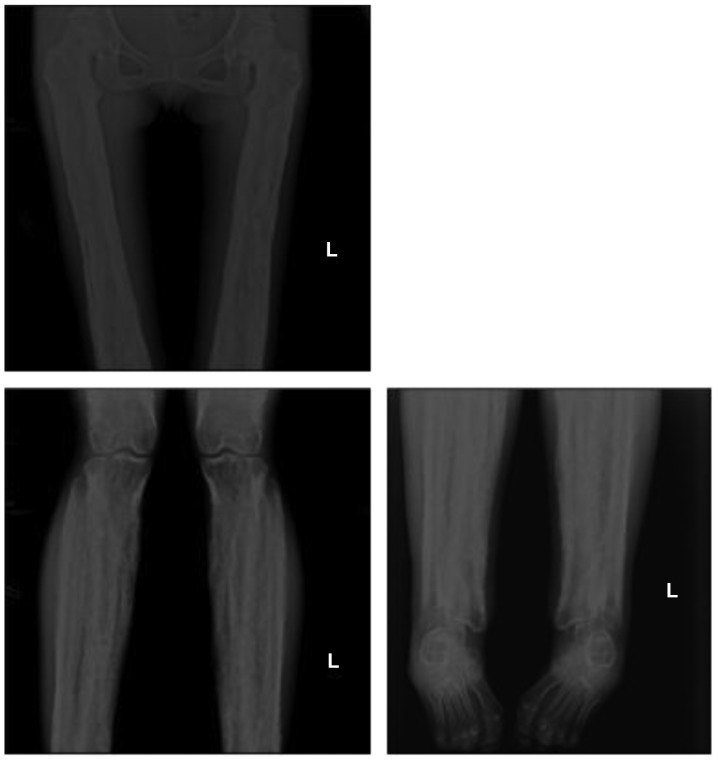
Figure 4.
Anteroposterior radiographs of the proband femur, tibia and fibula. Images revealed cortical thickening of the diaphysis with irregular endosteal sclerosis. The joint space of tibia and fibula was narrowed. Sparing of the metaphysis and epiphysis was noted. L, left.
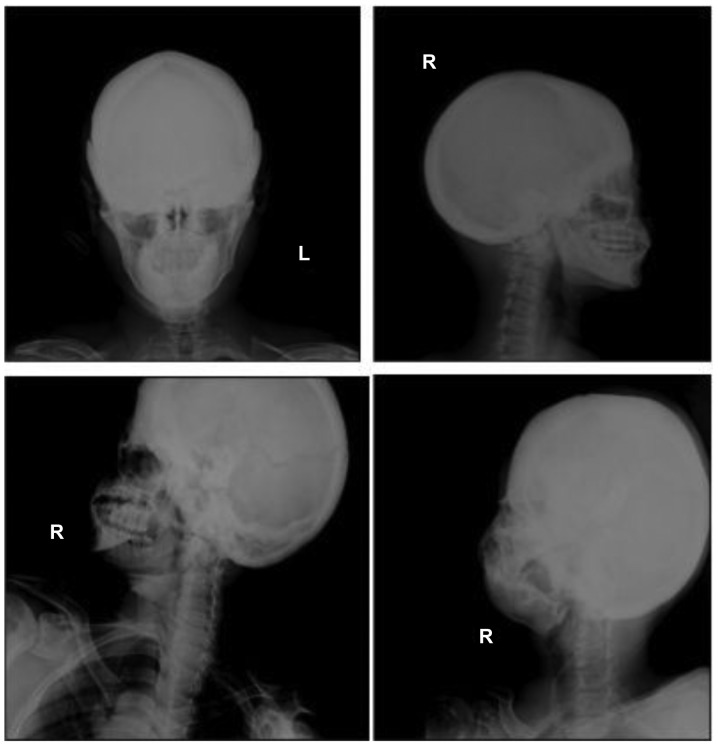
Figure 5.
Radiographs of the proband skull and facial bone in anteroposterior and lateral view. Periosteal thickening and sclerosis of the skull and facial bone were observed. The diploe of the skull disappeared. The mastoid and sella turcica also presented cortical sclerosis. L, left; R, right.
Identification of TGFβ1 mutations
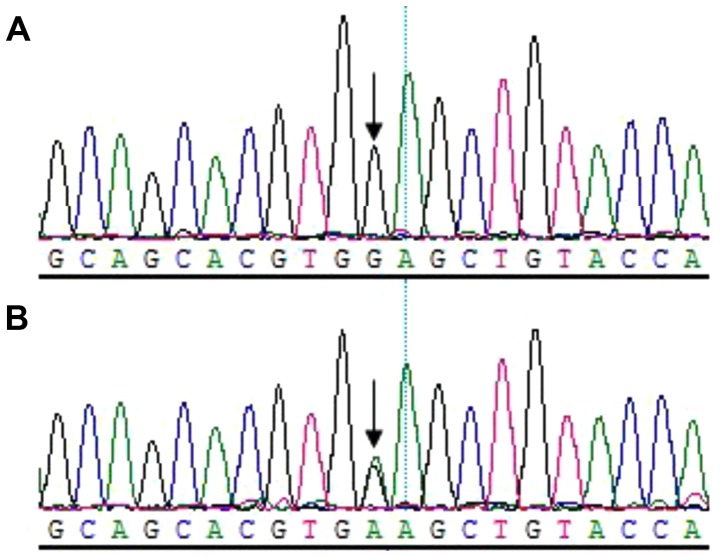
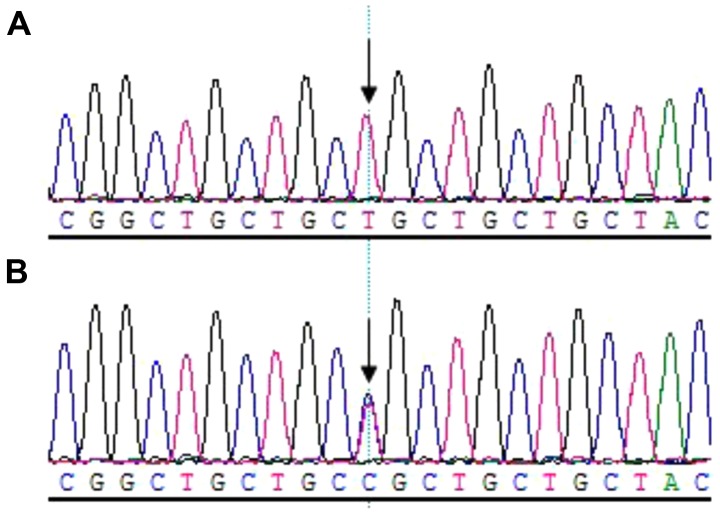
Sequencing analysis revealed that all patients harbored the c.505G→A (p.Glu169Lys) mutation within exon 2 of TGFβ1; however, not the healthy relatives in the family (Fig. 6). Additionally, a C→T transition mutation at code29 (c.29C→T, p.Pro10Leu) within exon1 of TGFβ1 was identified in one allele of the patient IV.2. The family member III.5 (father of the patient IV.2), who was of a normal phenotype, harbored the 29 C→T transition mutations in both alleles (Fig. 7).
Figure 6.
c.505G→A mutation within exon 2 of TGFβ1. The sequencing results of (A) III.5 (wild-type) and (B) the proband (mutant) were assessed.
Figure 7.
Missense mutation c.29C→T in exon1 of TGFβ1. The sequencing results revealed that the family member III.5, who was of a normal phenotype, was (A) a homozygote of code29 T allele and that (B) the patient IV.2 (daughter of III.5) was a heterozygote of the non-synonymous 29C/T single nucleotide polymorphism.
Discussion
CED is a rare bone disorder (9). The skeletal abnormalities associated with the disease lead to a low quality of life (10,11). However, due to the rarity and variable penetrance of CED, diagnosis of the disease is a great challenge for clinicians (7,8). The clinical and genetic data from the reported CED families may guide clinicians to make the diagnosis of novel cases.
Janssens et al (2) prepared a comprehensive review of 100 cases from 24 CED families spread worldwide and concluded that patients with CED most commonly present pain in extremities (68%), waddling gait (48%), easy fatigability (44%) and muscle weakness (39%) (2). In the present study, 3/4 patients complained of pain in the extremities. Waddling gait, easy fatigability and muscle weakness were identified for all patients in the family. Of note, patient IV.2 appeared to be less severely affected, not manifesting pain in extremities or the progressive symptoms (dizziness, vision change and hearing loss), unlike her elder relatives who were affected by CED. The present study inferred that the differential diagnosis between the IV.2 and other patients in the family reflects the variable penetrant nature of CED. Therefore, clinicians must take note of young patients who may be neglected due to incomplete penetrance of the disease.
TGFβ1 encodes an important cytokine that serves regulatory roles in bone formation and resorption. Therefore, abnormal activity of TGFβ1 causes bone diseases (12). It has been previously demonstrated that mutations in TGFβ1 are the genetic cause of CED (5,6). To date, a series of TGFβ1 mutations associated with the pathogenesis of CED have been identified. Among them are the L10-12 duplication, Y81H, R156C, R218C/H and C223R/S mutation (2). These mutations eventually lead to increased cytokine activity via different activation mechanisms (12). Sequencing analysis revealed that all patients in the Chinese family possessed the c.505G→A (p. Glu169Lys) mutation within exon 2 of TGFβ1; however, not the healthy relatives. The present study noticed that Wu et al previously reported the E169K mutation as a genetic cause for CED, without discussing the clinical symptoms (13). Regarding the genetic diagnosis, the present results further confirmed that this mutation is a causative mutation of CED. At the molecular level, the E169K mutation may be responsible for an increase in the activity of TGFβ. However, further mechanistic studies must be performed to confirm this hypothesis. With the exception of the c.505G→A (p.Glu169Lys) mutation, patient IV.2 harbored another missense mutation c.29C→T (p.Pro10Leu) in exon 1 of TGFβ1. However, the present study hypothesized that this transition belongs to a non-synonymous single nucleotide polymorphism and may not be associated with the disease, since the III.5 (father of IV.2), who had a normal phenotype, is a homozygote of the T allele of 29C/T single nucleotide polymorphism.
ALP is a marker of bone turnover (14). It is reported that the levels of serum ALP were elevated in a large fraction of patients with CED (3). The present study observed that the levels of serum ALP in all patients in the Chinese family were in the normal range, suggesting that serum ALP may not necessarily reflect bone metabolism in Chinese patients with CED. Notably, it was revealed that the level of PTH markedly increased in the patients. It is well known that PTH/PTH-receptor signaling serves a role in skeletal development, bone turnover and mineral ion homeostasis (15). Previously, Jang et al (16) demonstrated that intermittent PTH treatment increases the number of periosteal osteoblasts by delaying the transformation of mature osteoblasts into lining cells on the periosteal surfaces (16). This finding and the present observation may imply the potential role of PTH in diagnosis of CED.
In conclusion, the present study identified a Chinese family with CED based on the results of clinical, biochemical and radiological examinations. The present study reminds us that a comprehensive evaluation must be performed for the patients prior to the final diagnosis of CED, due to its rarity and variable phenotypes. The present data are useful to clinicians for the diagnosis and genetic counseling of CED.
Acknowledgements
The authors would like to thank the Chinese family for their participation in the present study. This study was supported by grants from the Science and Technology Project of Hunan Province (grant no. 2015JC3127) and the Health and Family Planning Commission of Hunan Province (grant nos. B2016083 and C2016038).
References
- 1.Wallace SE, Wilcox WR. Gene Reviews (R) University of Washington; Seattle (WA): 1993. Camurati-Engelmann Disease. [PubMed] [Google Scholar]
- 2.Janssens K, Vanhoenacker F, Bonduelle M, Verbruggen L, Van Maldergem L, Ralston S, Guañabens N, Migone N, Wientroub S, Divizia MT, et al. Camurati-Engelmann disease: Review of the clinical, radiological and molecular data of 24 families and implications for diagnosis and treatment. J Med Genet. 2006;43:1–11. doi: 10.1136/jmg.2005.033522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartuseviciene A, Samuilis A, Skucas J. Camurati-Engelmann disease: Imaging, clinical features and differential diagnosis. Skeletal Radiol. 2009;38:1037–1043. doi: 10.1007/s00256-008-0642-1. [DOI] [PubMed] [Google Scholar]
- 4.Carlson ML, Beatty CW, Neff BA, Link MJ, Driscoll CL. Skull base manifestations of Camurati-Engelmann disease. Arch Otolaryngol Head Neck Surg. 2010;136:566–575. doi: 10.1001/archoto.2010.68. [DOI] [PubMed] [Google Scholar]
- 5.Janssens K, Gershoni-Baruch R, Guañabens N, Migone N, Ralston S, Bonduelle M, Lissens W, Van Maldergem L, Vanhoenacker F, Verbruggen L, Van Hul W. Mutations in the gene encoding the latency-associated peptide of TGF-beta 1 cause Camurati-Engelmann disease. Nat Genet. 2000;26:273–275. doi: 10.1038/81563. [DOI] [PubMed] [Google Scholar]
- 6.Kinoshita A, Saito T, Tomita H, Makita Y, Yoshida K, Ghadami M, Yamada K, Kondo S, Ikegawa S, Nishimura G, et al. Domain-specific mutations in TGFB1 result in Camurati-Engelmann disease. Nat Genet. 2000;26:19–20. doi: 10.1038/79128. [DOI] [PubMed] [Google Scholar]
- 7.de Vernejoul MC. Sclerosing bone disorders. Best Pract Res Clin Rheumatol. 2008;22:71–83. doi: 10.1016/j.berh.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Lucke T, Illsinger S, Das AM, Schirg E, Hartmann H. Pitfalls in paediatric gait disturbances: Painless bone diseases. Eur J Pediatr. 2006;165:909–912. doi: 10.1007/s00431-006-0180-6. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MM., Jr The new bone biology: Pathologic, molecular, and clinical correlates. Am J Med Genet A. 2006;140:2646–2706. doi: 10.1002/ajmg.a.31368. [DOI] [PubMed] [Google Scholar]
- 10.Ayyavoo A, Derraik JG, Cutfield WS, Hofman PL. Elimination of pain and improvement of exercise capacity in Camurati-Engelmann disease with losartan. J Clin Endocrinol Metab. 2014;99:3978–3982. doi: 10.1210/jc.2014-2025. [DOI] [PubMed] [Google Scholar]
- 11.Toumba M, Neocleous V, Shammas C, Anastasiadou V, Allgrove J, Phylactou LA, Skordis N. A family with Camurati-Engelman disease: The role of the missense p. R218C mutation in TGFbeta1 in bones and endocrine glands. J Pediatr Endocrinol Metab. 2013;26:1189–1195. doi: 10.1515/jpem-2013-0150. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Zhang BH, Liu YJ, Hu YQ, He JW, Zhang ZL. Transforming growth factor-β1 gene mutations and phenotypes in pediatric patients with Camurati-Engelmann disease. Mol Med Rep. 2013;7:1695–1699. doi: 10.3892/mmr.2013.1367. [DOI] [PubMed] [Google Scholar]
- 13.Wu S, Liang S, Yan Y, Wang Y, Li F, Deng Y, Huang W, Yuan W, Luo N, Zhu C, et al. A novel mutation of TGF beta1 in a Chinese family with Camurati-Engelmann disease. Bone. 2007;40:1630–1634. doi: 10.1016/j.bone.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Hernàndez MV, Peris P, Guañabens N, Alvarez L, Monegal A, Pons F, Ponce A, Muñoz-Gómez J. Biochemical markers of bone turnover in Camurati-Engelmann disease: A report on four cases in one family. Calcif Tissue Int. 1997;61:48–51. doi: 10.1007/s002239900293. [DOI] [PubMed] [Google Scholar]
- 15.Cheloha RW, Gellman SH, Vilardaga JP, Gardella TJ. PTH receptor-1 signalling-mechanistic insights and therapeutic prospects. Nat Rev Endocrinol. 2015;11:712–724. doi: 10.1038/nrendo.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang MG, Lee JY, Yang JY, Park H, Kim JH, Kim JE, Shin CS, Kim SY, Kim SW. Intermittent PTH treatment can delay the transformation of mature osteoblasts into lining cells on the periosteal surfaces. J Bone Miner Metab. 2015;34:532–539. doi: 10.1007/s00774-015-0707-x. [DOI] [PubMed] [Google Scholar]