Abstract
Objective:
Achillea is a traditional medicinal herb which contains different phenol and flavonoid compounds that are responsible for Achillea pharmacological effects. We aimed to determine phenol and flavonoid contents, besides antioxidant activities of different extracts from Achillea eriophoraa (A. eriophora) DC. and Achillea biebersteinii (A. biebersteinii) Afan. (endemic species in Iran) and to investigate their effects on human cells.
Materials and Methods:
Achillea extracts, were prepared by maceration and shaking methods, from different parts (aerial parts, stem, leaves and inflorescence) of two species using methanol and ethanol as solvents. Total phenol and flavonoid contents were measured by spectrophotometry, and antioxidant activities of the extracts were determined by DPPH radical scavenging, BCB and TBARS assays. Cytotoxicity and antioxidant activities of the extracts were investigated in Human Foreskin Fibroblast (HFF3) cells using MTT, comet and H2O2 assays.
Results:
Methanol extracts of A. biebersteinii prepared from leaves and inflorescence by maceration method exhibited maximum phenol (1657.58 ± 36.45 mg GAE/100 g DW) and flavonoid (264.00 ± 62.16 mg QUE/100 g DW) contents. Leaf methanol extract showed significantly higher antioxidant activity (0.0276 ± 0.003, 0.16 ± 0.016 and 13.96 ± 0.26 mg/ml for DPPH, BCB and TBARS IC50s, respectively) than those of the other extracts. Leaf extract of A. biebersteinii was not cytotoxic even at the highest examined dose (512 µg/ml) and inhibited cell toxicity induced by H2O2 (98% viability for the cells pretreated with plant extract in the presence of H2O2). Comet assay also confirmed high DNA protective activity of leaf extracts.
Conclusion:
Achillea extracts possess remarkable antioxidant activity, and could be good natural alternatives to synthetic antioxidants in pharmaceutical and food industries.
Key Words: Phenol, Flavonoids, DPPH, MTT assay, Comet, HFF3 cells
Introduction
A chillea, which belongs to the family Compositae (Asteraceae), is a genus with over 100 species all around the world. Although these medicinal perennial rhizomatous plants are native to Europe and Western Asia, they are also found in Australia, New Zealand and North America (Chevallier, 2000 ▶). The two studied species Achillea eriophora (A. eriophora) DC. And Achillea biebersteinii (A. biebersteinii) Afan. are endemic plants in Iran.
Achillea, known as “Bumadaran” in Persian, is one of the most widely used medicinal plants in Iran. It is used as hypoglycemic, nerve tonic, anti-hemorrhoid, anti-diarrhea, antacid, carminative, appetizer, anthelmintic and anti-bacterial remedies (Amiri and Joharchi, 2013 ▶; Ghorbani, 2005 ▶; Pirbalouti and Golparvar, 2007 ▶; Zargari, 1993 ▶). These pharmacological properties have been mainly attributed to the phenolic and polyphenolic compounds, which are well known as antioxidant agents (Evans, 2009 ▶; Harborne and Williams, 2000 ▶; Weber et al., 2006 ▶). Clinical evidences have revealed that antioxidants are effective in the treatment of various diseases, including atherosclerosis, arthritis, ischemia and reperfusion injury of many tissues, central nervous system injury, gastritis, and cancer, and are beneficial to the wound healing process (Cook and Samman, 1996 ▶; Kumpulainen and Salonen, 1999 ▶). These activities are in accordance with reported pharmaceutical properties for Achillea.
Antioxidant activity of different species of Achillea including Achillea millefolium L. (Candan et al., 2003 ▶), Achillea ligustica All. (Conforti et al., 2005 ▶), Achillea wilhelmsii (Ozgen et al. 2004 ▶) and A. biebersteinii (Tawaha et al., 2007 ▶) have been previously investigated. However, the protective effects of A. eriophorea and A. biebersteinii against oxidative stress in HFF3 cells have not yet been reported. In this study, two extraction methods and solvents were used to obtain phenol and flavonoid-enriched extracts from different parts of A. eriophorea and A. biebersteinii. The antioxidant activity of the methanol extracts prepared by maceration from different parts of the plants, was evaluated using different assays. To the extent of our knowledge, this is the first report about the antioxidant activity of leaf methanol extracts of A. eriophorea and A. biebersteinii in human fibroblast cells.
Materials and Methods
Chemicals
Folin-Ciocaltue, sodium carbonate, methanol, ethanol, gallic acid, aluminum chloride, 1,1-diphenyl-2-picrylhydrazyl (DPPH), beta-carotene, linoleic acid, tween40, chloroform, butylated hydroxyl toluene (BHT), 2,2’-azobis-(2-amidinopropane) dihydrochloride (ABAP), acetic acid, tiubarbituric acid, sodium dodecyl sulfate (SDS), butanol, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), H2O2, ethylenediaminetetraacetic acid (EDTA), dimethyl sulfoxide (DMSO) and all solvents were purchased from Merck (Germany). Ascorbic acid and potassium acetate were purchased from Sigma–Aldrich (St. Louis, MO, USA). Dulbecco's Modified Eagle's Medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco Life Technologies (Grand Island, NY, USA).
Sample collection and extract preparation
Plants were collected at the flowering stage in May 2011 from pastures of Khorassan Razavi (N 36.291886, E 58.583121; 1618 m above sea level) and South Khorasan provinces (N 36.34020, E 40.710158; 1591 m above sea level), Iran. The voucher specimens (No. 30348 and 22004 for A. eriophora and A. biebersteinii, respectively) were deposited in Herbarium of Ferdowsi University, Mashhad, Iran.
Grinded dry materials from different parts of plants (stem, leaves, aerial parts and inflorescence), were extracted with methanol and ethanol (1:20 w/v) using maceration and shaking. Extracts were filtered through the regular filter paper and evaporated under vacuum.
Total phenol and flavonoid assay
The total phenolic content of all extracts was determined spectrophotometrically according to Folin-Ciocalteu method (Pattanayak et al., 2012), and total flavonoid content was determined using aluminum chloride colorimetric method (Chang et al., 2002 ▶). The results were expressed in terms of gallic acid equivalent (GAE mg/g of dry weight) for phenolic content and quercetin equivalent (QE mg/g of dry weight) for flavonoid content, which are the two common reference compounds.
Antioxidant assays
DPPH radical scavenging microplate assay
The antioxidant properties of methanol extracts (0.5-7 mg/ml) or gallic acid as a standard, were investigated by reducing the stable DPPH radical (Yang et al., 2011 ▶). The absorbance was then measured at 492 nm using an ELISA reader (Awareness Technology, USA). The antioxidant index (AI %) was calculated as [(1 – A1 – A2/ A0) × 100]. Where A0 is the absorbance of the control reaction (without sample), A1 is the absorbance of sample/gallic acid, and A2 is the absorbance of sample without DPPH. Analyses were run in triplicate, and IC50 values (presenting the concentration with 50% antioxidant index) were calculated.
Beta-carotene bleaching microplate assay (BCB)
A modified method of Dapkevicius et al. (1998) ▶ was used to test bleaching ability of the extracts against beta-carotene (Dapkevicius et al., 1998 ▶). Briefly, 1 mg of beta-carotene was dissolved in chloroform (5 ml) and then, linoleic acid (25 µl) and Tween 40 (200 mg) were added to 1 ml of this mixture. After removing chloroform using a rotary evaporator at 40ºC, the remaining where solved in oxygenated distilled water (50 ml) and vigorously shacked. An aliquot of 250 µl of the above-prepared beta carotene–linoleic acid emulsion was applied to each well of a 96-well plate. Next, 30 µl of different concentrations of the extracts (0.5-7 mg/ml) or BHT as a standard (1-100 µg/ml) were added to each well in triplicate. An equal amount of the extracts or BHT was used as blank. The microplates were incubated at 55ºC and their optical densities were determined at 492 nm using an ELISA reader. Reading the absorbance of all samples was carried out at the start (t=0) and after 105 min of incubation. The antioxidant activity coefficient (AAC) was estimated according to the following formula:
ACC = [(AT105 - AB105) / (AB0–AB105)]
Where AT105 and AB105 are absorbance of sample and blank after 105 min, respectively. AB0 is the absorbance of blank at t=0.
TBARS assay
A modified TBARS assay (Bazzaz et al., 2011 ▶), using egg yolk homogenates as lipid rich media, was applied to measure the antioxidant capacity of the extracts. Briefly, 500 µl of yolk homogenate (10% w/v in distilled water) and 100 µl of sample solution (5-50 mg/ml concentrations of the extracts or standard), were added to a test tube and made up to 1 ml with distilled water, followed by addition of 50 µl of ABAP aqueous solution (0.07 M; for induction of lipid peroxidation), 1.5 ml of 20% acetic acid (pH 3.5) and 1.5 ml of 0.8% (w/v) thiobarbituric acid in 1.1% (w/v) SDS. The mixture was vortexed, and heated at 95ºC for 60 min. After cooling, 5 ml butan-1-ol was added and extensively vortexed and centrifuged at 2500 g for 10 min. The absorbance of the upper organic layer was measured at 532 nm using a spectrophotometer. Butanol and BHT were used as the blank and positive control, respectively. All testes were carried out in triplicate. Values were expressed as antioxidant index (AI%) according to the following formula:
AI% = (1 – t / c) × 100
Where t and c are the absorbance of the test sample and the fully oxidized control, respectively.
Cell culture
Cytotoxicity assay
HFF3 (Human Foreskin Fibroblast) cells (a generous gift from Royan Institute, Tehran, Iran) were seeded at 8000 cells/well in 96-well plates in DMEM supplemented with 10% FBS. After 24 h of incubation in a humidified 5% CO2/air environment at 37ºC, when cells became 70-80% confluent, they were treated with different concentrations of the extracts (1-512 µg/ml) diluted in DMEM containing 1% FBS. Following 24, 48 and 72 h of incubation with the extracts, culture media were aspirated and replaced with DMEM containing 1% FBS and 20 µl of MTT solution (0.5 mg/ml). After 4 h of incubation, media were removed and purple colored crystals were dissolved in DMSO. Absorbance of each well was measured at 450 nm using an ELISA reader.
Antioxidant activity of plant extracts on HFF3 cells
The hydrogen peroxide (H2O2) assay was modified to assess the protective effects of the extracts in HFF3 cells against the oxidative damage induced by H2O2 (Kumar and Gupta, 2011 ▶). Fibroblast cells were seeded in 96-well plates at 8000 cells/well in DMEM containing 10% FBS, and grown at 37ºC to near confluence. Cells were serum-deprived and pretreated with leaf extracts 1 µg/ml for 2 h. Pretreated cells were then exposed to different concentrations of H2O2 (10, 100, 250, 500 and 1000 mM), for 24 h. The degree of protection of fibroblast cells by extracts against H2O2 damage was then quantified by MTT assay.
Comet assay
Single cell gel electrophoresis was performed (Rassouli et al., 2011 ▶) on HFF3 cells treated with H2O2 and leaf extracts. HFF3 cells were grown in DMEM containing 10% FBS in 6-well plates, for 24 h. The attached cells were pretreated for 2 h with 5 ml DMEM (1% FBS) containing leaf extracts 1 µg/ml of both species. After this pretreatment, H2O2 (500 mM) was added and cells were incubated for 24 h. Cells were then washed with 5 ml cold PBS, and detached by trypsin/EDTA solution for further analysis. Each data point represents the average DNA in tail of at least 150 measurements (comets). Comets were analyzed with Tri Teck Comet Score version 1.5.
Statistical analysis
Phenol and flavonoid contents were analyzed using univariate ANOVA, and Duncan as multiple range mean comparing test. One-way ANOVA was used to analyze the IC50 values of antioxidant testes, means of H2O2 and comet assay results. Also, Duncan was performed as post-hoc analysis. Results were expressed as mean ± S.D. All analyses were performed using STATISTICA (Statsoft, 2011) software.
Results
Total phenol and flavonoid contents
Total phenol and flavonoids content in the Achillea species ranged from 149 to 1657 mg gallic acid equivalent (GAE) /100 g dry weight, and 59-264 mg quercetin equivalent (QUE) /100 g dry weight, respectively (Table 1). The highest total phenol content was shown by inflorescence extract followed by leaf extract of A. biebersteinii. Also, the highest total flavonoid content was recorded for leaves extract of both species. Methanol extracts prepared by maceration method, possessed higher phenol and flavonoid content as compared to those prepared with ethanol and shaking methods.
Table 1.
Effect of extraction methods (maceration and shaking) and solvent type on total phenol and total flavonoid content in different parts of Achilea eriophora and Achilea biebersteinii from Iran
| Plant organ | Solvent | Total phenolmg GAE/100 g dry plant tissue | Total flavonoidmg QUE/100 g dry plant tissue |
|---|---|---|---|
| Stem ( A. eriophora ) | methanol | 316.79 ± 43.32 | 92.13±4.02 |
| 278.02 ± 110.33 | 88.12±8.48 | ||
| ethanol | 229.93 ± 9.02 | 70.04±2.75 | |
| 225.37 ± 37.59 | 59.25±4.30 | ||
| Leaves | methanol | 1050.82 ± 184.20 | 244.06±1.27 |
| 890.17 ± 75.27 | 232.08±8.23 | ||
| ethanol | 622.76 ± 138.90 740.92 ± 84.48 |
226.42±1.71 196.58±6.71 |
|
| Aerial parts | methanol | 667.74 ± 73.30 | 216.45±2.15 |
| 581.09 ± 98.45 | 201.69±44.36 | ||
| ethanol | 327.15 ± 46.80 | 93.81±2.89 | |
| 365.29 ± 142.42 | 81.83±12.99 | ||
| Inflorescence | methanol | 812.23 ± 64.39 | 216.56±1.67 |
| 807.46 ± 105.01 | 190.13±16.35 | ||
| ethanol | 570.72 ± 51.53 | 156.97±4.88 | |
| 566.16 ± 55.75 | 133.89±10.76 | ||
| Stem ( A.biebersteinii ) | methanol | 293.36 ± 77.24 | 77.79±4.40 |
| 284.45 ± 51.04 | 74.00±4.09 | ||
| ethanol | 149.91 ± 16.39 | 74.19±7.17 | |
| 164.84 ± 12.84 | 71.01±1.54 | ||
| Leaves | methanol | 1168.98 ± 146.10 | 249.99±12.01$ |
| 1156.34 ± 76.92 | 264.00±62.16$ | ||
| ethanol | 694.48 ± 56.81 | 236.36±3.25 | |
| 719.15 ± 26.30 | 207.82±5.08 | ||
| Aerial parts | methanol | 848.30 ± 121.90 | 203.86±6.63 |
| 821.14 ± 51.98 | 210.21±21.79 | ||
| ethanol | 454.02 ± 57.88 | 214.91±4.00 | |
| 389.13 ± 81.48 | 188.15±1.93 | ||
| Inflorescence | methanol | 1657.58 ± 36.45* | 226.09±4.83 |
| 1441.79 ± 215.27 | 197.49±28.53 | ||
| ethanol | 1347.47 ± 61.37 | 184.99±8.13 | |
| 1228.68 ± 50.85 | 169.25±6.73 |
In each row, presented data belong to maceration and shaking methods, respectively.
p<0.05. Data are means ± SD of five replicates.
Evaluation of antioxidant activities by different methods
Since among all the extracts, leaf and inflorescence methanol extract showed the highest phenol and flavonoid contents, maceration extraction and methanol solvents were selected for evaluation of antioxidant activities. The antioxidant capacity of Achillea extracts, as presented in Table 2, were determined by the following three methods: DPPH, BCB and TBARS assays. According the three different assays, leaf extract of A. biebersteinii, showed the highest antioxidant activity in three selected assays. IC50 values for leaf extract of A. biebersteinii were 0.27, 0.16 and 13.96 mg/ml, in DPPH, BCB and TBARS tests, respectively. Inflorescence extract of A. eriophora showed the lowest DPPH radical scavenging activity. Also, at concentrations below 100 mg/ml of inflorescence extracts in both species, TBARS AI did not reach 50%.
Table 2.
Antioxidant activities of the Achillea extracts
| Plant Species | Plant organ |
IC50 (mg/ml)
|
||
|---|---|---|---|---|
| DPPH | BCB | TBARS | ||
| Achillea eriophora | Leaves | 0.703±0.023 b | 1.46±0.03 a | 23.83±0.5 a |
| Inflorescence | 0.91±0.001 a | 1.78±0.441 a | > 100 | |
| Achillea biebersteini | Leaves | 0.276±0.003 d | 0.16±0.016 b | 13.96±0.26 b |
| Inflorescence | 0.33±0.006 c | 1.63±0.176 a | > 100 | |
| Ascorbate | 0.016±0.0003 e | - | - | |
| BHT | - | 0.00015±0.00002 b | 4.93±0.74 c | |
Data are mean ± SD of three replicates.
, means in each column following different letters are significantly different (p<0.05) as determined by Duncan’s multiple rang test.
Effects of Achillea leaf extracts on viability of HFF3 cells
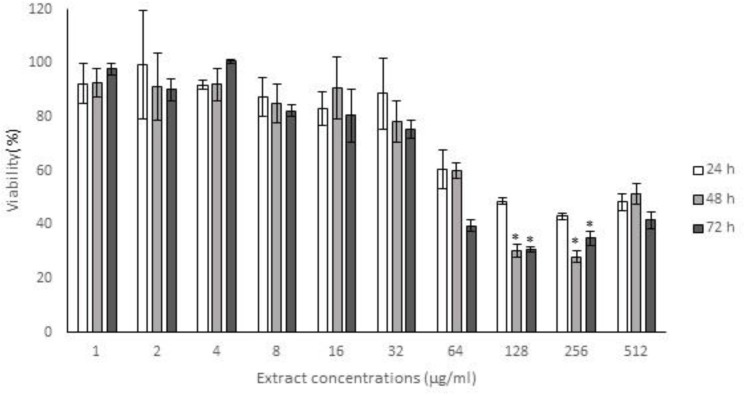
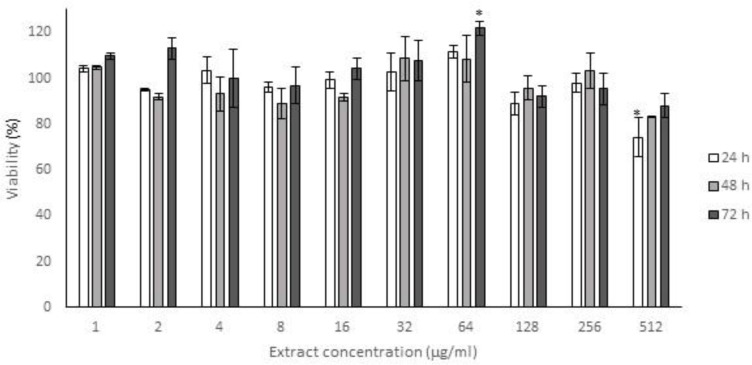
Antioxidant capacity and cytotoxic effect of methanol extracts of Achillea leaves were examined in vitro. Viability of HFF3 cells treated with different concentrations of A. eriophora extracts, reduced in a dose-dependent manner, but the highest examined dose (512 µg/ml) of A. biebersteinii extract showed no toxic effects. IC50 values obtained for A. eriophora extract were 120, 85 and 55 mg/ml after 24, 48 and 72 h treatments, respectively (Figures 1 and 2).
Figure 1.
The cytotoxicity of the methanol extract of the leaves of Achilea eriophora on the proliferation of human foreskin fibroblast cells (HFF3). The cells were treated with various concentrations (1-512 µg/ml) of the extract in a culture medium for 24, 48 and 72 h. Cells viability was determined and compared with the control (untreated cells) by the MTT assay. Each value represents the mean ± SD (n=3). *p<0.05 Starred values, are significantly different from other means
Figure 2.
The cytotoxicity of the methanol extract of the leaves of Achilea biebersteinii on the proliferation of human foreskin fibroblast cells (HFF3). The cells were treated with various concentrations (1-512 µg/ml) of the extract in a culture medium for 24, 48 and 72 h. Cells viability was determined and compared with the control (untreated cells) by the MTT assay. Each value represents the mean ± SD (n=3). *p<0.05 Starred values, are significantly different from other means
Antioxidant activity of leaf extracts on HFF3 cells
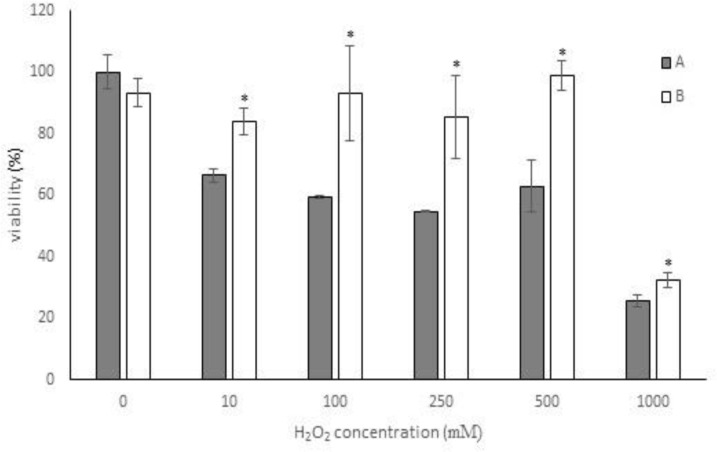
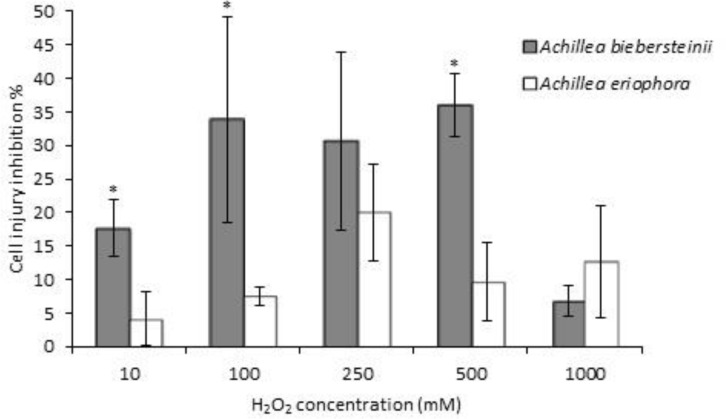
Achillea leaf extracts were selected due to their high phenol content and antioxidant activity, and their possible protective effects against H2O2-induced damages were investigated in vitro. The protective effects of the extracts on HFF3 cells were first confirmed by MTT assay. Reduced viability was recorded at different concentrations of H2O2 (54.73% at 250 mM H2O2, Figure 3), but pre-treatment with 1 µg/ml of the Achillea leaf extracts, could significantly (p<0.05) inhibit oxidative damage (Figure 3). Also results showed that pre-treatment with A. biebersteinii leaves extract were more effective than pre-treatment with A. eriophorea to inhibit cell injuries caused by H2O2 treatment (Figure 4). The oxidative damage to the DNA of HFF3 cells was examined using comet assay in H2O2-treated cells.
Figure 3.
The effect of methanol extract on H2O2 cytotoxicity in human foreskin fibroblast cells (HFF3). Cytotoxic effect of H2O2 at 0-1000 mM concentrations (A) were compared to cells pre-treated with 1 µg/ml A. biebersteinii leaves extract (B). Cells viability (%) was determined and compared with the control (untreated cells) using MTT assay. Each value represents the mean ± SD of three replications. *p<0.05 compared with the corresponding concentration of H2O2 without plant extract pre-treatment
Figure 4.
Achillea extracts inhibited HFF3 cells oxidative injury. HFF3 cells injury induced by H2O2 were inhibited by pretreatment with Achillea extracts (1 µg/ml). The percentage of cell injury inhibition was calculated on the basis of viability using MTT assay. Data are mean ± SD of three replicates. *p<0.05 compared with corresponding concentration of H2O2 with different plant extract pre-treatment
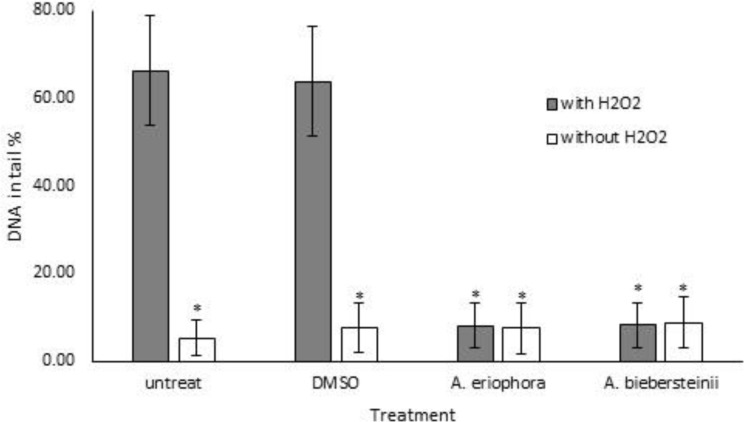
Comet results also confirmed protective effects of leaf extracts (1 µg/ml) against H2O2-induced oxidative damage. As shown in Figure 5 and 6, DNA in tail was reduced in cells pretreated with Achillea extracts after H2O2 treatment (8.9% and 7.6% for A. biebersteinii and A. eriophora, respectively), as compared to H2O2-treated cells in which the DNA in tail was about 66%.
Figure 5.
Effect of methanol extracts from the leaves of two Achillea species (1 µg/ml) on DNA damage induced by H2O2 (500 mM) in human foreskin fibroblast cells (HFF3) assessed by Comet assay. A. eriophora and A. biebersteinii groups were pre-treated with different extracts (1 µg/ml), DMSO group was pretreated with DMSO and untreated group was the null control without any pretreatment. DNA in tail in each cell was calculated by comet score. The data represent mean±SD of 150 cells. *p<0.05 Starred values compared with means in untreated and DMSO groups which are treated with H2O2
Figure 6.
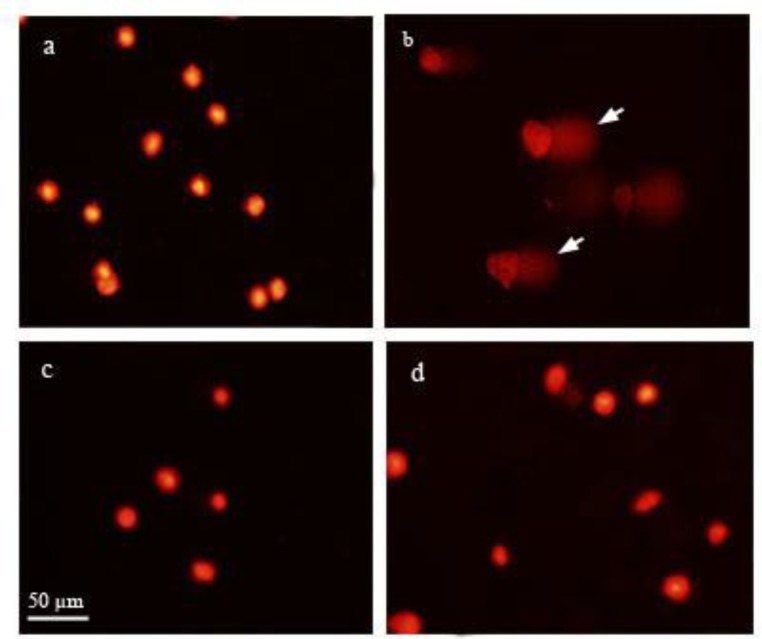
Comet tails of human foreskin fibroblast cells (HFF3) treated with H2O2 and Achillea extracts. Pre-treatment with the methanol extract of Achillea leaves (1 µg/ml) significantly (p<0.05) reduced comet tail of HFF3 cells induced by H2O2 (500 mM) treatment. Untreated cells (a), H2O2 treated cells (b), H2O2-incubated cells pretreated with Achillea eriophora (c), and A. biebersteinii extracts (d). Arrows indicate comet tails which contain damaged DNA
Discussion
There are different reports on the total phenolic contents of the leaf extract of A. biebersteinii. For example, Tawaha et al. reported that this value varied between 1600 and 2300 mg of GAE/100 g (Tawaha et al., 2007 ▶), while in the study of Sokmen and Özbek, it was calculated as 51 ± 0.6 mg/g (Sokmen and Özbek, 2006 ▶). These differences may be mainly due to diversities of the analytical methods, extraction methods, variety, developmental phase, and geographic origin of the plants. Similar to our results (Table 1), there is a study that reported the total phenol contents of A. eriophora to be were higher in leaves than inflorescence (Dokhani et al., 2005 ▶).
As indicated in the results, leaf extracts of A. biebersteinii with the highest antioxidant activity (as measured by three different methods), showed the highest phenol and flavonoid contents as well. It seems that higher antioxidant activity of A. biebersteinii extracts, as compared to A. eriophora extracts, could be attributed to their higher phenolic and flavonoid contents.
This study demonstrated that the cytotoxicity induced via oxidative stress (H2O2-induced stress) could be suppressed by pre-treatment with Achillea leaf extracts (1μg/ml). Protective effects of plant extracts against oxidative cell damage, have been previously reported by various researchers (Gião et al., 2010 ▶; Konyalioglu and Karamenderes, 2005 ▶; Yoo et al., 2008 ▶). Adetutu et al. (2011) ▶ and Annan and Houghton (2008) ▶ reported that fibroblast cells were protected against H2O2-induced oxidative damage using Bridelia ferruginea Benth. and Gossypium arboretum L., and Ficus asperifolia Miq. Extracts (Adetutu et al., 2011 ▶; Annan and Houghton, 2008 ▶). Adetutu et al. reached 82% protection, when the concentration of plant extract was 250 µg/ml (in the presence of 180 µM H2O2 as an oxidant), and Annan and Houghton reported 58% protection against oxidative damage at 50 µg/ml of F. asperifolia extract (in the presence of 100 µM H2O2); However, we observed 35.94% protection at a low concentration (1 µg/ml) of A. biebersteinii leaf extract (in the presence of 100-500 mM H2O2).
Anti-superoxide properties of flower infusion of A. biebersteinii in erythrocytes and leucocytes were reported. Cellular damages induced by 10 mM H2O2, were blocked after treatment with 500 µl of the infusion. Phenol and flavonoid compounds of infusion were responsible for the positive effects on enzymatic antioxidant system (Konyalioglu and Karamenderes, 2005 ▶). Behravan et al. (2011) ▶ reported a significant inhibitory effect of aqueous extract of Portulaca oleracea L. at 1 and 2.5 mg/ml on H2O2-induced DNA damage in human lymphocyte (percentage tail DNA 2.35% ± 0.16 and 1.29% ± 0.12, respectively). Pretreatment with Mentha arvensis L. (25 μg/ml), exhibited a significant protective effect (12.43% of tail DNA% compared to the control) in lymphocytes (Lin et al., 2013 ▶). As Achillea species are effective antioxidants, according to the result of antioxidant assays (DPPH, BCB, TBARS), our results suggest that it is likely that the inhibitory effect of Achillea on H2O2-induced DNA damage could be the result of interactions of different antioxidant compounds in the extracts, as reported for P. oleracea and M. arvensis (Behravan et al., 2011 ▶; Lin et al., 2013 ▶).
Although this study revealed remarkable antioxidant properties of A. biebersteinii and A. eriophora extracts, further studies are required to determine the chemical composition of the extracts and antioxidant activities of phenolic acids and flavonoids, as their main constituents. Other obvious important topics of research will be to determine the most effective compounds and their mode of action in cell-based assays.
A. eriophora and A. biebersteini, with remarkable antioxidant activities, especially on human fibroblast cell culture, could be considered as good sources of natural antioxidants and healthy replacements for the corresponding synthetic ones such as industrial food preservatives. Their high phenol and flavonoid contents might be responsible for the traditional usage of these medicinal plants; however, we recommend more detailed studies to determine their exact chemical composition and mechanism of action.
Acknowledgments
The authors appreciate the financial support provided by Ferdowsi University of Mashhad [number 3/16744].
Conflicts of Interest:
The authors report no declarations of interest.
References
- Adetutu A, Morgan WA, Corcoran O. Antibacterial, antioxidant and fibroblast growth stimulation activity of crude extracts of Bridelia ferruginea leaf, a wound-healing plant of Nigeria. J Ethnopharmacol. 2011;133:116–119. doi: 10.1016/j.jep.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Afsharypuor S, Asgary S, Lockwood G. Constituents of the essential oil of Achillea wilhelmsii from Iran. Planta Med. 1996;62:77–78. doi: 10.1055/s-2006-957810. [DOI] [PubMed] [Google Scholar]
- Amiri MS, Joharchi MR. Ethnobotanical investigation of traditional medicinal plants commercialized in the markets of Mashhad, Iran. Avicenna J Phytomed. 2013;3:254–271. [PMC free article] [PubMed] [Google Scholar]
- Annan K, Houghton PJ. Antibacterial, antioxidant and fibroblast growth stimulation of aqueous extracts of Ficus asperifolia Miq and Gossypium arboreum L wound-healing plants of Ghana. J Ethnopharmacol. 2008;119:141–144. doi: 10.1016/j.jep.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Asgary S, Naderi G, Sarrafzadegan N, Mohammadifard N, Mostafavi S, Vakili R. Antihypertensive and antihyperlipidemic effects of Achillea wilhelmsii. Drugs Exp Clin Res. 2000;26:89–94. [PubMed] [Google Scholar]
- Bashi DS, Fazly Bazzaz BS, Sahebkar A, Karimkhani MM, Ahmadi A. Investigation of optimal extraction, antioxidant, and antimicrobial activities of Achillea biebersteinii and A. wilhelmsii. Pharm Biol. 2012;50:1168–1176. doi: 10.3109/13880209.2012.662235. [DOI] [PubMed] [Google Scholar]
- Bazzaz BSF, Khayat MH, Emami SA, Asili J, Sahebkar A, Neishabory EJ. Antioxidant and antimicrobial activity of methanol, dichloromethane, and ethyl acetate extracts of Scutellaria litwinowii. ScienceAsia. 2011;37:327–334. [Google Scholar]
- Behravan J, Mosafa F, Soudmand N, Taghiabadi E, Razavi BM, Karimi G. Protective effects of aqueous and ethanolic extracts of Portula caoleracea L aerial parts on H2O2- induced DNA damage in lymphocytes by comet assay. J Acupunct Meridian Stud. 2011;4:193–197. doi: 10.1016/j.jams.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Benetis R, Radušienė J, Jakštas V, Janulis V, Malinauskas F. Development of an RP‐HPLC method for the analysis of phenolic compounds in Achillea millefolium L. J Liq Chromatogr Rel Technol. 2008;31:596–610. [Google Scholar]
- Candan F, Unlu M, Tepe B, Daferera D, Polissiou M, Sökmen A, Akpulat HA. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp Millefolium Afan. (Asteraceae). J Ethnopharmacol. 2003;87:215–220. doi: 10.1016/s0378-8741(03)00149-1. [DOI] [PubMed] [Google Scholar]
- Ćetković GS, Čanadanović-Brunet JM, Djilas SM, Tumbas VT, Markov SL, Cvetković DD. Antioxidant potential, lipid peroxidation inhibition and antimicrobial activities of Satureja montana L subsp kitaibelii extracts. International Journal of Molecular Sciences. 2007;8:1013–1027. [Google Scholar]
- Chandler R, Hooper S, Harvey MJ. Ethnobotany and phytochemistry of yarrow, Achillea millefolium, Compositae. Econ Bot. 1982;36:203–223. [Google Scholar]
- Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Chevallier A. Encyclopedia of herbal medicine. Dk Pub; 2000. [Google Scholar]
- Chiarugi P, Fiaschi T. Redox signalling in anchorage-dependent cell growth. Cell Signal. 2007;19:672–682. doi: 10.1016/j.cellsig.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Conforti F, Loizzo MR, Statti GA, Menichini F. Comparative radical scavenging and antidiabetic activities of methanolic extract and fractions from Achillea ligustica ALL. Biol Pharm Bull. 2005;28:1791–1794. doi: 10.1248/bpb.28.1791. [DOI] [PubMed] [Google Scholar]
- Cook N, Samman S. Flavonoids chemistry, metabolism, cardioprotective effects, and dietary sources. J N B. 1996;7:66–76. [Google Scholar]
- Dapkevicius A, Venskutonis R, van Beek TA, Linssen JP. Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania. J Sci Food Agric. 1998;77:140–146. [Google Scholar]
- Deba F, Xuan TD, Yasuda M, Tawata S. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidenspilosa Linn. var. Radiata. Food control. 2008;19:346–352. [Google Scholar]
- Dokhani S, Cottrell T, Khajeddin J, Mazza G. Analysis of aroma and phenolic components of selected Achillea species. Plant Foods Hum Nutr. 2005;60:55–62. doi: 10.1007/s11130-005-5100-9. [DOI] [PubMed] [Google Scholar]
- Evans WC. Trease and Evan's pharmacognosy. Elsevier Health Sciences; 2009. [Google Scholar]
- Falk A, Smolenski S, Bauer L, Bell C. Isolation and identification of three new flavones from Achillea millefolium L. J Pharm Sci. 1975;64:1838–1842. doi: 10.1002/jps.2600641119. [DOI] [PubMed] [Google Scholar]
- Ghorbani A. Studies on pharmaceutical ethnobotany in the region of Turkmen Sahra, north of Iran: (Part 1): General results. J Ethnopharmacol. 2005;102:58–68. doi: 10.1016/j.jep.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Gião MS, Leitão I, Pereira A, Borges AB, Guedes CJ, Fernandes JC, Belo L, Santos-Silva A, Hogg TA, Pintado ME. Plant aqueous extracts: Antioxidant capacity via haemolysis and bacteriophage P22 protection. Food Control. 2010;21:633–638. [Google Scholar]
- Giorgi A, Madeo M, Speranza G, Cocucci M. Influence of environmental factors on composition of phenolic antioxidants of Achillea collina Becker ex Rchb. Nat Prod Res. 2010;24:1546–1559. doi: 10.1080/14786419.2010.490656. [DOI] [PubMed] [Google Scholar]
- Giorgi A, Mingozzi M, Madeo M, Speranza G, Cocucci M. Effect of nitrogen starvation on the phenolic metabolism and antioxidant properties of yarrow (Achillea collina Becker ex Rchb) Food Chem. 2009;114:204–211. [Google Scholar]
- Goldberg AS, Mueller EC, Eigen E, Desalva SJ. Isolation of the anti‐inflammatory principles from Achillea millefolium (compositae) J Pharm Sci. 1969;58:938–941. doi: 10.1002/jps.2600580805. [DOI] [PubMed] [Google Scholar]
- Haase G, Dunkley W. Ascorbic acid and copper in linoleate oxidation II Ascorbic acid and copper as oxidation catalysts. J Lipid Res. 1969;10:561–567. [PubMed] [Google Scholar]
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- Hassan AM, Mohamed SR, El-Nekeety AA, Hassan NS, Abdel-Wahhab MA. Aquilegia vulgaris L extract counteracts oxidative stress and cytotoxicity of fumonisin in rats. Toxicon. 2010;56:8–18. doi: 10.1016/j.toxicon.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Hsouna AB, Trigui M, Culioli G, Blache Y, Jaoua S. Antioxidant constituents from Lawsonia inermis leaves: Isolation, structure elucidation and antioxidative capacity. Food Chem. 2011;125:193–200. [Google Scholar]
- Jacob RA. The integrated antioxidant system. Nutrition Research. 1995;15:755–766. [Google Scholar]
- Konyalioglu S, Karamenderes C. The protective effects of Achillea L species native in Turkey against H2O2-induced oxidative damage in human erythrocytes and leucocytes. J Ethnopharmacol. 2005;102:221–227. doi: 10.1016/j.jep.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Kumar S, Gupta S. Thymosin beta 4 prevents oxidative stress by targeting antioxidant and anti-apoptotic genes in cardiac fibroblasts. PLoS One. 2011;6:e26912. doi: 10.1371/journal.pone.0026912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpulainen JT, Salonen JT. Natural antioxidants and anticarcinogens in nutrition, health and disease: proceedings of the Second International Conference on Antioxidants and Anticarcinogens in Nutrition. Royal Society of Chemistry; 1999. [Google Scholar]
- Lampronti I, Saab AM, Gambari R. Antiproliferative activity of essential oils derived from plants belonging to the Magnoliophyta division. Int J Oncol. 2006;29:989. [PubMed] [Google Scholar]
- Lin K, Yang Y, Yang C, Huang M, Lo H, Liu K, Lin H, Chao P. Antioxidant activity of herbaceous plant extracts protect against hydrogen peroxide-induced DNA damage in human lymphocytes. BMC Research Notes. 2013;6:490–500. doi: 10.1186/1756-0500-6-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirheidari H. Application of plants in prevention and treatment of diseases. Office of Islamic culture pub; 1998. [Google Scholar]
- Moeina S, Moeinb M. Antioxidant activities and phenolic content of Juniperus excels extract. Iranian Journal of Pharmaceutical Sciences. 2010;6:133–140. [Google Scholar]
- Orhan I, Üstün O. Determination of total phenol content, antioxidant activity and acetylcholinesterase inhibition in selected mushrooms from Turkey. Journal of Food Composition and Analysis. 2011;24:386–390. [Google Scholar]
- Özgen U, Mavi A, Terzi Z, Coflkun M. Antioxidant activities and total phenolic compounds amount of some Asteraceae species. Turkish J Pharm sci. 2004;3:203–216. [Google Scholar]
- Pirbalouti A, Golparvar A. Evaluation of ethnobotany in the region of Chaharmahal & Bakhtyari, West Central Iran. Paper presented at The Abstract book of 48th annual meeting of the Society for Economic Botany; Chicago, Ill., USA. 2007. Jun, [Google Scholar]
- Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res. 2008;52:507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- Rassouli FB, Matin MM, Iranshahi M, Bahrami AR, Behravan J, Mollazadeh S, Neshati V, Kalalinia F. Investigating the enhancement of cisplatin cytotoxicity on 5637 cells by combination with mogoltacin. Toxicol In Vitro. 2011;25:469–474. doi: 10.1016/j.tiv.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Rezaeipoor R, Saeidnia S, Kamalinejad M. Immunosuppressive activity of Achillea talagonica on humoral immune responses in experimental animals. J Ethnopharmacol. 1999;65:273–276. doi: 10.1016/s0378-8741(98)00191-3. [DOI] [PubMed] [Google Scholar]
- Rice-evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995;22:375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- Saeidnia S, Gohari A, Mokhber-Dezfuli N, Kiuchi F. A review on phytochemistry and medicinal properties of the genus Achillea. DARU: Journal of Faculty of Pharmacy, Tehran University of Medical Sciences. 2011;19:173. [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Moreno C, A Larrauri J, Saura-Calixto F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res Int. 1999;32:407–412. [Google Scholar]
- Shahidi F, Janitha P, Wanasundara P. Phenolic antioxidants. Crit Rev Food SciNutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- Sokmen M, Özbek T. Biological activities of the essential oil and methanol extract of Achillea biebersteinii Afan (Asteraceae) Turk J Biol. 2006;30:65–73. [Google Scholar]
- Štajner D, Milić N, Mimica‐Dukić N, Lazić B, Igić R. Antioxidant abilities of cultivated and wild species of garlic. Phytother Res. 1998;12:S13–S14. [Google Scholar]
- Stojanović G, Radulović N, Hashimoto T, Palić R. In vitro antimicrobial activity of extracts of four Achillea species: The composition of Achillea clavennae L (Asteraceae) extract. J Ethnopharmacol. 2005;101:185–190. doi: 10.1016/j.jep.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Swain T, Hillis W. The phenolic constituents of Prunus domestica I The quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;10:63–68. [Google Scholar]
- Tawaha K, Alali FQ, Gharaibeh M, Mohammad M, El-Elimat T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007;104:1372–1378. [Google Scholar]
- Weber KC, Honório KM, Bruni AT, da Silva AB. The use of classification methods for modeling the antioxidant activity of flavonoid compounds. J Mol Model. 2006;12:915–920. doi: 10.1007/s00894-005-0083-x. [DOI] [PubMed] [Google Scholar]
- Yang H, Dong Y, Du H, Shi H, Peng Y, Li X. Antioxidant compounds from propolis collected in Anhui, China. Molecules. 2011;16:3444–3455. doi: 10.3390/molecules16043444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanparast R, Ardestani A, Jamshidi S. Experimental diabetes treated with Achillea santolina: Effect on pancreatic oxidative parameters. J Ethnopharmacol. 2007;112:13–18. doi: 10.1016/j.jep.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Yildirim A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L extracts. J Agric Food Chem. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- Yoo KM, Lee CH, Lee H, Moon B, Lee CY. Relative antioxidant and cytoprotective activities of common herbs. Food Chem. 2008;106:929–936. [Google Scholar]
- Yoo KM, Al-Farsi M, Lee H, Yoon H, Lee CY. Antiproliferative effects of cherry juice and wine in Chinese hamster lung fibroblast cells and their phenolic constituents and antioxidant activities. Food Chem. 2010;123:734–740. [Google Scholar]
- Yu L, Zhao M, Cui C, Yang B, Jiang Y, Zhao Q. Antioxidant, immunomodulatory and anti-breast cancer activities of phenolic extract from pine (Pinus massoniana Lamb) bark. Innovative Food Science & Emerging Technologies. 2008;9:122–128. [Google Scholar]
- Zargari A. Medicinal Plants. Tehran. Iran: University of Tehran Press; 1993. [Google Scholar]