Abstract
Preoperative differential diagnosis of gastric submucosal tumors has generally been difficult because they are covered with normal mucosa. However, recent advances in endoscopic ultrasound (EUS)-guided sampling of submucosal gastrointestinal lesions have made it possible to achieve preoperative differential diagnosis of gastric submucosal tumors. A 76-year-old woman was referred to our hospital with a gastric submucosal tumor. The tumor was observed in the antrum of the stomach. It was preoperatively diagnosed as a schwannoma after immunohistochemical evaluation of a biopsy specimen, obtained using endoscopic ultrasound-guided fine needle aspiration. A computed tomography scan of the abdomen revealed lymphadenopathies near the tumor indicating the possibility of lymph node metastasis from the gastric tumor. The patient underwent laparoscopic distal gastrectomy with D1 + lymph node dissection. The resected tumor was a submucosal tumor measuring 65 × 45 × 35 mm; it was histopathologically diagnosed as a schwannoma. Resected lymph nodes were enlarged in the absence of lymph node metastasis as a result of reactive lymphadenopathy. A definitive preoperative diagnosis of gastric schwannoma is possible using immunohistochemical staining techniques and EUS-guided sampling techniques. After definitive preoperative diagnosis of gastric schwannoma, minimal surgery is recommended to achieve R0 resection.
Keywords: gastric schwannoma, laparoscopic surgery, submucosal tumor
Mesenchymal tumors of the gastrointestinal (GI) tract mainly comprise a spectrum of spindle cell tumors that include gastrointestinal stromal tumors (GISTs), leiomyomas, leiomyosarcomas, and schwannomas.1 Immunohistochemical examination of the specimens obtained from a tumor is essential to make a definitive diagnosis. Preoperative differential diagnosis of gastric submucosal tumors has generally been difficult because they are covered with normal mucosa; this makes it difficult to obtain specimens. However, recent advances in endoscopic ultrasound (EUS)-guided sampling of submucosal GI lesions have made it possible to achieve preoperative differential diagnosis of gastric submucosal tumors;2 this enables physicians to easily decide on the optimal treatment strategy. In this report, we present a 76-year-old woman with preoperative diagnosis of gastric schwannoma with enlargement of the regional lymph nodes of the stomach.
PATIENT REPORT
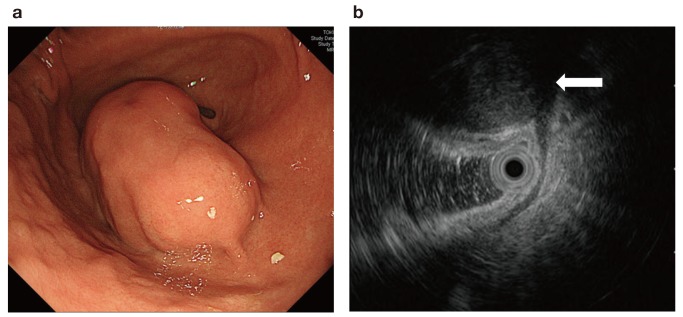
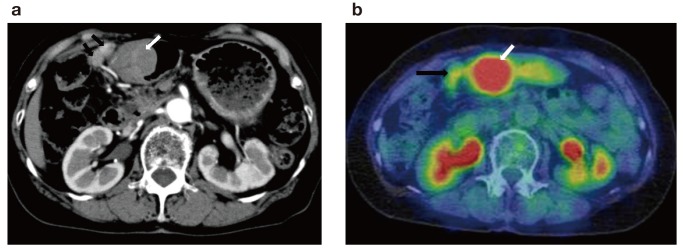
An asymptomatic 76-year-old woman was referred to our hospital because of the presence of a gastric submucosal tumor. The patient underwent appendectomy as a child and cholecystectomy in her 40s. There was no significant past medical, family, or psychosocial history. The upper GI series showed a smooth-marginated protruded lesion at the greater curvature of the antrum of stomach (Fig. 1); upper GI endoscopy revealed that this lesion measured 38 mm (Fig. 2a). Endoscopic ultrasonography (EUS) showed a well-defined hypoechoic mass arising from the proper muscle layer of the stomach; an endoscopic ultrasound-guided fine needle aspiration biopsy was performed (Fig. 2b). Histological evaluation revealed a spindle cell tumor with a fascicular pattern. Immunohistochemical analysis indicated that the tumor was positive for S-100 and negative for c-kit, CD34, α-SMA, and desmin. The preoperative histological diagnosis was gastric schwannoma. A computed tomography (CT) scan of the abdomen demonstrated a protruded lesion measuring 45 × 37 mm at the antrum of the stomach and lymphadenopathies near the tumor (Fig. 3a). The 18-fluorodeoxyglucose (FDG) positron emission tomography (PET) and CT scans indicated high uptake of 18-FDG in the tumor at the antrum of the stomach [standardized uptake value (SUV) max, 5.89–6.81] and slight uptake in the enlarged lymph node (SUVmax, 2.07–2.02)(Fig. 3b). Because of the possibility of lymph node metastasis, we performed laparoscopic distal gastrectomy with D1 + lymph node dissection.
Fig. 1.
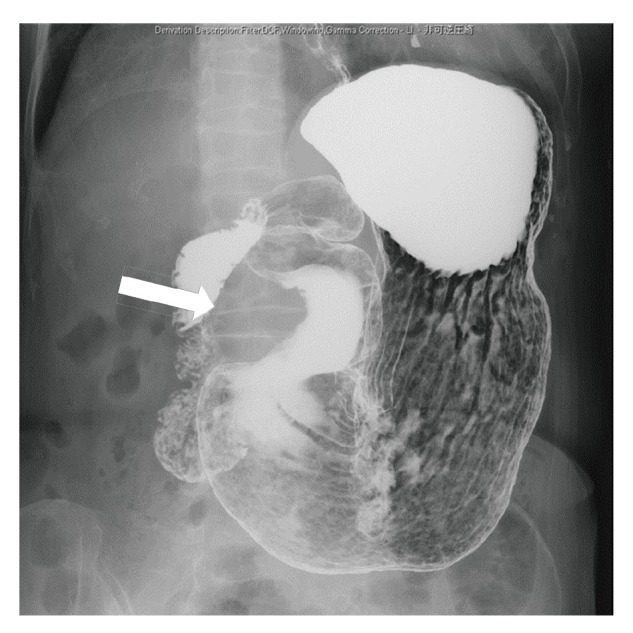
Upper gastrointestinal X-ray showing the smooth-marginated protruded lesion at the greater curvature of the antrum of the stomach (arrow)
Fig. 2.
Endoscope image. a: Upper gastrointestinal endoscopy showing the smooth protruded lesion measuring 38 mm at the greater curvature of the antrum of the stomach. b: Endoscopic ultrasonography image showing a well-defined hypoechoic mass arising from the proper muscle layer of the stomach (arrow)
Fig. 3.
CT image. a: CT image showing a protruded lesion measuring 45 × 37 mm at the antrum of the stomach (white arrow), and enlargement of the regional lymph nodes of the stomach (black arrow). b: 18-FDG positron emission tomography CT image showing high uptake of 18-FDG in the tumor at the antrum of the stomach (SUVmax, 5.89–6.81) (white arrow) and slight uptake in the lymph nodes (SUVmax, 2.07–2.02) (black arrow). CT, computed tomography; FDG, fluorodeoxyglucose; SUV, standardized uptake value.
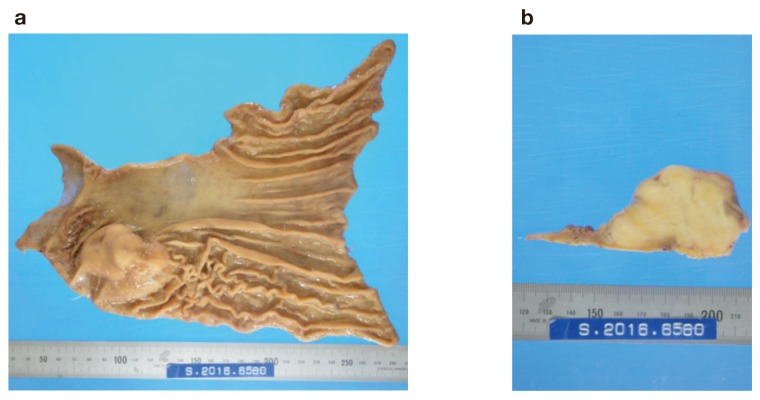
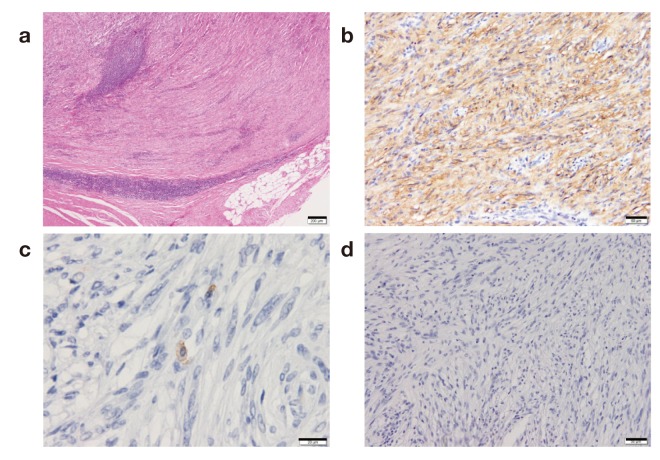
Macroscopically, the gastric mass covered by mucosa measured 65 × 45 × 35 mm, with the cut surface having the appearance of a firm lobulated yellowish white tumor. The tumor was located mainly in the proper muscle layer (Fig. 4). Microscopic examination of the resected and H and E-stained specimens revealed a spindle cell neoplasm arranged in a palisade manner with abundant collagen fibers. There was lymphoid cuffing (Fig. 5a). Immunohistochemical staining revealed that the tumor cells were positive for S-100 and negative for c-kit and neurofilaments (Figs. 5b–d). There were no tumor cells in the resected enlarged lymph nodes, indicating reactive lymphadenopathies. These histopathological and immunohistochemical findings are consistent with the presence of a gastric schwannoma with reactive lymphadenopathies.
Fig. 4.
The tumor was located mainly in the proper muscle layer. a: Gastric mass covered by mucosa measuring 65 × 45 × 35 mm, b: with the cut surface showing a firm lobulated yellowish white tumor.
Fig. 5.
Photomicrographs. a: There was lymphoid cuffing. Bar = 200 μm. b: Tumor cells were positive for S-100 and c: negative for c-kit. d: Immunostained neurofilaments. Bar = 50 μm.
DISCUSSION
Schwannomas, in other words neurinomas or neurilemmomas, are benign neurogenic tumors originating from Schwann cells, which normally wrap around the axons of the peripheral nerves. Theoretically, schwannomas can develop anywhere along the peripheral course of a nerve. However, they most commonly occur in the head and neck, but rarely in the GI tract. In the GI tract, the stomach is the most common gastrointestinal site of schwannoma, which constitute 0.2% of gastric neoplasms.3, 4 The typical presentation of gastric schwannoma is as a submucosal tumor with spindle cell histology. GIST, which are the most common mesenchymal tumor of the stomach, have the same presentation as gastric schwannoma. Despite strong morphological similarities, these tumors are heterogeneous regarding their immunophenotypes. In 1988, Daimaru et al. successfully identified schwannoma as a primary GI tumor based on positive S-100 staining.5 GIST also became a distinct GI tumor diagnostic category when it was discovered that nearly all GIST cells express c-kit protein.1, 6 Before the recognition of S-100 antigen and c-kit antigen in gastric schwannomas and in GISTs, these neoplasms were most often classified as leiomyomas, leiomyosarcomas, or gastrointestinal autonomic nerve tumors.1, 4–6 With the advent of immunohistochemical staining techniques and ultrastructural evaluation, it is now possible to identify these neoplasms based on their distinct immunophenotypes. Furthermore, recent advances in EUS-guided sampling of gastrointestinal submucosal lesions make it possible to make a preoperative differential diagnosis of gastric submucosal tumors. In fact, in our case preoperative diagnosis of gastric schwannoma was made by means of an immunohistochemical examination of specimens obtained from the tumor by EUS-guided sampling.
Surgical resection is the only possible treatment for gastric schwannoma.3, 7 The size and location of the tumor, as well as its relationship with the surrounding organs, are important factors in determining the type of operation. Local extirpation, wedge resection, partial, subtotal or even total gastrectomy, are all acceptable operations for the complete removal of the tumor.8 Laparoscopic techniques can also be used.9 Lymph node dissection is not required because it has been reported that gastric schwannoma is a benign tumor and is unlikely to metastasize to the lymph nodes. In our case, however, there were enlarged lymph nodes with moderate uptake of 18-FDG; this indicated the possibility of lymph node metastasis and the presence of another tumor such as malignant lymphoma that had developed in the lymph nodes. Shayanfar et al. reported a schwannoma that originated in the regional lymph nodes of the stomach.10 Furthermore, Murase et al. reported a malignant schwannoma of the esophagus with lymph node metastasis.11 Considering the possibility of the presence of tumor cells in the enlarged lymph node, we performed laparoscopic distal gastrectomy with D1+ lymph node dissection in the present case. Postoperative histopathological examination of the enlarged lymph nodes after resection revealed that there were no tumor cells present, indicating the presence of reactive lymphadenopathies. The correlation between gastric schwannoma and enlarged lymph nodes remains unclear. Because enlarged lymph nodes are regional lymph nodes and are located near the tumor, it is likely that gastric schwannoma can induce reactive lymphadenopathies. In this regard, the 18-FDG positron emission tomography and CT scans showed high uptake in gastric schwannoma in our case. Komatsu et al. reported the first case of gastric schwannoma that exhibited increased accumulation of 18-FDG on PET imaging.12 It has not been clarified why benign schwannomas show increased 18-FDG uptake on PET; Kawabe et al. reported that 18-FDG accumulated in inflammatory tissue involving lymphocyte proliferation in chronic tonsillitis.13 It has been reported that cuff-like lymphoid aggregates are recognized around the tumor in 97% of gastric schwannomas;14 this also occurred in the present case. Therefore, it is likely that lymphocyte proliferation in gastric schwannoma is related to enlargement of the regional lymph node observed in our case. Thus far, the majority of previous series in the literature have demonstrated no lymph node metastasis regarding gastric schwannoma.15, 16 Therefore, local resection of the stomach and extirpation of enlarged lymph nodes might be sufficient in the present case.
Gastric schwannoma is considered a benign tumor. It is unclear whether pathological parameters such as tumor size and mitotic rate have prognostic significance in this tumor, as in the cases of GISTs reported thus far; this is because gastric schwannoma is rare compared with GIST and experience concerning their long-term follow-up is limited. In this regard, Voltaggio et al. reported that some gastric schwannomas exceed 10 cm in size, and a minority have a mitotic rate > 5/50 high-power fields (HPFs); none of these 51 gastric schwannoma cases showed evidence of aggressive behavior.17 Despite this, these authors advised caution regarding gastric schwannomas with higher mitotic rates (> 10/50 HPFs), because experience from their long-term follow-up was limited.17 In fact, one case of malignant gastric schwannoma has been recently reported;18 in this case, immunohistochemistry revealed that the tumor cells were positive for S-100 protein and negative for c-kit and smooth muscle actin. Mitosis was scattered with 10 mitoses per 50 HPFs, indicating the aggressiveness of this tumor. This patient died at 5 months after surgery as a result of multiple liver metastases. Therefore, careful follow-up is required in gastric schwannoma patients with higher mitotic rates (> 10/50 HPFs) after surgery.
A definitive preoperative diagnosis of gastric schwannoma is possible using immunohistochemical staining techniques and EUS-guided sampling techniques. After definitive preoperative diagnosis of gastric schwannoma, minimal surgery is recommended to achieve R0 resection.
The authors declare no conflict of interest.
REFERENCES
- 1. Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293-301. [DOI] [PubMed] [Google Scholar]
- 2. Mekky MA, Yamao K, Sawaki A, Mizuno N, Hara K, Nafeh MA, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913-9. [DOI] [PubMed] [Google Scholar]
- 3. Melvin WS, Wilkinson MG. Gastric schwannoma. Clinical and pathologic considerations. Am Surg. 1993;59:293-6. [PubMed] [Google Scholar]
- 4. Sarlomo-Rikala M, Miettinen M. Gastric schwannoma-a clinicopathological analysis of six cases. Histopathology. 1995;27:355-60. [DOI] [PubMed] [Google Scholar]
- 5. Daimaru Y, Kido H, Hashimoto H, Enjoji M. Benign schwannoma of the gastrointestinal tract: a clinicopathologic and immunohistochemical study. Hum Pathol. 1988;19:257-64. [DOI] [PubMed] [Google Scholar]
- 6. Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38 Suppl 5:S39-51. [DOI] [PubMed] [Google Scholar]
- 7. Prevot S, Bienvenu L, Vaillant JC, de Saint-Maur PP. Benign schwannoma of the digestive tract: a clinicopathologic and immunohistochemical study of five cases, including a case of esophageal tumor. Am J Surg Pathol. 1999;23:431-6. [DOI] [PubMed] [Google Scholar]
- 8. Bandoh T, Isoyama T, Toyoshima H. Submucosal tumors of the stomach: a study of 100 operative cases. Surgery. 1993;113:498-506. [PubMed] [Google Scholar]
- 9. Basso N, Rosato P, De Leo A, Picconi T, Trentino P, Fantini A, et al. Laparoscopic treatment of gastric stromal tumors. Surg Endosc. 2000;14:524-6. [DOI] [PubMed] [Google Scholar]
- 10. Shayanfar N, Shahzadi S. Schwannoma In A Perigastric Lymph Node: A Rare Case Report. Iran J Pathol. 2008;3:43-6. [Google Scholar]
- 11. Murase K, Hino A, Ozeki Y, Karagiri Y, Onitsuka A, Sugie S, et al. Malignant schwannoma of the esophagus with lymph node metastasis: literature review of schwannoma of the esophagus. J Gastroenterol. 2001;36:772-7. [DOI] [PubMed] [Google Scholar]
- 12. Komatsu D, Koide N, Hiraga R, Furuya N, Akamatsu T, Uehara T, et al. Gastric schwannoma exhibiting increased fluorodeoxyglucose uptake. Gastric Cancer. 2009;12:225-8. [DOI] [PubMed] [Google Scholar]
- 13. Kawabe J, Okamura T, Shakudo M, Koyama K, Wanibuchi H, Sakamoto H, et al. Two cases of chronic tonsillitis studied by FDG-PET. Ann Nucl Med. 1999;13:277-9. [DOI] [PubMed] [Google Scholar]
- 14. Hou YY, Tan YS, Xu JF, Wang XN, Lu SH, Ji Y, et al. Schwannoma of the gastrointestinal tract: a clinicopathological, immunohistochemical and ultrastructural study of 33 cases. Histopathology. 2006;48:536-45. [DOI] [PubMed] [Google Scholar]
- 15. Fujiwara S, Nakajima K, Nishida T, Takahashi T, Kurokawa Y, Yamasaki M, et al. Gastric schwannomas revisited: has precise preoperative diagnosis become feasible?. Gastric cancer. 2013;16:318-23. [DOI] [PubMed] [Google Scholar]
- 16. Zheng L, Wu X, Kreis ME, Yu Z, Feng L, Chen C, et al. Clinicopathological and immunohistochemical characterisation of gastric schwannomas in 29 cases. Gastroenterol Res Pract. 2014;2014:202960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Voltaggio L, Murray R, Lasota J, Miettinen M. Gastric schwannoma: a clinicopathologic study of 51 cases and critical review of the literature. Hum Pathol. 2012;43:650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takemura M, Yoshida K, Takii M, Sakurai K, Kanazawa A. Gastric malignant schwannoma presenting with upper gastrointestinal bleeding: a case report. J Med Case Rep. 2012;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]