Abstract
Behcet's disease is a multi-system inflammatory disorder, and ocular Behcet's disease (OBD) is one of the most common causes of uveitis in China. A number of studies have indicated that Th17 cells, a subset of interleukin-17 (IL-17)-producing CD4+ T-helper cells, serve important roles in the pathogenesis of OBD. Berberine (BBR) is an isoquinoline derivative alkaloid isolated from Chinese herbs, and has been used traditionally for the treatment of gastrointestinal disorders. The aim of the present study was to investigate the effect of BBR on Th17 cell proliferation and cytokine secretion, and the expression and activation of the signal transducer and activator of transcription 3 (STAT3) transcription factor in OBD in vitro. Blood samples were obtained from healthy controls and patients with active ocular Behcet's disease. Peripheral blood mononuclear cells (PBMCs) or CD4+ T cells were cultured for three days with or without BBR and in the presence of anti-CD3 and anti-CD28 antibodies. IL-17 expression in cell sample supernatants was determined by enzyme-linked immunosorbent assay, and cell viability was measured using the Cell Counting kit-8 assay. The number of CD4+IL-17+ cells and the expression level of phosphorylated (p)-STAT3 in CD4+ T cells was determined using flow cytometry analysis. The expression of IL-17 was increased in patients with active OBD following the activation of PBMCs and CD4+ T cells with anti-CD3 and anti-CD28 antibodies when compared with healthy controls. However, no significant difference in cell viability following exposure to BBR was observed in PBMCs derived from healthy controls or patients with OBD. Following incubation with BBR, the expression of IL-17 was reduced and the number of CD4+IL-17+ cells was decreased in patients with active OBD and healthy controls. Furthermore, the expression of p-STAT3 was significantly decreased in the presence of BBR in healthy controls. In conclusion, the results of the present study demonstrate that BBR may suppress the Th17 response in patients with OBD by reducing STAT3 phosphorylation. BBR may be a potential therapeutic agent for the treatment of OBD.
Keywords: berberine, ocular Behcet's disease, Th17, signal transducer and activator of transcription 3
Introduction
Berberine (BBR) is an isoquinoline derivative alkaloid isolated from Chinese herbs, such as those from the Berberis and Coptis genera (1). Aside from its traditional use as a treatment for gastrointestinal disorders, BBR has been demonstrated to exhibit inhibitory functions in a number of additional diseases, including cancer (2), microbial infection (3), inflammatory and autoimmune diseases (such as cardiovascular disease) (4), collagen-induced arthritis (5), experimental type I diabetes in mice (6) and experimental autoimmune encephalomyelitis (EAE) (7). Numerous reports have demonstrated that BBR may suppress the Th17 response in certain autoimmune diseases (6–8). However, it remains unclear whether BBR exerts this function by suppressing the Th17 response in Behcet's disease.
Behcet's disease is a multi-system inflammatory disorder characterized by recurrent uveitis, oral aphthae, genital ulcers or skin lesions (9). Ocular Behcet's disease (OBD) is one of the most common causes of uveitis in China (9). A number of studies have indicated that Th17 cells, a subset of interleukin-17 (IL-17)-producing CD4+ T helper cells, and their relevant cytokines, serve important roles in the pathogenesis of experimental autoimmune uveitis (EAU), as well as in clinical uveitis (including OBD) (10–12). IL-17, the characteristic cytokine produced by Th17 cells, has been observed to be upregulated in patients with active OBD, and neutralization of IL-17 using a specific antibody or through the inhibition of Th17 cells, led to the amelioration of EAU (10,13). Corticosteroids and cyclosporin A, which are frequently used as effective immunosuppressive drugs to treat clinical uveitis, may significantly downregulate the Th17 cell response in patients with OBD (14).
The signal transducer and activator of transcription 3 (STAT3) serves a critical role in Th17 differentiation and IL-17 production (15). STAT3 activation is characterized by STAT3 phosphorylation, and previous studies have demonstrated that patients with active Behcet's disease exhibit elevated levels of STAT3 phosphorylation (16,17).
The aim of the present study was to investigate the effect of BBR on the Th17 response in OBD. The results demonstrated that patients with active OBD exhibited an increased Th17 response. In vitro experiments revealed that BBR significantly downregulated the expression of IL-17 and the number of Th17 cells in patients with active OBD, and BBR could inhibit the phosphorylation of STAT3. This suggests that BBR may suppress intraocular inflammation in Behcet's disease, likely through downregulating the phosphorylation of STAT3, thereby inhibiting the Th17 response.
Materials and methods
BBR
BBR was purchased from Sigma-Aldrich; Merck Millipore (Darmstadt, Germany), and was dissolved in dimethyl sulfoxide (DMSO) before the stock solution (50 mM) was stored at −20°C.
Patients and healthy controls
A total of 16 patients with active OBD, who were admitted to The Second Xiangya Hospital of Central South University (Changsha, China) from June 2014 to April 2015 (9 males and 7 females; average age, 34.8 years), and 18 healthy controls (HC; 10 males and 8 females; average age, 36.4 years) were included in the present study. Behcet's disease was diagnosed according to the diagnostic criteria of the International Study Group for Behcet's Disease (18). The patients presented with active uveitis, as evidenced by the presence of: i) Intraocular inflammation, which was characterized by the presence of floating cells in the anterior chamber or vitreous (100%); and ii) retinal vasculitis, which was characterized by fundus fluorescein angiography (100%). The extraocular systemic findings included recurrent oral aphthous ulcers (100%), recurrent genital ulcers (43.8%), skin lesions (56.3%) and arthritis (18.8%). The present study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University (Changsha, China). All procedures complied with the Declaration of Helsinki (19), and informed consent was obtained from all healthy individuals and patients with Behcet's disease that were included in the study.
Isolation and culture of cells
Anticoagulated whole blood samples were obtained from HCs and patients with active OBD. PBMCs were isolated using Ficoll-Hypaque density gradient centrifugation. Blood cells were diluted 1:1 with PBS. Ficoll-Hypaque solution (3–4 ml) was added into 15-ml centrifuge tubes, and the diluted blood cells (6–8 ml) were slowly layered on top of the Ficoll-Hypaque solution. The tube was centrifuged at 4°C at 800 × g for 25 min without the break. Following centrifugation, the PBMC layer appeared as a white, cloudy band between the blood cell and Ficoll-Hypaque layers, and was transferred to a new centrifuge tube and washed in PBS three times. Peripheral CD4+ T cells were isolated using human CD4 microbeads followed by magnetic-activated cell sorting according to the manufacturer's instructions (Miltenyi Biotec, Inc., San Diego, CA, USA). The purity of isolated cells identified by flow cytometry was >94%. PBMCs and CD4+ T cells were resuspended in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing L-glutamine (2 mM), penicillin/streptomycin (100 U/ml), and 10% fetal calf serum (Gibco; Thermo Fisher Scientific, Inc.), to a final concentration of 1×106 cells/ml. Cells were cultured for 3 days in the presence of BBR (5 µM) or DMSO and anti-CD3 (catalog no. 130-093-387) and anti-CD28 (catalog no. 130-093-375) antibodies (0.5 and 0.1 µg/ml, respectively; Miltenyi Biotec, Inc.) at 37°C, 100% humidity and 5% CO2.
Enzyme-linked immunosorbent assay (ELISA)
The levels of IL-17 in PBMCs and CD4+ T cell sample supernatants were measured using DuoSet ELISA Development Systems assay kits with a detection limit of 15.6 pg/ml according to the manufacturer's protocol (catalog no. DY317; R&D Systems, Inc., Minneapolis, MN, USA).
Cell viability assay
The PBMC suspension was seeded onto a 96-well plate (1×106 cells/ml), and treated with BBR (5 µM) or DMSO, and cultured for 3 days in the presence of anti-CD3 (0.5 µg/ml) and anti-CD28 (0.1 µg/ml) antibodies. Cell viability was measured using the Cell Counting kit-8 (Sigma-Aldrich; Merck Millipore). Absorbance was read at 450 nm using an ELISA plate reader.
Flow cytometry
CD4+ T cells were cultured in the presence of BBR or DMSO, and anti-CD3 (0.5 µg/ml) and anti-CD28 antibodies (0.1 µg/ml). In order to determine the number of IL-17+ cells, CD4+ T cell samples were stimulated with phorbol 12-myristate 13-acetate (100 ng/ml) and ionomycin (1 µg/ml; Sigma-Aldrich; Merck Millipore) for 5 h. During the final 4 h, brefeldin A (10 µg/ml; Sigma-Aldrich; Merck Millipore) was added. Fixation/Permeabilization Concentrate (1 part; catalog no. 00-5123-43; eBioscience; Affymetrix, Inc., Santa Clara, CA, USA) was mixed with Fixation/Permeabilization Diluent (3 parts; catalog no. 00-5223-56; eBioscience; Affymetrix, Inc.), and 1 ml of this Fixation/Permeabilization solution was added to stimulated cells for 30 min at 4°C. Subsequently, 2 ml 1X Permeabilization Buffer (catalog no. 00-8333; eBioscience; Affymetrix, Inc.) was added to wash the cells. Cells were resuspended in 100 µl 1X Permeabilization Buffer, and stained with a fluorescein isothiocyanate (FITC)-conjugated mouse anti-human IL-17 antibody (catalog no. 11-7179-41; eBioscience; Affymetrix, Inc.) or a FITC-conjugated mouse IgG1 κ isotype control (catalog no. 11-4714-41; eBioscience; Affymetrix, Inc.). The IL-17+ cells in the CD4+ T cell population were defined as Th17 cells.
Detection of phosphorylated (p)-STAT3 expression in CD4+ T cells was achieved by culturing cells in the presence of BBR (5 µM) or DMSO, together with anti-CD3 (0.5 µg/ml) and anti-CD28 antibodies (0.1 µg/ml) for 30 min. Cells were subsequently fixed and permeabilized as described above, and stained with a phycoerythrin (PE)-conjugated mouse anti-human pSTAT3 antibody (catalog no. 558557; BD Biosciences, San Jose, CA, USA) or a PE-conjugated mouse IgG1 isotype control (catalog no. 12-4714; eBioscience; Affymetrix, Inc.).
Flow cytometry analysis was performed using a FACSAria cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star, Inc., San Carlos, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation. Statistical analysis was performed using SPSS (version 13.0; SPSS, Inc., Chicago, IL, USA). Differences between sample groups were analyzed using Student's t-test, Mann-Whitney U-test and the Wilcoxon signed-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
BBR inhibited the production of IL-17 but had no effect on the viability of PBMCs
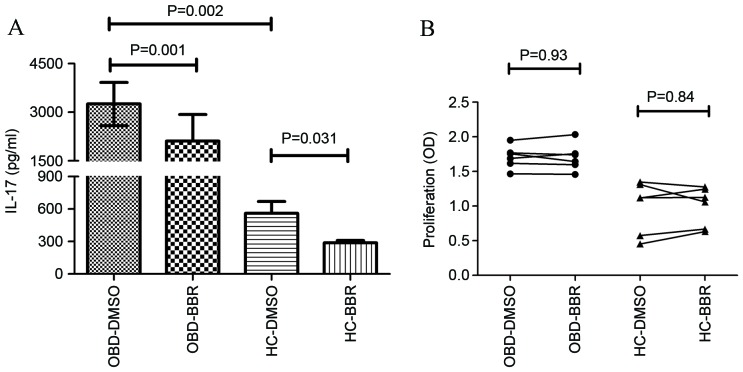
PBMCs obtained from HC and patients with active OBD were cultured in the presence of BBR (5 µM) or DMSO, together with anti-CD3 and anti-CD28 antibodies for 3 days, in order to investigate the effect of BBR on IL-17 production. Patients with active OBD demonstrated a significant increase in IL-17 expression compared with HC (3,251.98±666.02 vs. 561.61±108.37 pg/ml; P=0.002; Fig. 1A). As shown in Fig. 1A, BBR significantly inhibited the expression of IL-17 in PBMCs from patients with active OBD (from 3,251.98±666.02 to 2,113.63±815.07 pg/ml; P=0.0001) and HC (from 561.61±108.37 to 287.66±23.67 pg/ml; P=0.031). In order to investigate whether BBR inhibited IL-17 production by reducing PBMC cell viability, the effect of BBR exposure on PBMC cell viability was then examined. As shown in Fig. 1B, BBR did not have a significant effect on the viability of PBMC cells in HC or OBD groups at a concentration of 5 µM.
Figure 1.
Effect of BBR on PBMC IL-17 production and cell viability. (A) The expression of IL-17 in PBMCs derived from HC (n=6) and patients with active OBD (n=6) was significantly decreased in the presence of BBR (5 µM) compared with DMSO, as determined by flow cytometry analysis. (B) No significant difference in PBMC cell viability was observed between HC (n=6) and OBD (n=6) groups. Data are presented as the mean ± standard deviation. BBR, berberine; IL-17, interleukin 17; PBMC, peripheral blood mononuclear cells; HC, healthy control; OBD, ocular Behcet's disease; DMSO, dimethyl sulfoxide.
BBR inhibited the production of IL-17 in CD4+ T cells from HCs and patients with active OBD
The expression of IL-17 in CD4+ T cells was significantly higher upon stimulation with anti-CD3 and anti-CD28 antibodies in patients with active OBD compared with HCs (1,981.73±869.59 vs. 253.31±77.15 pg/ml; P=0.002; Fig. 2). Exposure to BBR significantly decreased the production of IL-17 by CD4+ T cells in active OBD patients (from 1,981.73±869.59 to 1,410.39±545.41 pg/ml; P=0.031) and HCs (from 253.31±77.15 to 185.11±67.80 pg/ml; P=0.001; Fig. 2).
Figure 2.
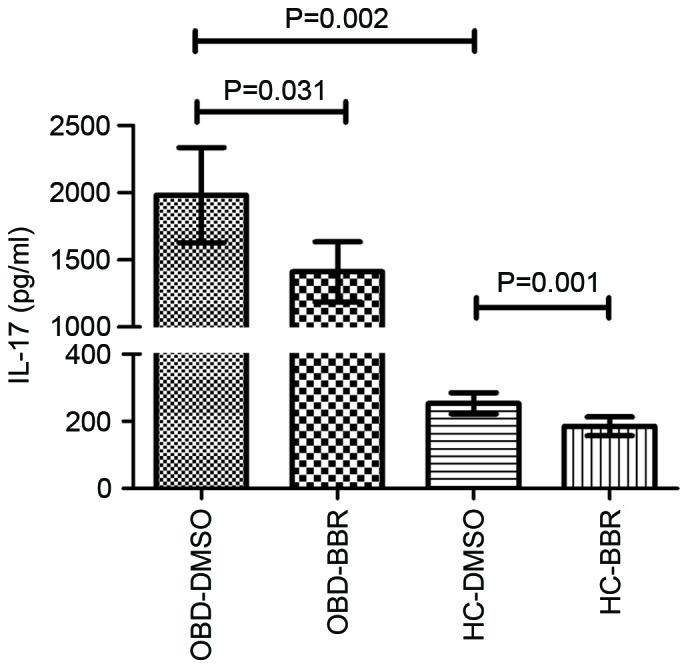
Effect of BBR on IL-17 expression in CD4+ T cells. In the presence of BBR, the expression of IL-17 in CD4+ T cells from HCs (n=6) and patients with active OBD (n=6) was significantly reduced compared with DMSO-treated cells. Data are presented as the mean ± standard deviation. BBR, berberine; IL-17, interleukin 17; HC, healthy control; OBD, ocular Behcet's disease; DMSO, dimethyl sulfoxide.
BBR inhibited the number of Th17 cells in patients with active OBD and healthy controls
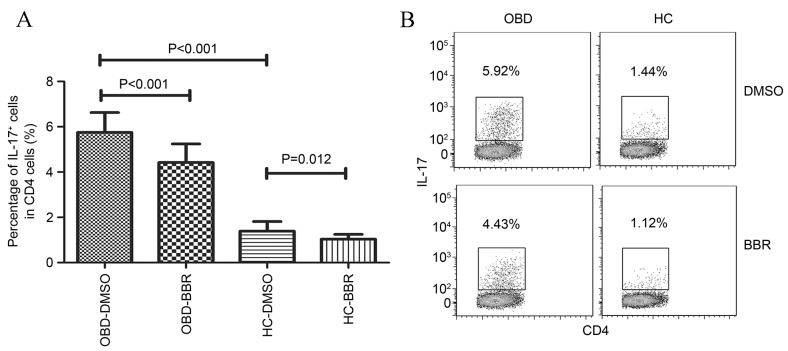
As shown in Fig. 3, the number of Th17 cells (IL-17-producing CD4+ T cells) was significantly higher in patients with active OBD compared with HCs (5.75±0.88 vs. 39±0.43%; P<0.001). In addition, exposure to BBR significantly inhibited the number of Th17 cells in patients with active OBD (from 5.75±0.88 to 4.42±0.82%; P<0.001) and HCs (from 1.39±0.43 to 1.03±0.22%; P=0.012; Fig. 3).
Figure 3.
Effect of BBR on the number of Th17 (IL-17+) cells. (A) The results represent the percentages of IL-17+ cells among CD4+ T cells obtained from HCs and patients with active OBD, following treatment with BBR or DMSO. (B) Representative plots of IL-17+ cells in a HC and a patient with OBD. The percentage of IL-17+ cells among CD4+ cells is indicated in each plot. Data are presented as the mean ± standard deviation. BBR, berberine; IL-17, interleukin 17; HC, healthy control; OBD, ocular Behcet's disease; DMSO, dimethyl sulfoxide.
BBR inhibited the activation of STAT3
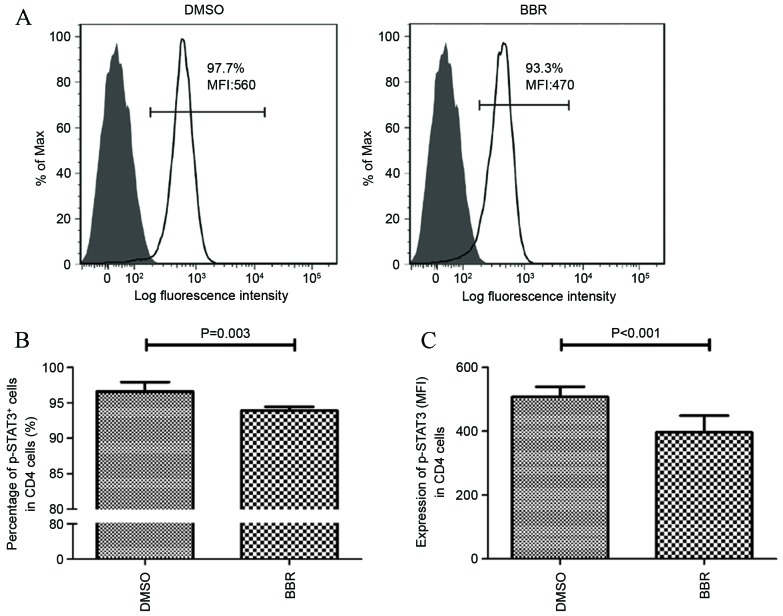
Due to the observation that STAT3 activation is an important factor involved in the differentiation of Th17 cells and the production of IL-17 (15), the effect of BBR on modulating STAT3 phosphorylation was investigated. As shown in Fig. 4A and B, exposure of CD4+ T cells, obtained from HCs, to BBR significantly decreased the number of p-STAT3-positive cells from 96.6±1.24 to 93.9±0.54% (P=0.0.003). The expression of p-STAT3 was then determined using the mean fluorescence intensity (MFI) parameter. The results demonstrated that the MFI value of p-STAT3 expression in BBR-treated CD4+ T cells was significantly lower compared with that of the DMSO-treated cells (506.67±32.75 vs. 396.33±58.49; P<0.001; Fig. 4C).
Figure 4.
Effects of BBR on STAT3 activation. (A) Representative histograms illustrate the expression of p-STAT3 in anti-CD3/28-stimulated CD4+ T cells in a HC treated with DMSO or BBR. (B) The number of p-STAT3+ cells expressed as a percentage of CD4+ T cells from HC following exposure to BBR or DMSO. (C) The expression of p-STAT3 expressed as the mean fluorescence intensity (MFI) in CD4+ T cells from HCs following exposure to BBR or DMSO. Data are presented as the mean ± standard deviation. BBR, berberine; p-STAT3, phosphorylated signal transducer and activator of transcription 3; DMSO, dimethyl sulfoxide; HC, healthy control.
Discussion
In the present study the potential role of BBR as a potential therapeutic agent for OBD was investigated. The results demonstrated that BBR suppressed IL-17 expression and the number of Th17 cells in PBMCs obtained from patients with active OBD and HCs. In addition, BBR inhibited the activation of STAT3. These results suggest that BBR may suppress the Th17 response in patients with OBD by downregulating the phosphorylation of STAT3, and therefore provides a rationale for investigating the potential use of BBR for the treatment of patients with OBD in a clinical setting.
BBR is an isoquinoline alkaloid isolated from certain Chinese herbs, such as Berberis, Hydrastis canadensis and Coptidis rhizoma (1). The role of BBR in the treatment of a number of inflammatory and autoimmune disorders has been reported previously (4,6,7,20,21). In addition, the role of BBR in uveitis has been investigated; in endotoxin-induced uveitis, Berberis aristata, whose primary components include BBR, inhibited ocular inflammation (22). Furthermore, BBR demonstrated an immunoregulatory role in the Vogt-Koyanagi-Harada ocular autoimmune disease (8). These studies support a role for BBR as an anti-inflammatory agent.
As Th17 cell-associated inflammatory responses are critically involved in the pathogenesis of OBD (23), the present study was designed to investigate the effect of BBR on the function of Th17 cells in OBD. The effect of BBR on the secretion of IL-17 by PBMCs was first investigated. Consistent with Chi et al (24), a significant increase in the expression of IL-17 was observed in patients with active OBD compared with HCs. Following exposure to BBR, IL-17 expression in PBMCs was significantly suppressed. Cell viability is an important parameter that can influence cytokine production (25). Therefore, we investigated whether BBR-mediated suppression of IL-17 expression was due to the suppression of T-cell viability. The results demonstrated that BBR did not influence T cell viability when exposed to a concentration of 5 µM. This result is largely consistent with a previous report, in which 50 µM BBR did not increase the death rate of lymphocytes (26).
The results of the present study indicate a suppressive effect of BBR on the secretion of IL-17 by PBMCs. As Th17 cells primarily produce IL-17, CD4+ T cells were purified from PBMCs in order to verify the suppressive effect of BBR on IL-17 secretion by Th17 cells. The results revealed that BBR may inhibit the secretion of IL-17 by Th17 cells.
Previous studies have demonstrated that STAT3 activation is critical for Th17 differentiation and IL-17 production (15). STAT3 activation is characterized by its phosphorylation, which leads to the regulation of downstream target genes that mediate intrinsic and extrinsic cellular functions (15). In the present study STAT3 phosphorylation was significantly inhibited in CD4+ T cells following BBR exposure, which may have been responsible for the suppressive effect of BBR on the function of Th17 cells. This result is consistent with those presented by Qin et al (7), which used a mouse model of EAE, and demonstrated that Th17 cell differentiation was markedly inhibited by BBR treatment in vivo and in vitro, and that this was associated with decreased phosphorylation of STAT3 in Th17 cells.
Despite these observations, a number of important questions for the use of BBR as a potential treatment for OBD remain. Namely, the selection of a safe but effective dose of BBR for the treatment of OBD. Our results demonstrate that BBR is not a cytotoxic or a general immunosuppressive agent that affects T cell survival in vitro. In addition, BBR is currently used for the treatment of numerous gastrointestinal diseases in China, and to the best of our knowledge, no unexpected safety concerns have yet been identified. A recent randomized, placebo-controlled, double-blind trial concerning the effects of BBR gelatin on recurrent aphthous stomatitis, has demonstrated that the use of BBR gelatin is safe and effective (27). Although there is no evidence to suggest that increasing the dose of BBR may cause severe adverse effects, there have been no reports on the safety of BBR treatment in large doses and in the long-term, thus, the appropriate dose of BBR for the treatment of OBD in vivo remains a critical factor that requires further investigation. In addition, the concentration of BBR in the plasma is an important aspect for improving the symptoms of autoimmune disease (28). Therefore further studies are required to define the optimal plasma BBR concentrations for the treatment of OBD in vivo.
Another important consideration for the use of BBR as a treatment for OBD, is based on the observation that BBR did not completely suppress the Th17 cell response in the present study. According to these results, the decreased levels of IL-17 expression and Th17 cell number following exposure of PBMCs obtained from OBD patients to BBR, were not as low as observed in the HC group. This suggests that BBR treatment alone may be insufficient to control the disease. Therefore, further studies are required to investigate whether BBR in combination with other currently used immunosuppressive agents may modulate an aberrant immune response, and may be a more suitable therapeutic approach for the treatment of patients with OBD and additional autoimmune diseases mediated by an abnormal Th17 immune response.
In conclusion, the results of the present study indicate that BBR exhibits an inhibitory effect on the Th17 cell response. BBR-mediated suppression of STAT3 activation abrogates downstream signaling events, and results in the decreased expression of IL-17 and the number of Th17 cells, which is one of the potential mechanisms underlying the immunosuppressive effects of BBR.
Acknowledgements
The authors would like to thank all of the patients and healthy donors enrolled in the study. The present study was supported by the Development Program of Hunan Provincial Science & Technology Department (grant no. 2014FJ3006).
Glossary
Abbreviations
- BBR
berberine
- OBD
ocular Behcet's disease
- PBMC
peripheral blood mononuclear cells
- STAT3
signal transducer and activator of transcription 3
- DMSO
Dimethyl sulfoxide
- ELISA
Enzyme-linked immunosorbent assay
- EAE
experimental autoimmune encephalomyelitis
- EAU
experimental autoimmune uveitis
- HC
healthy controls
- MFI
mean fluorescence intensity
References
- 1.Ye M, Fu S, Pi R, He F. Neuropharmacological and pharmacokinetic properties of berberine: A review of recent research. J Pharm Pharmacol. 2009;61:831–837. doi: 10.1211/jpp.61.07.0001. [DOI] [PubMed] [Google Scholar]
- 2.Song YC, Lee Y, Kim HM, Hyun MY, Lim YY, Song KY, Kim BJ. Berberine regulates melanin synthesis by activating PI3K/AKT, ERK and GSK3β in B16F10 melanoma cells. Int J Mol Med. 2015;35:1011–1016. doi: 10.3892/ijmm.2015.2113. [DOI] [PubMed] [Google Scholar]
- 3.Saha P, Bhattacharjee S, Sarkar A, Manna A, Majumder S, Chatterjee M. Berberine chloride mediates its anti-leishmanial activity via differential regulation of the mitogen activated protein kinase pathway in macrophages. PLoS One. 2011;6:e18467. doi: 10.1371/journal.pone.0018467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Chi L, Peng L, Pan N, Hu X, Zhang Y. The anti-atherogenic effects of berberine on foam cell formation are mediated through the upregulation of sirtuin 1. Int J Mol Med. 2014;34:1087–1093. doi: 10.3892/ijmm.2014.1868. [DOI] [PubMed] [Google Scholar]
- 5.Hu Z, Jiao Q, Ding J, Liu F, Liu R, Shan L, Zeng H, Zhang J, Zhang W. Berberine induces dendritic cell apoptosis and has therapeutic potential for rheumatoid arthritis. Arthritis Rheum. 2011;63:949–959. doi: 10.1002/art.30202. [DOI] [PubMed] [Google Scholar]
- 6.Cui G, Qin X, Zhang Y, Gong Z, Ge B, Zang YQ. Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J Biol Chem. 2009;284:28420–28429. doi: 10.1074/jbc.M109.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin X, Guo BT, Wan B, Fang L, Lu L, Wu L, Zang YQ, Zhang JZ. Regulation of Th1 and Th17 cell differentiation and amelioration of experimental autoimmune encephalomyelitis by natural product compound berberine. J Immunol. 2010;185:1855–1863. doi: 10.4049/jimmunol.0903853. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Qi J, Wang Q, Du L, Zhou Y, Yu H, Kijlstra A, Yang P. Berberine suppresses Th17 and dendritic cell responses. Invest Ophthalmol Vis Sci. 2013;54:2516–2522. doi: 10.1167/iovs.12-11217. [DOI] [PubMed] [Google Scholar]
- 9.Yang P, Fang W, Meng Q, Ren Y, Xing L, Kijlstra A. Clinical features of chinese patients with Behçet's disease. Ophthalmology. 2008;115:312–318. doi: 10.1016/j.ophtha.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 10.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 11.Chi W, Yang P, Li B, Wu C, Jin H, Zhu X, Chen L, Zhou H, Huang X, Kijlstra A. IL-23 promotes CD4+ T cells to produce IL-17 in Vogt-Koyanagi-Harada disease. J Allergy Clin Immunol. 2007;119:1218–1224. doi: 10.1016/j.jaci.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Peng Y, Han G, Shao H, Wang Y, Kaplan HJ, Sun D. Characterization of IL-17+ interphotoreceptor retinoid-binding protein-specific T cells in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2007;48:4153–4161. doi: 10.1167/iovs.07-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: Conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Yang P, Lin X, Ren X, Zhou H, Huang X, Chi W, Kijlstra A, Chen L. Inhibitory effect of Cyclosporin A and corticosteroids on the production of IFN-gamma and IL-17 by T cells in Vogt-Koyanagi-Harada syndrome. Clin Immunol. 2009;131:333–342. doi: 10.1016/j.clim.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O'Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 16.Tulunay A, Dozmorov MG, Ture-Ozdemir F, Yilmaz V, Eksioglu-Demiralp E, Alibaz-Oner F, Ozen G, Wren JD, Saruhan-Direskeneli G, Sawalha AH, Direskeneli H. Activation of the JAK/STAT pathway in Behcet's disease. Genes Immun. 2014;16:170–175. doi: 10.1038/gene.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi J, Yang Y, Hou S, Qiao Y, Wang Q, Yu H, Zhang Q, Cai T, Kijlstra A, Yang P. Increased Notch pathway activation in Behçet's disease. Rheumatology (Oxford) 2014;53:810–820. doi: 10.1093/rheumatology/ket438. [DOI] [PubMed] [Google Scholar]
- 18.Criteria for diagnosis of Behçet's disease, corp-author. International Study Group for Behçet's Disease. Lancet. 1990;335:1078–1080. [PubMed] [Google Scholar]
- 19.Rickham PP. Human experimentation. Code of ethics of the world medical association. Declaration of Helsinki. Br Med J. 1964;2:177. doi: 10.1136/bmj.2.5402.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma X, Jiang Y, Wu A, Chen X, Pi R, Liu M, Liu Y. Berberine attenuates experimental autoimmune encephalomyelitis in C57 BL/6 mice. PLoS One. 2010;5:e13489. doi: 10.1371/journal.pone.0013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marinova EK, Nikolova DB, Popova DN, Gallacher GB, Ivanovska ND. Suppression of experimental autoimmune tubulointerstitial nephritis in BALB/c mice by berberine. Immunopharmacology. 2000;48:9–16. doi: 10.1016/S0162-3109(99)00189-7. [DOI] [PubMed] [Google Scholar]
- 22.Gupta SK, Agarwal R, Srivastava S, Agarwal P, Agrawal SS, Saxena R, Galpalli N. The anti-inflammatory effects of Curcuma longa and Berberis aristata in endotoxin-induced uveitis in rabbits. Invest Ophthalmol Vis Sci. 2008;49:4036–4040. doi: 10.1167/iovs.07-1186. [DOI] [PubMed] [Google Scholar]
- 23.Na SY, Park MJ, Park S, Lee ES. Up-regulation of Th17 and related cytokines in Behçet's disease corresponding to disease activity. Clin Exp Rheumatol. 2013;31(3):32–40. Suppl 77. [PubMed] [Google Scholar]
- 24.Chi W, Yang P, Zhu X, Wang Y, Chen L, Huang X, Liu X. Production of interleukin-17 in Behcet's disease is inhibited by cyclosporin A. Mol Vis. 2010;16:880–886. [PMC free article] [PubMed] [Google Scholar]
- 25.Hedegaard CJ, Krakauer M, Bendtzen K, Sorensen PS, Sellebjerg F, Nielsen CH. The effect of beta-interferon therapy on myelin basic protein-elicited CD4+ T cell proliferation and cytokine production in multiple sclerosis. Clin Immunol. 2008;129:80–89. doi: 10.1016/j.clim.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Xu L, Liu Y, He X. Inhibitory effects of berberine on the activation and cell cycle progression of human peripheral lymphocytes. Cell Mol Immunol. 2005;2:295–300. [PubMed] [Google Scholar]
- 27.Jiang XW, Zhang Y, Zhu YL, Zhang H, Lu K, Li FF, Peng HY. Effects of berberine gelatin on recurrent aphthous stomatitis: A randomized, placebo-controlled, double-blind trial in a Chinese cohort. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:212–217. doi: 10.1016/j.oooo.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Pang B, Zhao LH, Zhou Q, Zhao TY, Wang H, Gu CJ, Tong XL. Application of berberine on treating type 2 diabetes mellitus. Int J Endocrinol. 2015;2015:905749. doi: 10.1155/2015/905749. [DOI] [PMC free article] [PubMed] [Google Scholar]