Abstract
It is known that inhaled anesthetics induce neuroinflammation and facilitate postoperative cognitive dysfunction (POCD) in aged individuals; however, the mechanisms by which they mediate these effects remain elusive. Inhalation of the isoflurane anesthetic leads to opening of the mitochondrial permeability transition pore and loss of mitochondrial membrane potential. Therefore, mitochondrial retrograde signaling, which is an adaptive mechanism that facilitates the transmission of signals from dysfunctional mitochondria to the nucleus to activate target gene expression, may be activated during isoflurane inhalation. Therefore, the present study was designed to investigate the role of mitochondrial retrograde signaling in isoflurane-induced hippocampal neuroinflammation and cognitive impairment in aged rats. As calcineurin (CaN) serves an important role in the initiation of mitochondrial retrograde signaling, and nuclear factor-κB (NF-κB) is involved in CaN signaling, their effects on isoflurane-induced hippocampal neuroinflammation and cognitive impairment were investigated. Reactive oxygen species and mitochondrial membrane potential fluorescence staining, western blotting, colorimetric analysis, ELISA, immunofluorescence and the Morris water maze test were used in the present study. The results indicate that isoflurane induced hippocampal mitochondrial dysfunction and activated CaN, which subsequently lead to the putative activation of NF-κB. These resulted in the elevation of interleukin-1β (IL-1β) expression (a typical marker of neuroinflammation), and was associated with cognitive impairment in aged rats. In addition, CaN and NF-κB inhibition attenuated isoflurane-induced neuroinflammation and subsequent cognitive impairment. In conclusion, the results of the present study demonstrate the role of mitochondrial retrograde signaling and associated protein factors in inhaled anesthetic-induced neuroinflammation and cognitive impairment. These protein factors may therefore present promising therapeutic targets for the prevention of POCD.
Keywords: calcineurin, nuclear factor-κB, isoflurane, neuroinflammation, postoperative cognitive dysfunction, mitochon drial retrograde signaling
Introduction
Postoperative cognitive dysfunction (POCD) is one of the most common postoperative complications in elderly patients (1). A previous study demonstrated that, in patients >60 years of age that had undergone major non-cardiac surgery, 25.8% developed cognitive dysfunction after 1 week and 9.9% developed cognitive dysfunction after 3 months (2). In addition, a significant association between early and late-onset POCD and advancing age was observed. POCD is associated with impairment of daily functional activities, an increased risk of dismissal from employment, as well as increased expenses, and risk of morbidity and mortality (2). However, the etiology and pathogenesis of POCD remain elusive.
Neuroinflammation, which involves the activation of microglia, astrocytes and neurons (3), as well as an elevation in the production of proinflammatory cytokines (4), has been reported to serve a role in the pathogenesis of neurodegenerative diseases. Although neuroinflammation is known to be involved in the pathogenesis of POCD (5), multiple perioperative factors, including surgical trauma (6) and inhaled anesthetics (7), may facilitate this process. Nuclear factor-κB (NF-κB) is activated as a result of canonical and non-canonical signaling pathways, and has been reported to mediate neuroinflammation in multiple pathological processes, such as Alzheimer's disease (AD) (8) and cerebral ischemia reperfusion injury (9). In a previous study, the canonical NF-κB pathway was observed to be involved in isoflurane-induced neuroinflammation (5); however, the upstream regulatory factors involved in this signaling pathway remain elusive and impede further studies.
Deficiencies in mitochondrial respiratory chain components, the generation of reactive oxygen species (ROS) and mitochondrial DNA mutations result in mitochondrial membrane potential (ΔΨm) loss, and may induce mitochondrial retrograde signaling (10). This signaling pathway is an adaptive mechanism that transmits signals from dysfunctional mitochondria to activate nuclear gene expression. Zhang et al (11) reported that inhaled anesthetics induce ROS generation and mitochondrial permeability transition pore (mPTP) opening, and reduce ΔΨm and ATP production in cells in vitro. Therefore, inhaled anesthetics may initiate mitochondrial retrograde signaling. ΔΨm initiates mitochondrial Ca2+ uptake (12), and its loss leads to cytosolic Ca2+ elevation and the activation of calcineurin (CaN). Ca2+/CaN signaling serves an important role in mitochondrial retrograde signaling (13), and NF-κB is also involved in the process (14). Therefore, inhaled anesthetics may activate CaN signaling, which may subsequently facilitate multiple pathological processes, such as neuroinflammation.
In the present study, an anesthesia model in aged rats was established using the inhaled anesthetic, isoflurane. This study aimed to elucidate the role of mitochondrial retrograde signaling in isoflurane-induced hippocampal neuroinflammation and cognitive impairment in aged rats, and to explore the effects of CaN and NF-κB on neuroinflammation and cognitive impairment, in order to provide potential therapeutic targets for the prevention and treatment of POCD.
Materials and methods
Animals
A total of 102 male Sprague-Dawley rats (age, 18 months; weight, 550–600 g; obtained from Dongchuang Laboratory Animal Center, Changsha, China) were used in the present study. Rats were maintained on a standard pellet food and water ad libitum, a with a 12:12 h light-dark cycle, and a temperature of 24±2°C for 2 weeks prior to isoflurane exposure.
Study protocol
The study protocol was approved by the Peking University Biomedical Ethics Committee, Experimental Animal Ethics Branch (approval no. LA201412). Rats were randomly divided into isoflurane (n=12) or control groups (n=6), and received isoflurane (Baxter International, Inc., Deerfield, IL, USA) or vehicle gas (50% O2 and 50% air), respectively. CaN and interleukin-1β (IL-1β) expression levels, and NF-κB activation, including inhibitor of NF-κB (IκBα) phosphorylation and NF-κB RelA (also known as p65) nuclear translocation in the hippocampus were assessed at 6 or 12 h after isoflurane exposure.
Rats were treated with the CaN inhibitor cyclosporin A (CsA) (15) and the NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC) (16) to investigate the role and interaction of CaN and NF-κB in isoflurane-induced neuroinflammation. To achieve this, rats were randomly divided into control, CsA, PDTC, isoflurane, isoflurane + CsA or isoflurane + PDTC groups (n=6 for each group). Rats in CsA and isoflurane + CsA groups received intraperitoneal CsA (7 mg/kg; Abcam, Cambridge, UK), and rats in the PDTC and isoflurane + PDTC groups received 100 mg/kg intraperitoneal PDTC (Sigma-Aldrich, St. Louis, MO) in 0.9% saline (total volume 0.5 ml) at 30 min prior to isoflurane exposure. Rats in the control and isoflurane groups received an identical volume of 0.9% saline delivered by intraperitoneal injection. Hippocampal CaN and IL-1β expression levels, and IκBα phosphorylation were then assessed, and the spatial learning and memory function of the rats were evaluated.
Isoflurane exposure
Isoflurane exposure was performed according to the methods described previously (17). Briefly, rats received 2% isoflurane for 4 h in an anesthetic chamber. During this time, the arterial oxygen saturation, heart rate, blood pressure and rectal temperature were monitored to ensure that they were maintained within the physiological range. The rats then received 100% oxygen until they regained consciousness. Isoflurane was well tolerated, with all monitored variables remaining within the physiological range. Rats in the control, CsA and PDTC groups only received vehicle gas.
ROS assay
An ROS assay kit (Cell Biolabs, Inc., San Diego, CA, USA) was used to determine ROS levels in the rat hippocampus. Briefly, rats were sacrificed by decapitation at the end of the experiments. The brain tissues were removed rapidly, and hippocampus was dissected out on ice for subsequent experiments. Subsequently, the hippocampus was homogenized and centrifuged at 7,155 × g for 5 min at 4°C, and the total protein concentration of the supernatant was determined using a bicinchoninic acid assay (Applygen Technologies Inc., Beijing, China). Protein samples were subsequently incubated with catalyst and dichlorodihydrofluorescein solutions included in the ROS assay kit. The fluorescence was read at 480/530 nm using a microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
ΔΨm assay
A tetramethylrhodamine ethyl ester perchlorate (TMRE) assay (Sigma-Aldrich, EMD Millipore, Billerica, MA, USA) was used to determine ΔΨm levels in the rat hippocampus. Briefly, the hippocampus was homogenized and centrifuged at 7,155 × g for 5 min at 4°C, and the total protein concentration of the sample supernatant was determined using a bicinchoninic acid assay. The samples were subsequently incubated with 50 nM TMRE solutions for 15 min at room temperature, and the fluorescence was read at 549/574 nm using a microplate reader (Thermo Fisher Scientific, Inc.).
Western blot analysis
Western blot analysis was performed to determine the protein expression levels of CaN and IκBα in the rat hippocampus. Briefly, the hippocampus was homogenized and centrifuged at 7,155 × g for 5 min at 4°C, and the total protein concentration of the supernatant was determined using a bicinchoninic acid assay. Proteins were separated by 10% SDS-PAGE, prior to transfer to the polyvinylidene fluoride membranes. Membranes were then incubated with the anti-CaN (dilution, 1:1,000; cat. no. ab3673; Abcam), anti-IκBα and anti-phosphorylated (p)-IκBα (dilution, 1:1,000; cat. nos. 4812 and 9246; Cell Signaling Technology, Inc., Danvers, MA, USA) and anti-β-actin (dilution, 1:10,000; cat. no. 4970; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) primary antibodies overnight at 4°C, followed by incubation with the IRDye® 800CW-conjugated goat anti-rabbit secondary antibody (dilution, 1:10,000; cat. no. 926-32211; LI-COR Biosciences, Inc., Lincoln, NE, USA) for 2 h at room temperature. Immunoreactivity was visualized by scanning membranes with an Odyssey 9120 infrared imaging system (LI-COR Biosciences, Inc.). The densitometric analysis was conducted using β-actin as a control for loading differences, the protein levels in the experimental groups were calculated as percentages of the control group.
CaN activity
A colorimetric assay was performed using a CaN activity kit (cat. no. ab139461; Abcam) to determine CaN activity in the rat hippocampus. Briefly, the hippocampus was homogenized and centrifuged at 7,155 × g for 5 min at 4°C, and the total protein concentration of the supernatant was determined using a bicinchoninic acid assay. Protein lysates and calcineurin assay buffer were mixed, calcineurin substrate was added and reaction proceeded for 10 min. Green assay reagent was added and optical density (OD)620nm was read on a microplate reader (Thermo Fisher Scientific, Inc.). OD620nm data were converted into released phosphate amount, and calcineurin activity was calculated as a ratio of phosphate amount/reaction time.
Enzyme-linked immunosorbent assay (ELISA)
ELISA was performed using an sandwich ELISA kit (cat. no. ab100768; Abcam) to determine the IL-1β protein expression level in the rat hippocampus. Briefly, the hippocampus was homogenized and centrifuged at 7,155 × g for 5 min at 4°C, and the total protein concentration of the supernatant was determined using a bicinchoninic acid assay. Protein lysates, biotinylated IL-1β antibody, horseradish peroxidase-streptavidin and TMB One-Step Substrate reagent were incubated in turn in 96-well plates for 2.5, 1 h, 45 min and 30 min at room temperature. Stop solution was then added and OD450nm was read on a microplate reader (Thermo Fisher Scientific, Inc.).
Immunofluorescence
Immunofluorescence was performed in order to determine the nuclear translocation of RelA. Briefly, the rat hippocampus was fixed with 4% paraformaldehyde for 24 h, cryoprotected with 30% sucrose for 48 h, and sectioned using a cryostat (Leica Microsystems, Inc., Buffalo Grove, IL, USA). Coronal sections (of 10-µm thickness) were incubated with a RelA primary antibody (dilution, 1:50; cat. no. 8242; Cell Signaling Technology, Inc.) overnight at 4°C, followed by incubation with a goat anti-rabbit IgG-fluorescein isothiocyanate-conjugated secondary antibody (dilution, 1:200; cat. no. sc-2012; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. Nuclei were subsequently counterstained with DAPI (1:5,000; Roche Diagnostics GmbH, Mannheim, Germany). Images were captured using a confocal fluorescence microscope (Zeiss GmbH, Jena, Germany). Due to the results of a previous study demonstrating that the CA1 region of hippocampus serves an important role in memory formation (18), this region was analyzed for RelA expression.
Morris water maze
The spatial learning ability and memory of rats was evaluated using a Morris water maze test as described previously (5). The place navigation test was performed 24 h following isoflurane exposure, at which point rats received four training trials daily for 5 days. In each trial, rats were randomly placed in water at one of four equally spaced starting positions. The time to locate a submerged platform (the escape latency, defined by a cut-off time of 120 sec) and the swim speed were recorded. A probe trial (duration, 120 sec) was performed 24 h after the last trial, during which time the platform was removed and the target quadrant dwell time was recorded.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (version 5.0; GraphPad Software, Inc., La Jolla, CA, USA). Data are expressed as the mean ± standard deviation or the mean ± standard error. One-way or two-way analysis of variance (ANOVA) was used to compare ROS, MMP, CaN, IκBα, p-IκBα, IL-1β, and probe test results between groups. Two-way repeated-measures ANOVA followed by a post-hoc Bonferroni test was used to compare place navigation test results. P<0.05 was considered to indicate a statistically significant difference.
Results
Isoflurane induces mitochondrial dysfunction in the hippocampus of aged rats and is associated with increased CaN activity
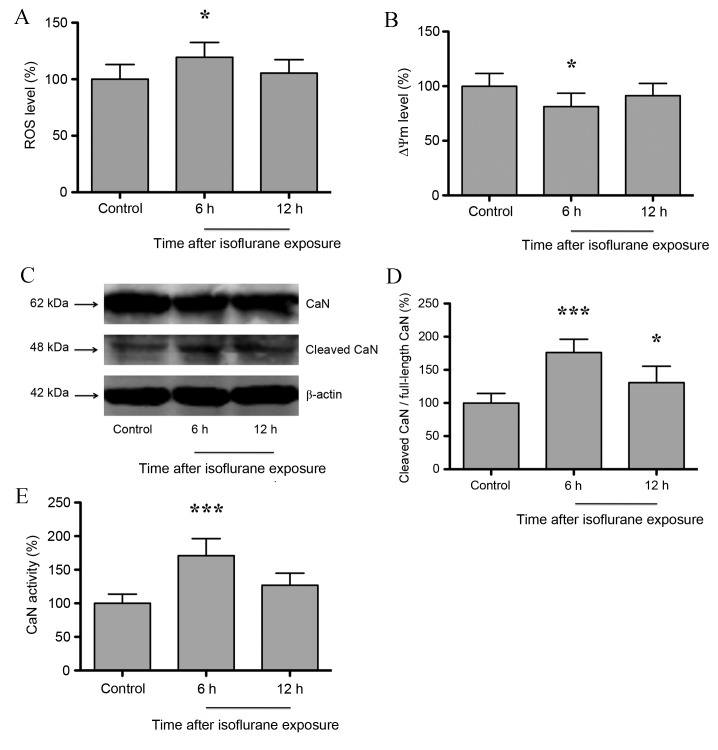
Zhang et al (11) demonstrated that isoflurane induced mitochondrial dysfunction in neuroglioma/neurons. As shown in Fig 1A, hippocampal ROS and ΔΨm levels increased significantly at 6 h following isoflurane exposure compared with untreated controls (P<0.05), but returned to baseline levels at 12 h post-isoflurane exposure (P>0.05). In addition, ΔΨm levels decreased significantly at 6 h (P<0.05), and returned to baseline levels at 12 h following isoflurane exposure (P>0.05; n=6; Fig. 1B). Consistent with Zhang et al (11), the results of the present study indicate that isoflurane induces mitochondrial dysfunction in the hippocampus of aged rats. Hypoxia, genetic or pharmacological intervention-induced mitochondrial dysfunction may activate mitochondrial retrograde signaling, and CaN serves an important role in the initiation of mitochondrial retrograde signaling (10). As shown in Fig. 1C and D, the expression of cleaved CaN in the rat hippocampus increased significantly at 6 h (P<0.001) following isoflurane exposure, and was maintained at a relatively high level at 12 h after exposure when compared with untreated controls (P<0.05; n=6;). In addition, CaN activity was observed to increase significantly at 6 h (P<0.001) following isoflurane exposure, but returned to baseline levels following 12 h (P>0.05; n=6; Fig. 1E). These results indicate that isoflurane induces mitochondrial dysfunction and subsequent CaN activation in the hippocampus.
Figure 1.
Isoflurane exposure induced mitochondrial dysfunction and CaN activation in the hippocampus of aged rats. (A) The ROS level increased significantly at 6 h after exposure. (B) The ΔΨm level decreased significantly at 6 h after exposure. (C) Western blot analysis and (D) quantification of full-length and cleaved CaN protein expression levels, indicating that the expression levels of cleaved CaN increased significantly at 6 h following isoflurane exposure. (E) CaN activity increased significantly at 6 h after exposure. Data are expressed as the mean ± standard deviation (n=6). *P<0.05 and ***P<0.001 vs. the control group. CaN, calcineurin; NF-κB, nuclear factor-κB; ROS, reactive oxygen species; ΔΨm, mitochondrial membrane potential.
Isoflurane exposure leads to increased IκBα phosphorylation and RelA nuclear translocation in the rat hippocampus
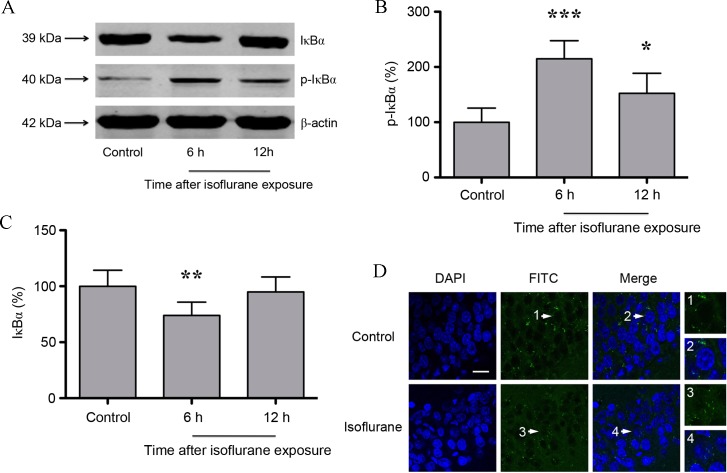
NF-κB has been demonstrated to be involved in CaN signaling (19), and consists of RelA, RelB, c-Rel, p50 and p52 protein dimers. RelA/p50 is the most abundant NF-κB heterodimer, and its nuclear translocation and transcriptional activity is inhibited by IκBα (20). Thus, hippocampal IκBα and p-IκBα protein expression following isoflurane exposure was determined. As shown in Fig. 2A-C, the protein expression levels of p-IκBα increased significantly, whereas IκBα expression decreased significantly at 6 h (P<0.01 and P<0.001, respectively) post-isoflurane exposure. IκBα expression returned to baseline levels (P>0.05) at 12 h following isoflurane exposure, whereas p-IκBα expression was maintained at a significantly higher level (P<0.05) compared with untreated controls (n=6; Fig. 2A-C). With IκBα phosphorylation and subsequent degradation, NF-κB dimers may enter the nucleus to regulate target gene expression. Therefore, the nuclear translocation of RelA was investigated at 6 h following isoflurane exposure. In untreated controls, RelA was primarily distributed in the cytosol of the pyramidal cell layer in the CA1 region of hippocampus. However, following isoflurane exposure, RelA exhibited a nuclear distribution (Fig. 2D).
Figure 2.
(A) Western blot analysis and quantification of (B) p-IκBα and (C) IκBα protein expression levels. IκBα levels were significantly decreased, and p-IκBα levels were significantly increased, at 6 h following isoflurane exposure. (D) Immunofluorescence images showing RelA (FITC, green) and DAPI (blue) counter-staining. In controls, RelA was primarily distributed in the cytosol of the pyramidal cell layer in the CA1 region. At 6 h following isoflurane exposure, RelA was distributed in the nucleus and cytosol. The white arrows indicate the typical RelA distribution, which is shown in the high-magnification images (magnification, ×400; scale bar, 20 µm). Data are presented as the mean ± standard deviation (n=6). *P<0.05, **P<0.01 and ***P<0.001 vs. the control group. p-IκBα, phosphorylated inhibitor of κB; FITC, fluorescein isothiocyanate.
Inhibition of CaN attenuates isoflurane-induced hippocampal IκBα phosphorylation and the nuclear translocation of RelA, whereas NF-κB inhibition has no effect on CaN activity
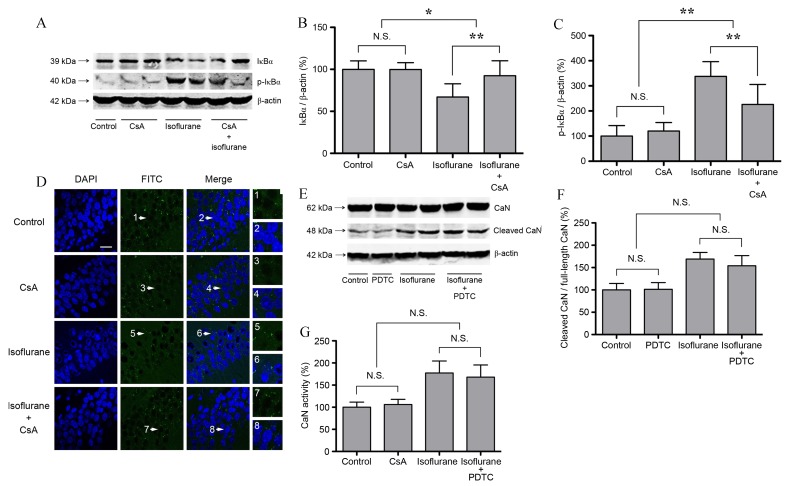
In order to investigate the effect of increased CaN expression and activity on NF-κB following isoflurane exposure, untreated and isoflurane-treated rats were treated with CsA. In isoflurane + CsA-treated rats at 6 h following treatment, the protein expression levels of IκBα increased significantly, whereas the expression levels of p-IκBα decreased significantly when compared with rats treated with isoflurane alone (P<0.01; Fig. 3A-C). CsA treatment alone did not affect IκBα or p-IκBα expression (P>0.05; n=6; Fig. 3A-C). It is therefore possible that CsA attenuated isoflurane-induced hippocampal NF-κB activation. As shown in Fig. 3D, CsA attenuated isoflurane-induced RelA nuclear translocation. However, CsA treatment alone did not affect RelA distribution, which was primarily localized to the cytosol of the pyramidal cell layer in the CA1 region of hippocampus (Fig. 3D). Subsequently, rats were treated with the NF-κB inhibitor, PDTC, in order to investigate the effect of NF-κB on CaN. As shown in Fig. 3E-G, PDTC did not affect the expression level of cleaved CaN or its activity following isoflurane exposure or in isoflurane-untreated controls (P>0.05; n=6). The results suggest that CaN is an upstream mediator of NF-κB activation in the hippocampus following isoflurane exposure.
Figure 3.
CaN activation mediates isoflurane-induced NF-κB activation in the hippocampus of aged rats. CsA (7 mg/kg) or PDTC (100 mg/kg) was administered 30 min prior to isoflurane exposure through intraperitoneal injection. (A) Western blot analysis and quantification of (B) IκBα and (C) p-IκBα protein expression levels relative to β-actin. IκBα expression decreased and p-IκBα expression increased significantly at 6 h following isoflurane exposure. CsA attenuated isoflurane-induced alterations in IκBα expression, and CsA treatment alone did not affect IκBα expression. (D) Immunofluorescence images, demonstrating that the nuclear distribution of RelA increased following isoflurane exposure in the pyramidal cell layer of the CA1 hippocampal region. CsA attenuated isoflurane-induced RelA nuclear translocation, whereas CsA treatment alone did not affect RelA distribution. The white arrows indicate the typical RelA distribution, which are provided as high magnification images (magnification, ×400; scale bar, 20 µm). (E) Western blot analysis and (F) quantification of cleaved CaN expression. The expression of cleaved CaN increased significantly at 6 h following isoflurane exposure. PDTC did not affect the expression of cleaved CaN in untreated controls or isoflurane treated rats. (G) CaN activity increased significantly at 6 h following isoflurane exposure, and PDTC did not affect CaN activity after isoflurane exposure or in the control group. Data are expressed as mean ± standard deviation (n=6). *P<0.05 and **P<0.01 vs. the control group. CaN, calcineurin; NF-κB, nuclear factor-κB; CsA, cyclosporin A; PDTA, pyrrolidine dithiocarbamate; p-IκBα, phosphorylated inhibitor of κB; N.S., not significant.
IL-1β is a downstream target of the CaN/NF-κB signaling pathway in the hippocampus, and its expression is increased in response to isoflurane exposure
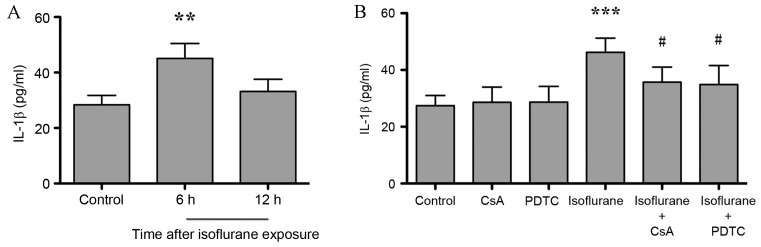
NF-κB regulates the expression of genes involved in inflammation and the innate immune response (21). As shown in Fig. 4A, the expression level of hippocampal inflammatory factor IL-1β increased significantly at 6 h (P<0.001) compared with untreated controls, and returned to baseline levels at 12 h after isoflurane exposure (P>0.05; n=6). Treatment of isoflurane-exposed rats with CsA and PDTC significantly attenuated isoflurane-induced IL-1β elevation (P<0.05) and no difference between isoflurane + CsA and isoflurane + PDTC groups was observed (P>0.05, Fig. 4B). CsA or PDTC treatment alone did not affect IL-1β expression levels, when compared with untreated controls (P>0.05, Fig. 4B). These results indicate that CaN/NF-κB signaling may mediate neuroinflammation in the hippocampus following isoflurane exposure.
Figure 4.
CsA and PDTC attenuates isoflurane exposure-induced hippocampal IL-1β elevation in aged rats. (A) IL-1β expression levels increased significantly at 6 h following isoflurane exposure. (B) CsA and PDTC attenuated isoflurane-induced IL-1β elevation to a similar level, whereas CsA or PDTC treatment alone did not affect IL-1β expression levels. Data are presented as the mean ± standard deviation (n=6). **P<0.01 and ***P<0.001 vs. control group; #P<0.05 vs. isoflurane-only treated grSoups. CsA, cyclosporin A; PDTC, pyrrolidine dithiocarbamate; IL-1β, interleukin 1β.
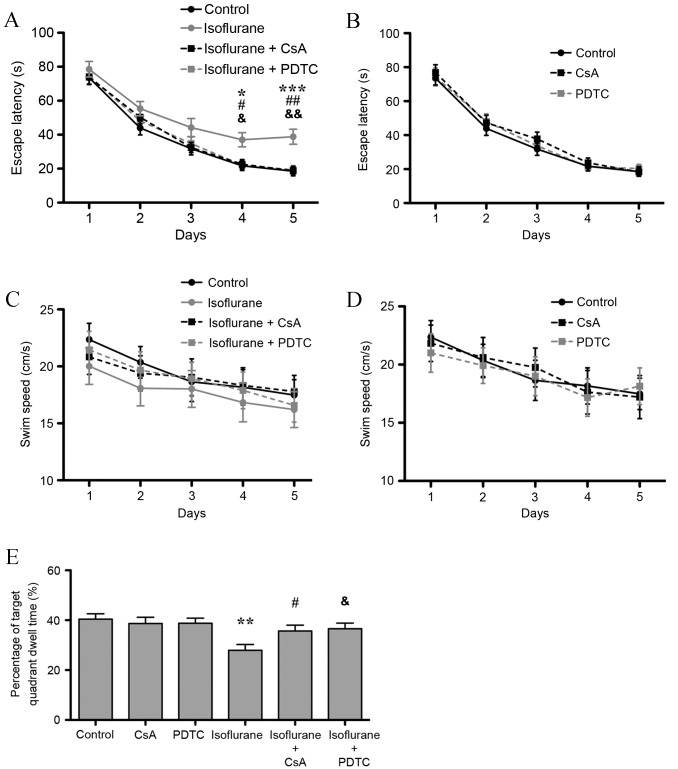
Isoflurane exposure decreased the memory and spatial learning abilities of rats
Neuroinflammation is involved in AD, the stress response (22) and in hypoxic-ischemic encephalopathy (23), and may lead to cognitive impairment. Due to the observed increase in the expression of IL-1β (a marker of neuroinflammation) in the hippocampus of aged rats following isoflurane exposure, the cognitive function of isoflurane-treated rats was further investigated. The results of the Morris water maze's place navigation test demonstrated that the group (isoflurane exposure, CsA and PDTC treatment) and repeated (Morris water maze training time) factors significantly affected escape latency (P=0.014 and P<0.001), and no interaction was found (P>0.05). The Bonferroni test demonstrated that exposure to isoflurane prolonged escape latency on the 4th and 5th days following isoflurane exposure (P<0.05 and P<0.001, respectively; Fig. 5A). Furthermore, CsA and PDTC treatment attenuated isoflurane-induced escape latency prolongation also on the 4th and 5th days (P<0.05 and P<0.01, respectively), and no difference between isoflurane + CsA and isoflurane + PDTC groups was observed (P>0.05; Fig. 5A). CsA or PDTC treatment alone did not affect escape latency (Fig. 5B). As shown in Fig. 5C and D, no significant difference in swim speed was observed among all the groups (P>0.05). In the probe test, isoflurane treatment significantly decreased target quadrant dwell time compared with untreated controls (P<0.01; Fig. 5E). CsA or PDTC treatment attenuated the isoflurane-induced decrease in target quadrant dwell time (P<0.05), and no difference between isoflurane + CsA and isoflurane + PDTC groups was observed (P>0.05, Fig. 5E). However, treatment with CsA or PDTC alone did not affect target quadrant dwell time (P>0.05, Fig. 5E). The results indicate that isoflurane induced hippocampal mitochondrial dysfunction and activated the CaN/NF-κB signaling pathway, which led to neuroinflammation and subsequent cognitive impairment in aged rats.
Figure 5.
CsA and PDTC attenuates isoflurane exposure-induced spatial memory impairment in aged rats. (A) Isoflurane exposure increased escape latency, and CsA and PDTC attenuated isoflurane-induced increase in escape latency to a similar level. (B) CsA or PDTC treatment alone did not affect escape latency. (C) Isoflurane, isoflurane + CsA or isoflurane + PDTC treatment did not affect swim speed. (D) CsA or PDTC treatment did not affect swim speed. (E) Isoflurane exposure decreased target quadrant dwell time, and CsA and PDTC attenuated the isoflurane-induced decrease in target quadrant dwell time to a similar level. CsA or PDTC treatment alone did not affect target quadrant dwell time. Data are presented as the mean ± standard error (n=12). *P<0.05, **P<0.01 and ***P<0.001 isoflurane group vs. the control group; #P<0.05 and ##P<0.01 isoflurane + CsA group vs. isoflurane group; &P<0.05 and &&P<0.01 isoflurane + PDTC group vs. isoflurane group. CsA, cyclosporin A; PDTC, pyrrolidine dithiocarbamate.
Discussion
Ca2+ is involved in the regulation of multiple cellular functions (24), and mitochondrial Ca2+ uptake and release serve fundamental roles in a number of physiological processes, such as ATP generation and cellular metabolism (25). In addition, disruption of mitochondrial Ca2+ may induce apoptotic processes through mPTP opening (26). The results of the present study indicate that isoflurane impairs mitochondrial function, and mitochondrial impairment may result in cytosolic Ca2+ elevation. Furthermore, isoflurane may induce overactivation of inositol 1,4,5-trisphosphate or ryanodine receptors located on the endoplasmic reticulum (ER) membrane (27), leading to Ca2+ escaping from the ER. Based on the results presented in the current study and a previous study (28), CaN, a Ca2+-dependent serine/threonine protein kinase, is activated following cytosolic Ca2+ elevation, which may initiate mitochondrial retrograde signaling.
NF-κB signaling participates in mitochondrial biogenesis and metabolism (29). In the central nervous system, NF-κB functions as a pleiotropic regulator of target gene expression, hippocampal neurogenesis (30) and neurodegeneration (31). The results of the present study suggest that isoflurane activates NF-κB following mitochondrial dysfunction and CaN activation, and CaN is an upstream regulator of NF-κB activation. A complex interaction between CaN and NF-κB has been reported previously (32,33). CaN may dephosphorylate Bcl-10, which leads to IKKβ phosphorylation, IκBα degradation, RelA nuclear translocation and DNA binding in T helper cells (19). These results are consistent with those of the current study demonstrating that an increase in CaN expression and activity in the hippocampus, is associated with a reduction in IκBα expression. However, it has also been reported that CaN inhibition may promote NF-κB activation in kidney tubular cells (34). In addition, NF-κB has been reported to activate regulator of calcineurin 1 gene expression (35), which may subsequently interact with calcineurin A and activate downstream signaling pathways thereby affecting central nervous system development. In contrast, the results of the present study indicate that NF-κB inhibition does not affect CaN activity following isoflurane exposure.
NF-κB is a nuclear transcription factor that regulates the expression of genes that are critical for the regulation of apoptosis, inflammation and the immune system. In the nervous system, NF-κB was observed to inhibit neurite growth through the phosphorylation of RelA on Ser-536 (36). RelA-containing dimers induce proapoptotic gene expression in ischemic neurons, and its inhibition exerts protective effects in neuronal cells (37). Alcohol induces IL-6, IL-1β and tumor necrosis factor-α elevation in the mouse brain through NF-κB signaling (38). In addition, NF-κB inhibition reduces proinflammatory inducible nitric oxide synthase and cytochrome c oxidase subunit 2 expression levels, and ameliorates inflammation and locomotor recovery during spinal cord injury (39). The results of the present study indicate that isoflurane elevates IL-1β expression (a typical marker of neuroinflammation) in the hippocampus of aged rats, and inhibition of CaN and NF-κB may attenuate this process. This indicates that the CaN/NF-κB-mediated mitochondrial retrograde signaling pathway may be involved in the upstream mechanism for isoflurane-induced neuroinflammation in the hippocampus.
The results of the present study indicate that isoflurane induces cognitive impairment in aged rats. The pathological consequences of anesthetic-induced cognitive impairment includes amyloid-β accumulation and tau phosphorylation (40). In addition, the immune system exacerbates POCD pathology (7), and inflammatory cytokines and microglia may be potential therapeutic targets. In the present study, inhibition of CaN and NF-κB attenuated hippocampal neuroinflammation and cognitive dysfunction, which confirms the role of neuroinflammation in inhaled anesthetic-induced cognitive impairment. This indicates that the CaN/NF-κB signaling pathway may present a potential therapeutic target for the treatment and/or prevention of hippocampal neuroinflammation and memory impairment in aged rats.
CaN dephosphorylates the Ca2+ sensor/translocation domain of nuclear factor of activated T-cells (NFAT), which leads to NFAT nuclear translocation and activation of target gene expression (41). A previous study indicated that consistent with intermediate to severe AD, the nuclear translocation of NFATc4 in the hippocampus may also involve in CaN-mediated memory impairment following isoflurane exposure (28). Moreover, the cAMP response element-binding protein, CCAAT enhancer binding protein δ, and the heterogeneous ribonucleoprotein A2 are reportedly involved in CaN-mediated mitochondrial retrograde signaling and contribute to the regulation of downstream target gene expression (42). Thus, the role of these factors in isoflurane-induced memory impairment, and the mechanisms underlying these processes, may require further investigation.
In conclusion, the results of the present study indicate that isoflurane induces hippocampal mitochondrial dysfunction and CaN activation, and CaN functions as an upstream mediator of NF-κB activation. In addition, the protein expression levels of IL-1β were elevated following isoflurane exposure, which is a classical marker of neuroinflammation, and may result in cognitive impairment in aged rats. Furthermore, both CaN and NF-κB inhibition attenuated isoflurane-induced neuroinflammation and subsequent cognitive impairment. Collectively, these results reveal the role of mitochondrial retrograde signaling and the factors involved in inhaled anesthetic induced-neuroinflammation and cognitive impairment. These factors may therefore present promising therapeutic targets for the treatment and/or prevention of POCD.
Acknowledgements
The present study was supported by the National Natural Science Foundation Of China (grant no. 81400869) and the Scientific Research Foundation for Returned Scholars (awarded to Dr Cheng Ni, Peking University Third Hospital, Beijing, China).
References
- 1.Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA. Neurological Outcome Research Group and the Cardiothoracic Anesthesiology Research Endeavors Investigators: Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 2.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS. ISPOCD Group: Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–555. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 3.Heneka MT, O'Banion MK. Inflammatory processes in Alzheimer's disease. J Neuroimmunol. 2007;184:69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, Benjamin EJ, Au R, Kiel DP, Wolf PA, Seshadri S. Inflammatory markers and the risk of Alzheimer disease: The framingham study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 5.Li ZQ, Rong XY, Liu YJ, Ni C, Tian XS, Mo N, Chui DH, Guo XY. Activation of the canonical nuclear factor-κB pathway is involved in isoflurane-induced hippocampal interleukin-1β elevation and the resultant cognitive deficits in aged rats. Biochem Biophys Res Commun. 2013;438:628–634. doi: 10.1016/j.bbrc.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Xu Z, Dong Y, Wang H, Culley DJ, Marcantonio ER, Crosby G, Tanzi RE, Zhang Y, Xie Z. Age-dependent postoperative cognitive impairment and Alzheimer-related neuropathology in mice. Sci Rep. 2014;4:3766. doi: 10.1038/srep03766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu X, Lu Y, Dong Y, Zhang G, Zhang Y, Xu Z, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-α, IL-6, and IL-1β. Neurobiol Aging. 2012;33:1364–1378. doi: 10.1016/j.neurobiolaging.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solberg NO, Chamberlin R, Vigil JR, Deck LM, Heidrich JE, Brown DC, Brady CI, Jagt TA Vander, Garwood M, Bisoffi M, et al. Optical and SPION-enhanced MR imaging shows that trans-stilbene inhibitors of NF-κB concomitantly lower Alzheimer's disease plaque formation and microglial activation in AbetaPP/PS-1 transgenic mouse brain. J Alzheimers Dis. 2014;40:191–212. doi: 10.3233/JAD-131031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T, Zhang T, Yu H, Shen H, Xia W. Adjudin protects against cerebral ischemia reperfusion injury by inhibition of neuroinflammation and blood-brain barrier disruption. J Neuroinflammation. 2014;11:107. doi: 10.1186/1742-2094-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guha M, Avadhani NG. Mitochondrial retrograde signaling at the crossroads of tumor bioenergetics, genetics and epigenetics. Mitochondrion. 2013;13:577–591. doi: 10.1016/j.mito.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning and memory. Ann Neurol. 2012;71:687–698. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 13.Guha M, Srinivasan S, Biswas G, Avadhani NG. Activation of a novel calcineurin-mediated insulin-like growth factor-1 receptor pathway, altered metabolism, and tumor cell invasion in cells subjected to mitochondrial respiratory stress. J Biol Chem. 2007;282:14536–14546. doi: 10.1074/jbc.M611693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biswas G, Tang W, Sondheimer N, Guha M, Bansal S, Avadhani NG. A distinctive physiological role for IkappaBbeta in the propagation of mitochondrial respiratory stress signaling. J Biol Chem. 2008;283:12586–12594. doi: 10.1074/jbc.M710481200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X, Nguyen BC, Dziunycz P, Chang S, Brooks Y, Lefort K, Hofbauer GF, Dotto GP. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature. 2010;465:368–372. doi: 10.1038/nature08996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crack PJ, Taylor JM, Ali U, Mansell A, Hertzog PJ. Potential contribution of NF-kappaB in neuronal cell death in the glutathione peroxidase-1 knockout mouse in response to ischemia-reperfusion injury. Stroke. 2006;37:1533–1538. doi: 10.1161/01.STR.0000221708.17159.64. [DOI] [PubMed] [Google Scholar]
- 17.Ni C, Tan G, Luo A, Qian M, Tang Y, Zhou Y, Wang J, Li M, Zhang Y, Jia D, et al. Melatonin premedication attenuates isoflurane anesthesia-induced β-amyloid generation and cholinergic dysfunction in the hippocampus of aged rats. Int J Neurosci. 2013;123:213–220. doi: 10.3109/00207454.2012.742895. [DOI] [PubMed] [Google Scholar]
- 18.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 19.Frischbutter S, Gabriel C, Bendfeldt H, Radbruch A, Baumgrass R. Dephosphorylation of Bcl-10 by calcineurin is essential for canonical NF-κB activation in Th cells. Eur J Immunol. 2011;41:2349–2357. doi: 10.1002/eji.201041052. [DOI] [PubMed] [Google Scholar]
- 20.Ferreiro DU, Komives EA. Molecular mechanisms of system control of NF-kappaB signaling by IkappaBalpha. Biochemistry. 2010;49:1560–1567. doi: 10.1021/bi901948j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee W, Moon M, Kim HG, Lee TH, Oh MS. Heat stress-induced memory impairment is associated with neuroinflammation in mice. J Neuroinflammation. 2015;12:102. doi: 10.1186/s12974-015-0324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirza MA, Ritzel R, Xu Y, McCullough LD, Liu F. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J Neuroinflammation. 2015;12:32. doi: 10.1186/s12974-015-0251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams GS, Boyman L, Chikando AC, Khairallah RJ, Lederer WJ. Mitochondrial calcium uptake. Proc Natl Acad Sci USA. 2013;110:10479–10486. doi: 10.1073/pnas.1300410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenár T, Csordás G, Madireddi P, Yang J, Müller M, et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol. 2012;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duchen MR. Mitochondria and Ca(2+) in cell physiology and pathophysiology. Cell calcium. 2000;28:339–348. doi: 10.1054/ceca.2000.0170. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology. 2008;109:243–250. doi: 10.1097/ALN.0b013e31817f5c47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni C, Li Z, Qian M, Zhou Y, Wang J, Guo X. Isoflurane induced cognitive impairment in aged rats through hippocampal calcineurin/NFAT signaling. Biochem Biophys Res Commun. 2015;460:889–895. doi: 10.1016/j.bbrc.2015.03.083. [DOI] [PubMed] [Google Scholar]
- 29.Bakkar N, Ladner K, Canan BD, Liyanarachchi S, Bal NC, Pant M, Periasamy M, Li Q, Janssen PM, Guttridge DC. IKKα and alternative NF-αB regulate PGC-1β to promote oxidative muscle metabolism. J Cell Biol. 2012;196:497–511. doi: 10.1083/jcb.201108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crampton SJ, O'Keeffe GW. NF-κB: Emerging roles in hippocampal development and function. Int J Biochem Cell Biol. 2013;45:1821–1824. doi: 10.1016/j.biocel.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 31.Camandola S, Mattson MP. NF-kappa B as a therapeutic target in neurodegenerative diseases. Expert Opin Ther Targets. 2007;11:123–132. doi: 10.1517/14728222.11.2.123. [DOI] [PubMed] [Google Scholar]
- 32.de la Fuente V, Federman N, Fustiñana MS, Zalcman G, Romano A. Calcineurin phosphatase as a negative regulator of fear memory in hippocampus: Control on nuclear factor-κB signaling in consolidation and reconsolidation. Hippocampus. 2014;24:1549–1561. doi: 10.1002/hipo.22334. [DOI] [PubMed] [Google Scholar]
- 33.Lim D, Iyer A, Ronco V, Grolla AA, Canonico PL, Aronica E, Genazzani AA. Amyloid beta deregulates astroglial mGluR5-mediated calcium signaling via calcineurin and Nf-kB. Glia. 2013;61:1134–1145. doi: 10.1002/glia.22502. [DOI] [PubMed] [Google Scholar]
- 34.González-Guerrero C, Ocaña-Salceda C, Berzal S, Carrasco S, Fernández-Fernández B, Cannata-Ortiz P, Egido J, Ortiz A, Ramos AM. Calcineurin inhibitors recruit protein kinases JAK2 and JNK, TLR signaling and the UPR to activate NF-κB-mediated inflammatory responses in kidney tubular cells. Toxicol Appl Pharmacol. 2013;272:825–841. doi: 10.1016/j.taap.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Zheng L, Liu H, Wang P, Song W, Sun X. Regulator of calcineurin 1 gene transcription is regulated by nuclear factor-kappaB. Curr Alzheimer Res. 2014;11:156–164. doi: 10.2174/1567205010666131212114907. [DOI] [PubMed] [Google Scholar]
- 36.Gutierrez H, O'Keeffe GW, Gavaldà N, Gallagher D, Davies AM. Nuclear factor kappa B signaling either stimulates or inhibits neurite growth depending on the phosphorylation status of p65/RelA. J Neurosci. 2008;28:8246–8256. doi: 10.1523/JNEUROSCI.1941-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inta I, Paxian S, Maegele I, Zhang W, Pizzi M, Spano P, Sarnico I, Muhammad S, Herrmann O, Inta D, et al. Bim and Noxa are candidates to mediate the deleterious effect of the NF-kappa B subunit RelA in cerebral ischemia. J Neurosci. 2006;26:12896–12903. doi: 10.1523/JNEUROSCI.3670-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Wei G, Di Z, Zhao Q. miR-339-5p inhibits alcohol-induced brain inflammation through regulating NF-κB pathway. Biochem Biophys Res Commun. 2014;452:450–456. doi: 10.1016/j.bbrc.2014.08.092. [DOI] [PubMed] [Google Scholar]
- 39.Rafati DS, Geissler K, Johnson K, Unabia G, Hulsebosch C, Nesic-Taylor O, Perez-Polo JR. Nuclear factor-kappaB decoy amelioration of spinal cord injury-induced inflammation and behavior outcomes. J Neurosci Res. 2008;86:566–580. doi: 10.1002/jnr.21508. [DOI] [PubMed] [Google Scholar]
- 40.Dong Y, Wu X, Xu Z, Zhang Y, Xie Z. Anesthetic isoflurane increases phosphorylated tau levels mediated by caspase activation and Aβ generation. PLoS One. 2012;7:e39386. doi: 10.1371/journal.pone.0039386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canellada A, Cano E, Sánchez-Ruiloba L, Zafra F, Redondo JM. Calcium-dependent expression of TNF-alpha in neural cells is mediated by the calcineurin/NFAT pathway. Mol Cell Neurosci. 2006;31:692–701. doi: 10.1016/j.mcn.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Guha M, Tang W, Sondheimer N, Avadhani NG. Role of calcineurin, hnRNPA2 and Akt in mitochondrial respiratory stress-mediated transcription activation of nuclear gene targets. Biochim Biophys Acta. 2010;1797:1055–1065. doi: 10.1016/j.bbabio.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]