Abstract
The notion of multiple memory systems based on conscious accessibility has been supported largely by neuropsychological patient studies. Specifically, it was widely held that amnesic patients have impaired explicit memory performance but spared implicit memory performance. However, recent patient studies have called the implicit/explicit memory distinction into question. In this study, normal participants were tested on a visual search task, once after an injection of midazolam, an anesthetic that induces temporary amnesia, and once after an injection of saline. Under the influence of midazolam, participants did not show facilitation in search times for repeated configurations (contextual cuing), although there was a general speed-up in performance across blocks in both the midazolam and saline conditions. Neither the contextual-cuing effect nor the procedural-learning effect was available to subjective experience, yet only one of these was affected by midazolam-induced amnesia. These data call into question the notion that memory systems divide on the basis of subjective experience of consciousness or reportability. Rather, the findings support the contention that anterograde amnesia affects learning that depends on building novel associations in memory and that this deficit does not hinge upon accessibility to consciousness.
Keywords: explicit memory, implicit memory, synthetic amnesia
The distinction between explicit (declarative) memory and implicit (nondeclarative) memory involves whether or not a memory is accompanied by conscious recollection, that is, an ability to report the memory. Studies of neuropsychological patients have shown that individuals who suffer from amnesia have impairment on explicit memory tasks but no performance deficit on measures of implicit memory (1, 2). Because of this selective impairment, it has been claimed that different memory systems are subserved by different brain regions (e.g., refs. 3 and 4). Accessibility to consciousness has been considered as the criterion for different memory systems (3, 5), although others have suggested using as the distinction whether the formation of new memories depends on the medial temporal lobe (4, 6).
Recently, the notion of multiple memory systems based on consciousness has been questioned. Studies of neuropsychological patients have begun to explore whether these patients have problems learning the association between pieces of information or have difficulty only in acquiring new explicit memories (7, 8). For example, amnesic patients showed a deficit on a visual search task such that performance was not facilitated when displays were repeated (7). When the contextual cues were repeated (i.e., when the configuration of the distractors in relation to the target remained constant), normal participants showed facilitation in locating the target without any awareness of display repetition. In other words, this enhancement, i.e., the contextual-cuing effect, was acquired implicitly. Therefore, amnesic patients, who were thought to manifest only explicit memory deficits, have now been shown to be impaired on an implicit memory task. Interestingly, general performance on the search task did improve with practice for both controls and patients. Thus, the deficit in neuropsychological patients seems to lie in whether a task requires associative or relational processing, as in contextual cuing, rather than in whether it relies on explicit memory.
However, another patient study recently showed that amnesiacs were still able to demonstrate an effect of contextual cuing (9), supporting the validity of a dichotomous memory distinction, i.e., an explicit (declarative) vs. implicit (nondeclarative) memory system. This result calls into question the previous result (7), which found that amnesic patients were impaired on the same implicit task. Thus, controversy still surrounds the question of whether memory dichotomies should be based on conscious accessibility and, conversely, whether conscious accessibility is the critical feature underlying the dichotomous neural bases of distinct memory systems.
Although patient studies have provided invaluable information to further the understanding of human memory, there are inherent problems involving neuropsychological patients that raise some doubt about conclusions based on the data in these patient studies. In particular, the pattern of brain damage across each individual in any given patient group is likely to vary significantly. By using a neuropharmacological approach that induces temporary amnesia in healthy participants, each participant can serve as his or her own control. Further, this type of approach finesses a criticism sometimes leveled against research on implicit memory with normal populations (e.g., refs. 10 and 11). Specifically, performance on tests of implicit memory is much less vulnerable to contamination by explicit memory (12, 13).
Benzodiazepines have been used to reduce anxiety in clinical settings, but they also have a temporary, functional amnesic effect on encoding information (14, 15). Benzodiazepines facilitate the action of γ-aminobutyric acid (GABA) by increasing the binding of GABA to GABAA receptors. GABA is the primary inhibitory neurotransmitter in the mammalian central nervous system, and GABAA receptors are expressed throughout the brain with a very high density in the hippocampal system, which has been established as critical for explicit memory (13, 16). Midazolam is a benzodiazepine that has the benefits of being metabolized quickly and of being water-soluble (13). These attributes help minimize potential side effects without disturbing other cognitive functions when midazolam is given in low doses, thereby providing a tool to investigate distinct forms of memory based on conscious accessibility in healthy participants (17, 18). Previous research (17, 19, 20) showed that midazolam severely impaired performance on explicit memory tasks but not on implicit memory tasks. However, a few studies (e.g., ref. 13) showed that midazolam might have a small effect on priming, although the degree of impairment has been much larger for tests of explicit memory, and the interpretation of the finding is open to debate.§
In this study we examine both implicit and explicit memory performance in healthy participants with a double-blind drug administration using a within-subject design to control participant variability. Of particular interest is whether there are types of implicit memory performance that are impaired under midazolam analogous to the recent findings with amnesic patients (7). Each participant performed two versions of a visual search task (adapted from refs. 21 and 22) one under the influence of midazolam and one under the influence of saline. The task required participants to locate a target in a display of distractors and then make a binary decision based on the identity of the target. The time and accuracy of making these judgments were examined as a function of practice at the task and whether the particular display had been seen previously.
Methods
Participants. The 30 participants were healthy, paid volunteers (age range 19-29). Three participants were excluded from all analyses because they fell asleep due to excessive sedation effects. Informed consent was obtained from all participants before participation, and the study was performed under a protocol approved by the Institutional Review Boards of Carnegie Mellon University and the University of Pittsburgh.
Design, Materials, and Tasks. This study used a 2 (Old configuration vs. New configuration) × 2 (midazolam vs. saline) within-subjects design with a visual search task. There were two dependent measures, accuracy and reaction time (RT). In the visual search task, each display contained 12 items, 1 target and 11 distractors, appearing within a grid of 8 × 6 locations. For one of the two tasks, the visual display contained a “T” rotated 90° either clockwise or counterclockwise, presented among rotated “L” distractors. Once the T was located, participants were to indicate the direction of the rotated T by pressing the corresponding key; the other task involved locating an upright “2” or “5” presented among rotated 2 or 5 distractors, and participants were to respond by pressing the corresponding key. Each task consisted of 24 blocks of 24 trials per block for a total of 576 trials. A minimum 10-s break, which could be extended if needed, was given at the end of each block.
The variable of interest was whether a display was repeated over blocks (Old configuration) or was a new, random display (New configuration). For Old configurations, the target appeared in the same location within an invariant configuration across blocks. Half of the trials in a block were old, and each of the 12 patterns repeated once per block. The other half of the trials in each block displayed a previously unseen, New configuration as a baseline control. To control target-location effects at search, the locations of the target were repeated across blocks for both Old and New configurations so that the target appeared equally in 24 possible locations. Thus, improvement in performance for Old configurations should be attributed to the benefit of contextual cuing, that is, learning the association between the target location and the repeated visual context. The other variable was drug (midazolam vs. saline). For each drug condition, performance was compared by block, between Old and New configurations.
To ensure the effectiveness of the drug manipulation and to determine whether midazolam affects only explicit tests or both implicit and explicit tests, we included two types of control tasks. First, we used an implicit, quadrant-guessing task, to see whether participants were unaware of repeated displays in the visual search task (23). This task involved presenting displays of distractors without a target present and asking participants to predict in which quadrant the target was likely to appear. Chance performance would be 25%, and at issue was whether participants were any better than chance at predicting a target location for Old configurations. Second, to see whether an implicit contextually cued visual search task is impaired in conditions that produce synthetic amnesia, we needed to ensure that explicit memory was indeed impaired because of the drug intervention. Participants were given word pairs to commit to memory immediately after injection both for the midazolam and saline conditions. At the end of the testing session, participants were prompted with a stimulus cue and were asked to recall the corresponding response term.
Procedure. The experiment consisted of two sessions. Each participant performed two versions of an implicit visual search task, one under the influence of midazolam and the other under the influence of saline, with an approximately 1-week interval between the two sessions. The order of drug administration and assignment of task to drug condition was counterbalanced with the assignment of participants to orders randomly determined. The experiment was conducted in a postoperative recovery area with the participant using a laptop computer. The experiment began with instructions as to the nature of the visual search task that the participants would perform, followed by a practice trial before injection.
Participants were advised, before injection, that they would be shown a set of paired associates for a subsequent cued-recall test. After being instructed, participants were given a single i.v. injection of either midazolam (0.03 mg/kg of the participant's body mass) or a matching volume of saline within a 2-min period. The drug administration procedure was double-blinded, thus neither the participants nor the experimenter was told which drug the participants were being given. The set of word pairs was presented to the participant immediately after the injection. After studying a list of 15 word pairs, participants performed one of the two versions of the visual search task.
After the search task, participants were given the quadrant-guessing task and asked to predict in which quadrant of the display they thought the target would be located if it were present. After the quadrant-guessing task, participants performed the cued-recall test by attempting to recall the response term to the stimulus cue for each pair studied earlier. Upon finishing the second session, participants were asked whether they had noticed any repetitions of the visual displays or whether they had used any explicit strategy to find the target during the visual search task.
Results
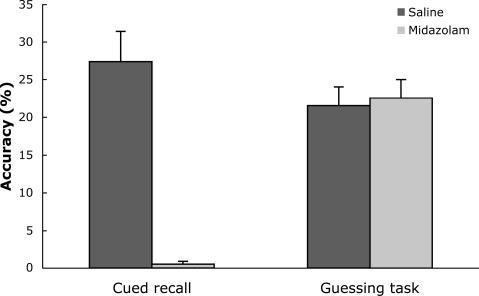
The results for both post-tests, an explicit memory (cued-recall) and an implicit memory (quadrant-guessing) task, are displayed in Fig. 1 as a function of drug condition. Explicit memory performance in the midazolam condition was severely impaired compared with performance in the saline condition, t(26) = 6.70, P < 0.001, confirming that midazolam disrupted explicit memory as expected. The other post-test, which involved quadrant guessing, did not differ reliably between the two drug conditions for the Old configurations, t(26) = 0.25, P > 0.8, and neither differed from chance (25%), both P > 0.15. In addition, participants did not report noticing any repetitions of displays, nor did they report attempting to find targets by looking for Old configurations. Thus, any facilitation for Old configurations should not be attributed to explicit memory.
Fig. 1.
Mean accuracy of cued-recall and guessing tasks in the saline and midazolam conditions. Error bars represent standard errors of the means.
Given that participants under the influence of midazolam were severely impaired on the explicit memory task and that participants in both conditions demonstrated no explicit awareness of any repeated patterns, we examined whether the administration of midazolam also diminished performance on an implicit task. Specifically, we looked to see whether the facilitation of repeated patterns in the visual search task was reduced. For each drug condition, performance (latency and accuracy of response) was compared by block, between Old and New configurations. For the RT analysis, only correct responses were included, and all RTs that exceeded three standard deviations of that participant's mean RT were discarded. Less than 1% of the data was removed because of outliers. To reduce statistical noise, blocks of trials were grouped into sets of four, yielding six epochs for analysis.
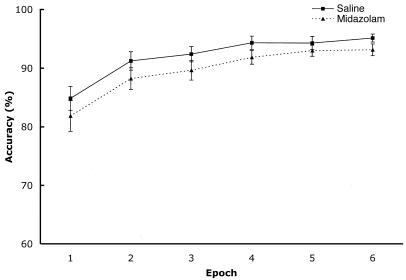
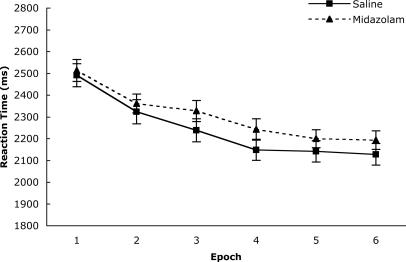
Collapsing over the Old versus New configuration factor, the percentage of correct trials is displayed in Fig. 2 as a function of drug condition and epoch. Fig. 3 displays the mean correct RTs as a function of these same factors. Both figures show general improvement in the task over time, such that accuracy increased over epochs, F(5,130) = 33.66, P < 0.001, and search time decreased over epochs, F(5,130) = 96.55, P < 0.001, regardless of drug condition. This result means that participants performed better with more experience in terms of both speed and accuracy, consistent with the literature on skill learning (e.g., ref. 24). There was no main effect of drug condition on accuracy or RT, both F < 1.7, but the pattern suggests that midazolam produces slightly degraded performance, as would be expected because of the drug's sedative effects.
Fig. 2.
Accuracy as a function of drug and epoch. Error bars represent standard errors of the means.
Fig. 3.
RT as a function of drug and epoch. Error bars represent standard errors of the means.
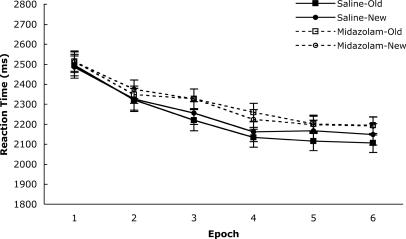
In an analysis that included Old versus New configurations as a factor, accuracy did not differ between Old and New configurations or between drug conditions. The treatment order (midazolam-saline vs. saline-midazolam) did not yield any differences. Fig. 4 displays the correct RTs for Old and New configurations as a function of epoch and drug condition. No differences were expected for New configurations as a function of drug condition, and no interaction was found between drug condition and epoch, F(5,130) = 0.37, P > 0.8. However, the epoch effect was robust, F(5,130) = 65.88, P < 0.001. The important contrasts involved comparisons between drug conditions for the Old configurations. There was no significant main effect of drug condition but there was an effect of epoch for the Old configurations, F(5,130) = 88.59, P < 0.001. Unlike for the New configurations, there was a significant interaction between drug and epoch for the Old configurations, F(5,130) = 2.51, P < 0.05, such that the improvement due to specific practice with Old configurations was limited to the saline condition.
Fig. 4.
RT to search targets in Old versus New configurations as a function of drug and epoch. Error bars represent standard errors of the means.
To determine whether the advantage of an Old configuration increased with additional repetitions, we compared priming scores for Old and New configurations for each epoch. Table 1 shows an RT-priming measure, comparing the speed in subsequent epochs with performance in the original epoch. That is, the RTs in epochs 2, 3, 4, 5, and 6 for each condition are subtracted from the corresponding RT in epoch 1. There was a reliable difference between Old and New configurations in the saline condition that came from the last two epochs, but there was no reliable difference between Old and New configurations for any epoch in the midazolam condition.
Table 1. Amount of RT priming (Epoch 1 - Epoch X, in milliseconds) as a function of Old vs. New configuration and epoch in each drug condition.
| Drug | Configuration | Epoch 2 | Epoch 3 | Epoch 4 | Epoch 5 | Epoch 6 |
|---|---|---|---|---|---|---|
| Midazolam | Old | 165.41 (27.92) | 186.44 (30.07) | 253.03 (31.84) | 311.35 (32.40) | 317.93 (37.20) |
| New | 137.76 (34.30) | 185.23 (31.49) | 288.45 (39.35) | 315.04 (37.45) | 321.89 (42.29) | |
| t(26) | 0.766 | 0.043 | -1.05 | -0.15 | -0.13 | |
| P (two-tailed) | 0.45 | 0.97 | 0.30 | 0.88 | 0.90 | |
| Saline | Old | 174.53 (25.57) | 275.81 (31.14) | 361.22 (36.14) | 380.28 (36.41) | 389.84 (36.84) |
| New | 160.84 (20.97) | 230.25 (30.18) | 323.87 (38.36) | 318.46 (31.59) | 337.14 (30.97) | |
| t(26) | 0.65 | 1.78 | 1.29 | 2.41 | 2.12 | |
| P (two-tailed) | 0.52 | 0.09 | 0.21 | 0.02 | 0.04 |
Numbers in parentheses are standard errors.
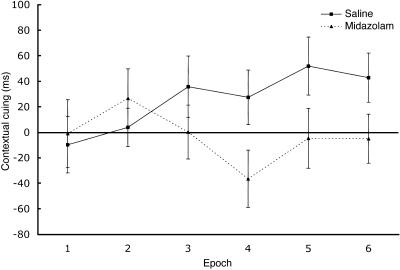
We also compared the benefit of contextual cuing, defined as the difference in RT for Old configurations compared to New configurations, for each epoch. Fig. 5 displays the contextual-cuing effects in the saline and midazolam conditions. Unlike the general improvement in skill learning shown in Fig. 3, the contextual-cuing effect was limited to the saline condition. There was a significant linear trend over epochs for the saline condition, F(1,26) = 6.22, P < 0.05; however, no such trend was found for the midazolam condition, P > 0.2. When epochs were grouped into the first half and the second half of the experiment, a significant interaction between drug and epoch was found, F(1,26) = 10.39, P < 0.005, such that the contextual-cuing scores did not differ between the two drug conditions for the first half of the experiment, t(26) = 0.07, but the scores were greater in the saline condition for the second half of the experiment, t(26) = 2.60, P < 0.05.
Fig. 5.
The contextual-cuing effect as a function of drug condition and Old/New configurations. Contextual cuing is defined as the difference in search performance between New and Old configuration conditions. Error bars represent standard errors of the means.
Discussion
In this study, healthy participants were tested on two versions of an implicit visual search task under midazolam and saline. Participants demonstrated a general improvement in search performance with practice at the tasks, regardless of drug condition; however, a specific facilitation for the Old configurations appeared only in the saline condition. Participants could not predict, above chance, the quadrant that contained the target for the Old configurations even in the saline condition, and they also reported being unaware of repeated patterns, confirming earlier conclusions that the learning of the contextual cues that facilitate visual search occurs implicitly (21, 23). Midazolam had an adverse effect on this implicit learning performance even though it had no adverse effect on other nondeclarative components of performance such as the general speed-up in the task that occurred with practice.
The finding that midazolam adversely affected implicit learning and produced the expected amnesic effects on tests of explicit memory converges with previous findings in studies with neuropsychological patients (e.g., refs. 7 and 8). It is important to note that, although participants showed impaired performance on both an explicit and an implicit memory task, skill learning was unaffected. Skill learning is considered a subsystem of implicit memory (6) and, consistent with patient studies, midazolam did not reduce the effect of practice. The result that synthetic amnesia, like organic amnesia, adversely affects both conscious and unconscious aspects of learning, although leaving other types of implicit learning (such as skill learning) unaffected, calls into question the notion that multiple memory systems should be distinguished on the basis of conscious accessibility.
An alternative conceptualization is based on the informational requirements of the task. Specifically, when the task requires the associative processing of information, regardless of whether that information is explicit or implicit in nature, amnesiacs are vulnerable (25-29). It has often been assumed that explicit memory is the only aspect of memory vulnerable to amnesia because many implicit memory tasks do not require associative processing (27). In contrast, explicit memory tasks frequently require a binding between the concept and the experimental context in order for memory to be accurate. However, when an implicit memory task (such as the visual search paradigm used in the current study) requires binding of cues and context, then even performance on an implicit task seems vulnerable to amnesia.
Not all implicit learning requires binding, and not all implicit learning is vulnerable to amnesia. Skill learning, which tends to be viewed as not open to introspection, is not affected by amnesia. The generalized skill learning in the visual search task that depends on strengthening of procedures was unaffected by the drug and was also unimpaired in amnesiacs. Past research has demonstrated that skills such as mirror tracing and rotary-pursuit task have shown no impairment with amnesia (30). Even acquisition of cognitive skills, such as artificial grammars, is not impaired for novel instances of the grammar (31). Likewise, sequence learning in amnesiacs is unimpaired for the basic task, failing only to show the normal facilitation from repetitions of complex patterns that involve learning associations (32). These results also support the distinction between rule learning and binding.
Although the hippocampus is generally thought to be the site of binding in mammals (e.g., ref. 33), it is premature to make claims concerning the specificity of the neuropharmacological effect of midazolam. GABAA receptors are distributed throughout the brain, not only in the hippocampal system. Although it would be appealing to assert that the locus of effect of midazolam is in the hippocampal regions, there is no evidence that any neuropharmacological drug is that specific, and, furthermore, there is the possibility of interactions among the various neurotransmitters (12). What can be asserted is that whatever brain regions are responsible for impaired performance of the implicit contextual-cuing effect on visual search, those regions do not seem to affect other implicit learning effects such as speed-up with practice.
In conclusion, the present study supports the view that anterograde amnesia affects learning that depends on building novel associations in memory. Considering the information-processing requirements of a task in this way provides an alternative framework for understanding memory dissociations.
Acknowledgments
We thank Kimberly Mason and Greig Williams for drug administration and monitoring participants, Kaia Vilberg for assistance with the Institutional Review Board protocol preparation, Jay Anderson for program modification, and Ferenc Gyulai for comments on the manuscript. We especially thank Marvin Chun (Department of Psychology, Yale University, New Haven, CT) for graciously providing experimental stimulus materials. This work was supported by National Institute of Mental Health Grant 2-R01-MH52808 (to L.M.R.).
Author contributions: H.P. and L.M.R. designed research; H.P. and E.T. performed research; H.P., E.T., and L.M.R. analyzed data; H.P. and L.M.R. wrote the paper; and J.Q., anesthesiologist on the project, screened subjects for appropriateness to receive drug and provided consultation on mechanisms of drug.
Abbreviations: GABA, γ-aminobutyric acid; RT, reaction time.
Footnotes
As the authors of ref. 13 also acknowledged, the observed implicit memory impairment may have been due to the elimination of a contamination from explicit memory that can affect implicit memory in the saline condition.
References
- 1.Scoville, W. B. & Milner, B. (1957) J. Neurochem. 20, 11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warrington, E. K. & Weiskrantz, L. (1974) Neuropsychologia 12, 419-428. [DOI] [PubMed] [Google Scholar]
- 3.Schacter, D. L. & Tulving, E. (1994) in Memory Systems 1994, eds. Schacter, D. L. & Tulving, E. (MIT Press, Cambridge, MA), pp. 1-38.
- 4.Squire, L. R. & Zola-Morgan, S. (1991) Science 253, 1380-1386. [DOI] [PubMed] [Google Scholar]
- 5.Schacter, D. L. (1987) J. Exp. Psychol. Learn. Mem. Cognit. 13, 501-508. [DOI] [PubMed] [Google Scholar]
- 6.Squire, L. R. (1992) Psychol. Rev. 99, 195-231. [DOI] [PubMed] [Google Scholar]
- 7.Chun, M. M. & Phelps, E. A. (1999) Nat. Neurosci. 2, 844-847. [DOI] [PubMed] [Google Scholar]
- 8.Ryan, J. D., Althoff, R. R., Whitlow, S. & Cohen, N. J. (2000) Psychol. Sci. 11, 454-461. [DOI] [PubMed] [Google Scholar]
- 9.Manns, J. R. & Squire, L. R. (2001) Hippocampus 11, 776-782. [DOI] [PubMed] [Google Scholar]
- 10.Hamann, S. B. & Squire, L. R. (1996) J. Exp. Psychol. Learn. Mem. Cognit. 22, 933-947. [DOI] [PubMed] [Google Scholar]
- 11.Schacter, D. L., Bowers, J. & Booker, J. (1989) in Implicit Memory: Theoretical Issues, eds. Lewandowsky, S., Dunn, J. C. & Kirsner, K. (Erlbaum, Hillsdale, NJ), pp. 47-65.
- 12.Ghoneim, M. M. (2004) Anesthesiology 100, 987-1002. [DOI] [PubMed] [Google Scholar]
- 13.Hirshman, E., Passannante, A. & Henzler, A. (1999) Brain Cognit. 41, 351-364. [DOI] [PubMed] [Google Scholar]
- 14.Goneim, M. M. & Mewaldt, S. P. (1975) Psychopharmacologia 44, 257-262. [DOI] [PubMed] [Google Scholar]
- 15.Goneim, M. M. & Mewaldt, S. P. (1990) Anesthesiology 72, 926-938. [PubMed] [Google Scholar]
- 16.Kraemer, S. P., Zilles, K., Schleicher, A., Gebhard, R., Robbins, T., Everitt, B. & Divac, I. (1995) Anat. Embryol. 191, 213-225. [DOI] [PubMed] [Google Scholar]
- 17.Polster, M., McCarthy, R., O'Sullivan, G. & Park, G. (1993) Brain Cognit. 22, 244-265. [DOI] [PubMed] [Google Scholar]
- 18.Mewaldt, S. P., Hinrichs, J. V. & Ghoneim, M. M. (1983) Mem. Cognit. 11, 557-564. [DOI] [PubMed] [Google Scholar]
- 19.Arndt, J., Passannante, A. & Hirshman, E. (2004) Memory 12, 158-173. [DOI] [PubMed] [Google Scholar]
- 20.Thomas-Anterion, C., Koenig, O., Navez, M. & Laurent, B. (1999) Psychopharmacology 145, 139-143. [DOI] [PubMed] [Google Scholar]
- 21.Chun, M. M. & Jiang, Y. (1998) Cognit. Psychol. 36, 28-71. [DOI] [PubMed] [Google Scholar]
- 22.Wang, Q., Cavanagh, P. & Green, M. (1994) Percept. Psychophys. 56, 495-500. [DOI] [PubMed] [Google Scholar]
- 23.Chun, M. M. & Jiang, Y. (2003) J. Exp. Psychol. Learn. Mem. Cognit. 29, 224-234. [DOI] [PubMed] [Google Scholar]
- 24.Anderson, J. R. & Lebiere, C. (1998) The Atomic Components of Thought (Erlbaum, Mahwah, NJ).
- 25.Cohen, N. J. & Eichenbaum, H. (1993) Memory, Amnesia and the Hippocampal System (MIT Press, Cambridge, MA).
- 26.Eichenbaum, H. (1997) Science 277, 330-332. [DOI] [PubMed] [Google Scholar]
- 27.Eichenbaum, H. (1999) Nat. Neurosci. 2, 775-776. [DOI] [PubMed] [Google Scholar]
- 28.Eichenbaum, H. & Cohen, N. J. (2001) From Conditioning to Conscious Recollection: Memory Systems of the Brain (Oxford Univ. Press, Oxford).
- 29.Ryan, J. D. & Cohen, N. J. (2003) Cognit. Affect. Behav. Neurosci. 3, 168-185. [DOI] [PubMed] [Google Scholar]
- 30.Corkin, S. (1968) Neuropsychologia 6, 255-265. [Google Scholar]
- 31.Knowlton, B. J. & Squire, L. R. (1994) J. Exp. Psychol. Learn. Mem. Cognit. 20, 79-91. [DOI] [PubMed] [Google Scholar]
- 32.Curran, T. (1997) J. Cognit. Neurosci. 9, 522-533. [DOI] [PubMed] [Google Scholar]
- 33.Wallenstein, G. V., Eichenbaum, H. & Hasselmo, M. E. (1998) Trends Neurosci. 21, 317-323. [DOI] [PubMed] [Google Scholar]