Abstract
γ-Tocopherol (γT), the predominant form of vitamin E in diets, but not α-tocopherol, the major vitamin E form in tissues and supplements, inhibits proliferation of prostate cancer cells (LNCaP and PC-3) and lung cancer cells (A549). In contrast, at similar concentrations, γT has no effect on normal prostate epithelial cells. Combinations of some vitamin E forms, such as γT and δ-tocopherol, exhibit additive or synergistic inhibitory effects. In this study, γT or its combination with δ-tocopherol induced apoptosis in androgen-sensitive prostate LNCaP, but not in androgen-resistant PC-3 cells, by the induction of cytochrome c release, activation of caspase 9 and caspase 3, cleavage of poly-ADP-ribose polymerase (PARP), and involvement of caspase-independent pathways. Myriocin and fumonisin B1, specific inhibitors of key enzymes (serine palmitoyltransferase and dihydroceramide synthase, respectively) in de novo synthesis of sphingolipids, significantly protected cells from γT-induced DNA fragmentation, cytochrome c release, PARP cleavage, and the formation of active caspase 3. Compared with vehicle-treated controls, γT treatment led to pronounced dihydroceramide and dihydrosphingosine accumulation, which preceded morphological and biochemical manifestations of apoptosis. In contrast, ceramide and shpingosine levels did not increase until day 3, when substantial cell death took place. Our study demonstrates that γT and mixed vitamin E forms induce cell death by interrupting the de novo sphingolipid pathway in a prostate cancer cell line. Thus, certain vitamin E forms may be valuable as anticancer agents.
Keywords: apoptosis, ceramide, dihydroceramide, δ-tocopherol, tocotrienol
Cancer is one of the leading causes of mortality. Radiation or chemotherapy, although often effective in causing remission, frequently lead to deleterious side effects. It is therefore important to develop effective anticancer agents with high selectivity for malignant cells and low toxicity. We suggest that some vitamin E forms may fall into this category, as well as being beneficial in human disease prevention.
Vitamin E is a generic term for at least eight structurally related molecules: α-tocopherol (αT), β-tocopherol, γ-tocopherol (γT), δ-tocopherol (δT), α-tocotrienol, β-tocotrienol, γ-tocotrienol (γTE), and δ-tocotrienol. Among them, αT is the predominant form of vitamin E in plasma and tissues and is the form that has drawn most attention in the past. Benefit from αT for cancer prevention has been suggested in some studies (1), but contradictory results exist in both animal and human intervention studies (2-5). Recently, studies by us and others have indicated that other forms of vitamin E appear to have unique properties that are not shared by αT but may be important to human health (6). For instance, γT, the major form of vitamin E in U.S. diets, but not αT, exhibits anti-inflammatory activities by inhibiting cyclooxygenasecatalyzed prostaglandin E2 formation in cell cultures and animals (7, 8). γT, unlike αT, is strongly nucleophilic and thus is more efficient than αT in trapping reactive nitrogen species (9-11). Consistently, the administration of γ-enriched tocopherols significantly lowered C-reactive protein, a biomarker of inflammation, in hemodialysis patients, but the administration of α-enriched tocopherols did not (12).
Recently, Helzlsouer et al. (13) reported that men in the highest quintile of plasma concentration of γT had a 5-fold reduced risk of prostate cancer compared with those in the lowest quintile. In the same study, significant protective effects of high concentrations of selenium and αT were observed only when γT concentrations were high. Consistently, γT and its metabolite, 2,7,8-trimethyl-2-(β-carboxyethyl)-6-hydroxychroman, is more potent than αT in inhibiting prostate cancer cell growth by the down-regulation of cyclins (14, 15). γT is also stronger than αT in the induction of peroxisome proliferator-activated receptor-γ in colon cancer cell lines (16). In addition, various tocotrienols have been shown to cause death in breast cancer cell lines (17) and exhibit antitumor effects in animals (18). These studies point toward the potential use of tocopherols and tocotrienols as anticancer agents, but the molecular mechanism behind the observed effects has not been elucidated.
In this study, we investigated the antiproliferation and proapoptotic effect of γT, and its combination with other forms of vitamin E, in prostate cancer cells (LNCaP and PC-3) and lung cancer cells (A549) by using a prostate epithelial cell (PrEC) as the normal counterpart. We found that γT dose-dependently inhibited proliferation of LNCaP and PC-3 but had no effect on PrEC. γT and its combination with δT induced apoptosis in LNCaP by interrupting de novo synthesis of sphingolipids.
Materials and Methods
Materials. αT (99%), γT (97-99%), and δT (93-97%) were purchased from Fluka and Sigma. 2,7,8-trimethyl-2-(β-carboxyethyl)-6-hydroxychroman (≥98%) and eicosanoids including 5R-hydroxy-eicosatetraenoic acid, 15R-hydroxyeicosatetraenoic acid, 13R-hydroxy-octadecadienoic acid, and prostaglandin E2 were from Cayman Chemical (Ann Arbor, MI). γ-TE was a gift from Klaus Kramer (BASF, Ludwigshafen, Germany). Tissue culture reagents were from GIBCO/BRL. The pancaspase inhibitor Z-Val-Ala-Asp(OMe)-CH2F (Z-VAD-fmk) and fatty acid free BSA were from Calbiochem. Fumonisin B1 from Fusarium moniliforme, myriocin from Mycelia Sterilia, DMSO, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), arachidonic acid (AA), linoleic acid (LA), and all other chemicals were from Sigma.
Cell Culture. Human prostate cancer cell lines (androgen-resistant PC-3 and androgen-sensitive LNCaP) and lung cancer cells (A549) were obtained from the American Type Culture Collection (Manassas, VA). These cells were maintained routinely in RPMI medium 1640/10% FBS. At the time of the experiments, cells were seeded in RPMI medium 1640/10% FBS at a density of 1.8-2.5 × 104 cells per well in 24-well plates. Twenty-four (PC-3 and A549) or 48 (LNCaP) h later, media were replaced with fresh RPMI medium 1640 containing 1-10% FBS and vitamin E forms. Human prostate epithelial cells, PrEC, were obtained from Clonetics (San Diego) and maintained in prostate epithelial cell growth medium (Clonetics). During experiments, PrEC cells were seeded at the same density (1.8-2.5 × 104) and treated with tocopherols in epithelial cell-growth medium.
Tocopherol Preparation. Tocopherols were dissolved in DMSO at 50-100 mM and then diluted into BSA (5 mg/ml). During preparation, samples were kept cold, and exposure to light was avoided. The final concentration of DMSO in all samples did not exceed 0.15%. In controls, the corresponding amounts of DMSO and BSA were added.
Evaluation of Cell Viability by MTT Assays. The number of viable cells was quantified by the estimation of dehydrogenase activity that reduces MTT to form an insoluble product, which was dissolved in DMSO and measured at 570 nm (19).
DNA Fragmentation Assay by Electrophoresis. Cells (5 × 105) were seeded in 10-cm dishes for 24-48 h in RPMI medium 1640/10% FBS and then treated with tocopherols at various concentrations for 2-4 days. Cells were harvested by collecting the floating and attached cells that were briefly trypsinized. DNA was isolated according to a previously published protocol established by Herrmann et al. (20), with minor modifications. The precipitated DNAs were separated on 1.2% agarose gels and visualized by ethidium bromide staining.
Evaluation of Apoptosis by Annexin V and Propidium Iodide Staining. Both floating and attached cells were collected by brief trypsinization. Cells were stained with an Annexin-V-Fluos staining kit (Roche Applied Science, Indianapolis), and apoptosis was evaluated by using FACSort (Becton Dickinson) with cellquest pro 5.1.1 (BD Biosciences). The externalization of phosphatidylserine of the plasma membrane, a marker of apoptosis, is recognized by annexin V conjugated with fluorescein; propidium iodide penetrates into the plasma membrane of cells that have lost membrane integrity.
Isolation of Cytosolic Fraction. Cells were homogenized in the extraction buffer (220 mM mannitol/68 mM sucrose/50 mM Pipes-KOH/50 mM KCl/5 mM EGTA/2 mM MgCl2/protease inhibitors) (21). The homogenate was centrifuged at 400 × g at 4°C for 10 min, and the supernatant was further centrifuged at 10,000 × g for 10 min; the resultant supernatant was used as the cytosolic fraction. The protein amount was determined with a bicinchoninic acid (BCA) protein assay kit (Pierce).
Western Blot. Cells were lysed in Tris-EDTA/1% SDS/1 mM DTT with protease inhibitor cocktails (Sigma), and the resulting solution was heated at 95°C for 5 min. To measure Akt phosphorylation, cells were collected by scraping. After a brief centrifugation, cell pellets were lysed in lysis buffer containing 2 mM Na3VO4. Equal amounts of protein (10-25 μg) were loaded on 10-12% precast SDS/PAGE gels (Bio-Rad). Resolved proteins were transferred onto a poly(vinylidene difluoride) membrane (Millipore) and probed by antibodies. Membranes were exposed to chemiluminescent reagent (PerkinElmer) and visualized on Kodak film with an M35A X-Omat processor (Kodak).
Lipid Extraction. Lipids were extracted as described in refs. 22 and 23. Briefly, cell pellets were resuspended in chloroform/methanol/1 M hydrochloric acid (100:100:1, vol/vol), vortexed, and tip-sonicated for 20 s. A two-phase separation was obtained after adding 0.25 vol of 1 M sodium chloride and then vortexing and centrifuging. The lower organic phase was recovered. Ten percent organic phase was used to determine total choline-containing phospholipids by an enzymatic colorimetric assay (Wako Chemicals, Neuss, Germany). The remaining 90% organic phase was dried in a speedvac and then resuspended in 0.5 ml of 1 M potassium hydroxide in methanol with added C16-sphingosine (250-500 pmol) as the internal standard. Samples were incubated at 90°C for 1 h to completely convert ceramide to sphingoid bases. Lipids were then extracted, dried, and kept at -20°C until further analysis. Endogenous free sphingoid bases were measured after mild alkaline hydrolysis (with 0.1 M sodium hydroxide in methanol at 37°C for 1 h) to prevent ceramide hydrolysis and then extracted by using the procedure described above.
Measurement of Sphingolipid Intermediates by Using HPLC with Fluorescent Detection. Sphingolipid intermediates were derivatized by using o-phthalaldehyde to form fluorescent compounds that were separated by HPLC and quantified with a fluorescence detector (22, 23).
Results
γT Inhibited Proliferation of Several Cancer Cell Lines but Did Not Affect Normal Prostate Epithelial Cells. γT, but not αT, inhibited growth of several human cancer cell lines, including prostate PC-3 and LNCaP and lung A549 cells, as indicated by a dose- and time-response effect in MTT assays (Fig. 1). The results from MTT assays were confirmed by the trypan blue exclusion technique (data not shown). In contrast, γT did not significantly affect PrEC normal prostate epithelial cells after 4-day treatment at 50 μM (Fig. 1D). 2,7,8-trimethyl-2-(β-carboxyethyl)-6-hydroxychroman, the metabolite of γT, had no significant effect on the growth of LNCaP or PrEC (data not shown). A previous study by Gysin et al. (15) reported that both αT and γT inhibited proliferation of LNCaP, although γT was more potent. In our study, we did not find a significant effect of αT. This discrepancy may be due to the different experimental conditions; specifically, the previous study examined inhibition of cell proliferation with respect to serum stimulation after cell-cycle synchronization (15), whereas our data were obtained with unsynchronized cells.
Fig. 1.
Effect of γT on proliferation of PC-3 (A), LNCaP (B), A549 (C), and PrEC (D). In 24-well plates, cells were seeded at a density of 1.8-2.5 × 104 cells per well. After 24-48 h, tocopherols (indicated in μM) and the corresponding amount of DMSO in controls were added. Cell viability (indicated as absorbance in A and B) was evaluated on days 1-4 by using MTT assays. A549 (C) or PrEC (D) cells were treated with vitamin E forms for 4 days, and the viability is expressed as the relative ratio of absorbance between treatments and controls. Data are the averages of two to three independent experiments. * (P < 0.05) and ** (P < 0.01) indicate a significant difference between treated and control cells.
In an earlier attempt to understand the mechanisms of the observed effects, we found that several factors appear to potentiate the antiproliferation potency of γT. For instance, more pronounced inhibition of growth was seen under conditions with relatively low serum (1-2% FBS) compared with higher serum concentrations (10% FBS) (data not shown). All of the subsequent experiments were performed in the presence of 1% FBS unless otherwise indicated. Because cyclooxygenase and lipoxygenase are believed to promote tumorigenesis (24-26), we decided to investigate whether the antiproliferation effect of γT is due to its inhibition of these enzymes (7, 8). Although the presence of low micromolar concentrations of AA and LA, the substrates of cyclooxygenase and lipoxygenase, partially reversed the inhibitory effect of γT (Fig. 2), eicosanoids derived from AA and LA, such as 5R-hydroxy-eicosatetraenoic acid and prostaglandin E2, did not significantly counteract the effect of γT at 50 μM (data not shown). Furthermore, under current experimental conditions, no detectable levels of cyclooxygenase 2 or lipoxygenase could be found in PC-3 or LNCaP by using a Western blot (unpublished data). The effect of γT on proliferation, therefore, appears to be independent of inhibition of these enzymes.
Fig. 2.
Effect of AA and LA on γT-induced antiproliferation. After cells were seeded for 24-48 h, tocopherols (50 μM) or DMSO were added with 10 μMAA or LA in PC-3 (A) and LNCaP (B). After a 4-day incubation, cell viability was evaluated by MTT assays and is expressed as the ratio of absorbance between treatments and controls. * (P < 0.05) indicates a significant counteracting effect of AA or LA on γT treatment.
In addition, the inhibitory effect of γT was not significantly reversed by a coincubation with 1-5 mM N-acetylcysteine (NAC) (data not shown), a precursor for intracellular synthesis of glutathione (GSH). This observation suggests that it is unlikely that the antiproliferation from γT was caused by the formation of a putatively oxidized product, γ-tocopheryl quinone, because this quinone is known to deplete GSH and induce apoptosis (27, 28), which should be blocked by NAC (29).
Combinations of Vitamin E Forms Exhibited Synergistic or Additive Effects. To evaluate whether various forms of vitamin E act synergistically, we examined the combinations of γT with αT, δT, or γTE that are rich in tissues and/or diets (30). Combinations of γT (25 μM) and δT (10 μM) or γT (25 μM) and γTE (2.5 μM) inhibited proliferation additively or synergistically (Fig. 3). Interestingly, these combinations exhibited antiproliferative potency similar to that of γT alone at 50 μM. αT, which is the most abundant vitamin E form in tissues and supplements, did not significantly enhance or counteract the inhibitory effect from γT alone (Fig. 3B).
Fig. 3.
Effect of combinations of vitamin E forms on cell growth in PC-3 (A) and LNCaP (B). Cells were treated with various forms of vitamin E for 3-4 days. The cell-culture conditions are the same as those described in Fig. 1.
γT Induced Apoptosis in LNCaP Cells but Not in PC-3 Cells. We found that γT or its combination with other forms of vitamin E, such as δT, induced apoptosis in LNCaP cells but not in PC-3 cells, as indicated by the DNA fragmentation, which was observed predominantly after 3-day treatment (Fig. 4A), although variable results were seen for the 2-day-treated samples (data not shown). Consistent with the proliferation assays, αT did not cause an enhancement in DNA fragmentation. The apoptosis was confirmed by annexin V and propidium iodide double staining (Fig. 4B). γT treatment, therefore, led to an increase in early (Fig. 4B, lower right; 21.4% in treated cells vs. 6.3% in controls) and late-stage (Fig. 4B, upper right; 8.9% in treated cells vs. 5.3% in controls) apoptosis compared with controls. A small but significant increase in cell population with a loss of membrane integrity also was observed (Fig. 4B, upper left).
Fig. 4.
γT (50 μM) or the combination of γT (25 μM) and δT (10 μM) induced apoptosis in LNCaP but not PC-3. Apoptosis was evaluated in cells after 3-day treatment with tocopherols by means of DNA fragmentation (A) and annexin V-propidium iodide double staining (B). Experimental procedures are described in Materials and Methods.
The mechanism involved in γT- and γT-plus-δT-induced apoptosis was investigated. A significant release of cytochrome c to the cytosolic compartment was observed after 48 h of incubation with γT in LNCaP but not PC-3 (Fig. 5A), suggesting damage to mitochondria. On the other hand, γT treatment did not cause Bax translocation (Fig. 5A). Consistent with cytochrome c release, caspase 9 was activated (Fig. 5B) and PARP was cleaved, as indicated by the appearance of the 85-kDa cleavage product and a decrease of the 116-kDa intact protein (Fig. 5C). In search of the downstream effectors, a slight activation of caspase 3 was evident with the appearance of the active form, p17 (Fig. 6A).
Fig. 5.
γT treatment led to cytochrome c (Cyto C) release, caspase 9 activation, and PARP cleavage. (A) γT caused a time-dependent release of cytochrome c in LNCaP cells but not in PC-3 cells, with no effect on Bax translocation. (B and C) After 72-h treatment, an activation of caspase 9 (B) and PARP cleavage (C) were observed in treated cells.
Fig. 6.
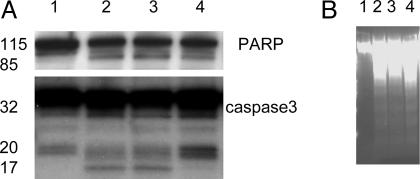
Z-VAD-fmk did not protect cells from γT-induced PARP cleavage (A) or DNA fragmentation (B), although it significantly inhibited the formation of active caspase 3. LNCaP cells were treated as follows: lane 1, DMSO (control); lane 2, γT (50 μM); lane 3, γT (25 μM) and δT (10 μM); lane 4, γT (50 μM) and Z-VAD-fmk (30 μM) for 3 days.
γT-Caused Cell Death Was Mediated by Caspase-Dependent and Caspase-Independent Mechanisms. To further evaluate the role of caspase-dependent pathways in γT-induced cell death, the irreversible pancaspase inhibitor z-VAD-fmk was used. The coincubation with z-VAD-fmk showed only minor protection from the γT-caused effect, as assayed by DNA fragmentation (Fig. 6B) and MTT assay; γT-treated cells with z-VAD-fmk showed 51 ± 10% viability vs. 41 ± 11% for γT alone. Although z-VAD-fmk completely blocked the formation of active caspase 3, p17, it did not significantly protect PARP from being cleaved (Fig. 6A).
γT-Induced Cell Death Was Mediated by Sphingolipid Intermediates. In search of the upstream signaling pathways that are responsible for γT-induced biological effects, we found that γT treatment had no significant effect on BCl-2, BCl-xL, or P53 expression at the studied time points, i.e., 12, 24 and 48 h (unpublished data). γT did not appear to activate caspase 8 (unpublished data), which ruled out the potential involvement of the death receptor-regulated pathway. In addition, γT-induced cell death did not appear to be mediated by the phosphatidylinositol 3 kinase (PI3K)-regulated pathway, because γT treatment (12-48 h) did not affect the phosphorylation of Akt (data not shown), a key downstream target of PI3K.
On the other hand, coincubation with myriocin or fumonisin B1, the specific inhibitors of serine palmitoyltransferase or dihydroceramide synthase (Scheme 1), respectively, resulted in a significant protection against apoptosis, as indicated by DNA fragmentation (Fig. 7A) and MTT assay; γT-treated cells with fumonisin or myriocin showed 65 ± 10% or 85 ± 11% viability, respectively, vs. 41 ± 11% for γT alone. These observations strongly suggest that γT-induced cell death is mediated by sphingolipid intermediates, which are believed to be important mediators of signaling-cell survival and death (31). The importance of sphingolipid intermediates in γT-mediated cell death is further confirmed by the observation that myriocin largely prevented γT-induced cytochrome c release, cleavage of PARP, and the formation of active caspase 3 (Fig. 7B).
Scheme 1.
Fig. 7.
Fumonisin and myriocin significantly protected cells from γT-induced DNA fragmentation (A), and myriosin prevented cytochrome c release, PARP cleavage, and caspase 3 activation (B). In A, LNCaP cells were treated with DMSO (ctrl), γT (50 μM), fumonisin (40 μM) (c + f), myriocin (6 μM) (c + M), γT (50 μM) with fumonisin (40 μM) (γT + f), or myriocin (6 μM) (γT + M) for 3 days. In B, cells were treated with γT (50 μM) with or without myriosin for 3 days.
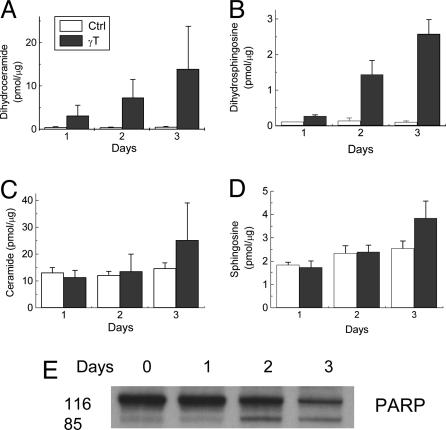
The effect of γT on sphingolipid intermediate levels was further investigated. Unexpectedly, γT treatment caused a 5- to 30-fold and 2- to 30-fold accumulation of dihydroceramide (Fig. 8A) and dihydrosphingosine (Fig. 8B), respectively, in a time-dependent manner. Importantly, the increase in dihydroceramide and dihydrosphingosine was observed on day 1, before any significant changes in cellular morphology, cytochrome c release (cf. Fig. 5), and PARP cleavage (Fig. 8E). However, ceramide (Fig. 8C) and sphingosine (Fig. 8D) levels did not increase until day 3, when apoptosis occurred in significant numbers of cells. In cells treated with a combination of γT and δT, a similar accumulation of dihyroceramide and dihydrosphingosine was observed (data not shown).
Fig. 8.
Time course for γT-caused changes in dihydroceramide (A), dihydrosphingosine (B), ceramide (C), and sphingosine (D), as well as PARP cleavage (E). The results of sphingolipids were expressed as the ratio of sphingolipid intermediates (in pmol) to total choline-containing phospholipids (in μg) (see Materials and Methods).
Discussion
Our current study demonstrates that γT, in contrast to αT, inhibits proliferation of prostate cancer cells but appears to have no effect on the growth of a normal prostate epithelial cell, PrEC. The combinations of γT and δT, or γT and γTE, exhibit additive or synergistic effects. γT and its combination with δT induced apoptosis in androgen-sensitive LNCaP cells but not in androgen-resistant PC-3 cells, as indicated by DNA fragmentation and annexin-V staining. γT treatment consistently led to the release of cytochrome c, activation of caspase 9 and caspase 3, and the cleavage of PARP in LNCaP. Cell death also appears to be mediated by caspase-independent pathways, because Z-VAD-fmk only slightly protected cells from γT-induced apoptosis. γT is shown to induce cell death by interrupting de novo synthesis of sphingolipids by several lines of evidence: (i) Myriocin and fumonisin, specific inhibitors of key enzymes in the de novo pathway (Scheme 1), largely block γT-induced DNA fragmentation, cytochrome c release, PARP cleavage, and the formation of active caspase 3. (ii) γT treatment leads to a substantial increase of dihydroceramide and dihydrosphingosine, which precedes biochemical and morphological manifestation of apoptosis. Ceramide and sphingosine increased only on day 3, when substantial cell death was observed. We therefore provide evidence that the cell death caused by vitamin E forms is linked to de novo synthesis of sphingolipids.
Sphingolipids, which are important membrane components, are generated by condensation of palmitoyl-CoA and serine (Scheme 1). Ceramide is the common intermediate for the formation of complex sphingolipids, including sphingomyelin and glycosphingolipids. Recently, ceramide has been proposed as a mediator in regulating stress response, particularly apoptosis (31-33). In addition, other sphingolipid intermediates, such as sphingosine, sphingosine-1-phosphate and dihydrosphingosine, have been shown to mediate cell survival (32, 34, 35). The use of inhibitors of the enzymes in the de novo synthesis pathway has provided insight regarding the importance of sphingolipid intermediates in cell fate. For instance, fumonisin, which inhibits dihydroceramide synthase, has been shown to attenuate apoptosis induced by varieties of chemical agents and other stresses (36, 37). Myriocin, the specific inhibitor of serine palmitoyl-CoA transferase, recently has been reported to block CD95-mediated apoptosis of T cells (35). In our study, fumonisin and myriosin partially and completely block γT-induced cell death, which strongly suggests that sphingolipid intermediates play an important role in γT-mediated effects.
Surprisingly, a large amount of dihydroceramide accumulation was observed before apoptosis, whereas total ceramide increased only when apoptosis was substantial. In addition to γT, certain other forms of vitamin E also caused a significant enhancement of dihydroceramide (unpublished data). Our findings are different from some previously published studies that showed a moderate increase of ceramide preceding apoptosis (31). These results, however, have been questioned by several recent studies that failed to observe an enhancement of ceramide until relatively late stages of apoptosis (38, 39). Few studies have reported the status of dihydroceramide during apoptosis. Interestingly, Tserng and Griffin (40) recently reported the same pattern of dihydroceramide, but not ceramide, accumulation along with cell death when HL-60 human leukemia cells were treated with natural ceramide or N-oleoylethanolamide, a ceramidase inhibitor, as well as its inactive homologue, N-palmitoylethanolamide (40). Our current data, together with those gathered by Tserng and Griffin, therefore, indicate that this pattern of dihydroceramide and dihydrosphingosine accumulation is independent of the cell types and the reagents applied and therefore may represent a previously unrecognized type of cellular response to stress.
It is currently not clear which sphingolipid intermediate is responsible for γT-induced cell death. Although we did not observe a significant increase of total ceramide until the late stage of apoptosis, it is possible that an increase of a specific ceramide, such as C16, preceded apoptosis (41-43). Further studies using HPLC and mass spectrometry are being undertaken to further elucidate the role of ceramide in γT-mediated effects. It is known that C2-dihydroceramide does not directly cause cell death (37), but it is not clear whether long-chain dihydroceramide induces apoptosis. Our current observation that fumonisin, which attenuates dihydroceramide accumulation (data not shown), partially attenuated apoptosis suggests that dihydroceramide may partially contribute to γT-caused biological effects. An increase in dihydrosphingosine could be another potential candidate, because this sphingolipid intermediate has been shown to induce apoptosis in certain types of cells (35).
The high level of dihydroceramide and dihydrosphingosine accumulation suggests that dihydroceramide desaturase is likely to be inhibited as a result of γT treatment (Scheme 1). The potential interaction of this enzyme with vitamin E forms and possibly other apoptotic agents needs to be characterized. Little information is currently available regarding the role of this enzyme in cell survival. We suggest that dihydroceramide desaturase may play a role in the cellular response to certain chemical treatments, which warrants further investigation.
Although the nature of γT-induced caspase-independent apoptosis is not clear, our data suggest that PARP can be cleaved by means of a caspase-independent mechanism (Fig. 6). The caspase-independent PARP cleavage has been reported previously in transforming growth factor β1-induced apoptosis in murine hepatocytes (44). In addition to apoptosis, the fact that γT caused a small but significant increase in membrane impairment suggests that small portions of cells may undergo necrosis (Fig. 4B).
It is noteworthy that the tumor cell lines we have tested appear to be more sensitive to γT-induced anti-proliferation or apoptosis compared with normal prostate epithelial cells. γT and combinations of vitamin E forms are therefore potentially attractive candidates as anticancer agents. The observation that androgen-sensitive LNCaP cells, but not androgen-resistant PC-3 cells, are more susceptible to γT-induced apoptosis indicates that these cells may have different mechanisms in coping with stresses, which has also been suggested by others (45). Future studies comparing the effect of vitamin E forms on sphingolipid metabolism in normal vs. cancer cells or apoptosis-sensitive (e.g., LNCaP) vs. -resistant (e.g., PC-3) cells may be useful to elucidate the regulatory role of different sphingolipids and related enzymes in cancer cell survival. These studies may also lead to the discovery of new therapeutic targets.
It is becoming clear that individual vitamin E forms possess different chemical and biological activities (6) and have distinct tissue distributions (46, 47). We therefore hypothesize that combinations of different forms of vitamin E may be superior to each alone. Consistently, combinations of γT and δT or γT and γTE showed additive or synergistic effects. Importantly, αT did not interfere with the effect of γT, although αT is known to deplete γT in vivo (48). Although high doses of γT supplementation would be necessary to achieve relatively high concentrations of γT, i.e., 50 μM, the combination effect indicates that a similar biological outcome may be achieved by a moderate supplementation with mixed vitamin E forms such as γT and δT or γTE. The significance of this observation awaits further investigation.
In summary, this study demonstrates that γT induces cell death in a prostate cancer cell line by interrupting de novo synthesis of sphingolipids. The combinations of vitamin E forms exhibit additive or synergistic effects. Our study, together with in vitro and epidemiological studies, strongly supports the notion that vitamin E forms may be useful as anticancer agents. Animal studies and clinical trials are necessary to further establish the role of various forms of vitamin E and their combinations in cancer therapy.
Acknowledgments
We thank Drs. Angelo Azzi, Maret Traber, and Stephan Ladisch for critical reviews of the manuscript. We thank Helen Song and Huiyi Chen for participating in some of the earlier studies. This work was supported by a National Institutes of Health Grant to the Cancer Research Laboratory at the University of California, Berkeley (to Q.J.), and National Institutes of Health Grants 1R01AT001821 (to Q.J. and B.N.A.), K05AT001323 (to B.N.A.), and R01CA77528 (to J.D.S.).
Author contributions: Q.J. designed research; Q.J., J.W., and H.F. performed research; J.D.S. and B.N.A. contributed new reagents/analytic tools; Q.J., J.W., H.F., J.D.S., and B.N.A. analyzed data; and Q.J. wrote the paper.
Abbreviations: AA, arachidonic acid; αT, α-tocopherol; γT, γ-tocopherol; δT, δ-tocopherol; γTE, γ-tocotrienol; LA, linoleic acid; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; PARP, poly-ADP-ribose polymerase; Z-VAD-fmk, pancaspase inhibitor Z-Val-Ala-Asp(OMe)-CH2F.
References
- 1.Eichholzer, M., Stahelin, H. B., Gey, K. F., Ludin, E. & Bernasconi, F. (1996) Int. J. Cancer 66, 145-150. [DOI] [PubMed] [Google Scholar]
- 2.Toth, B. & Patil, K. (1983) J. Natl. Cancer Inst. 70, 1107-1111. [PubMed] [Google Scholar]
- 3.Chung, H., Wu, D., Han, S. N., Gay, R., Goldin, B., Bronson, R. E., Mason, J. B., Smith, D. E. & Meydani, S. N. (2003) J. Nutr. 133, 528-532. [DOI] [PubMed] [Google Scholar]
- 4.Chan, J. M., Stampfer, M. J., Ma, J., Rimm, E. B., Willett, W. C. & Giovannucci, E. L. (1999) Cancer Epidemiol. Biomarkers Prev. 8, 893-899. [PubMed] [Google Scholar]
- 5.Moyad, M. A. (2002) Urology 59, 9-19. [DOI] [PubMed] [Google Scholar]
- 6.Jiang, Q., Christen, S., Shigenaga, M. K. & Ames, B. N. (2001) Am. J. Clin. Nutr. 74, 714-722. [DOI] [PubMed] [Google Scholar]
- 7.Jiang, Q., Elson-Schwab, I., Courtemanche, C. & Ames, B. N. (2000) Proc. Natl. Acad. Sci. USA 97, 11494-11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang, Q. & Ames, B. N. (2003) FASEB J. 17, 816-822. [DOI] [PubMed] [Google Scholar]
- 9.Cooney, R. V., Franke, A. A., Harwood, P. J., Hatch-Pigott, V., Custer, L. J. & Mordan, L. J. (1993) Proc. Natl. Acad. Sci. USA 90, 1771-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooney, R. V., Harwood, P. J., Franke, A. A., Narala, K., Sundström, A.-K., Berggren, P.-O. & Mordan, L. J. (1995) Free Radical Biol. Med. 19, 259-269. [DOI] [PubMed] [Google Scholar]
- 11.Christen, S., Woodall, A. A., Shigenaga, M. K., Southwell-Keely, P. T., Duncan, M. W. & Ames, B. N. (1997) Proc. Natl. Acad. Sci. USA 94, 3217-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Himmelfarb, J., Kane, J., McMonagle, E., Zaltas, E., Bobzin, S., Boddupalli, S., Phinney, S. & Miller, G. (2003) Kidney Int. 64, 978-991. [DOI] [PubMed] [Google Scholar]
- 13.Helzlsouer, K. J., Huang, H. Y., Alberg, A. J., Hoffman, S., Burke, A., Norkus, E. P., Morris, J. S. & Comstock, G. W. (2000) J. Natl. Cancer Inst. 92, 2018-2023. [DOI] [PubMed] [Google Scholar]
- 14.Galli, F., Stabile, A. M., Betti, M., Conte, C., Pistilli, A., Rende, M., Floridi, A. & Azzi, A. (2004) Arch. Biochem. Biophys. 423, 97-102. [DOI] [PubMed] [Google Scholar]
- 15.Gysin, R., Azzi, A. & Visarius, T. (2002) FASEB J. 16, 1952-1954. [DOI] [PubMed] [Google Scholar]
- 16.Campbell, S. E., Stone, W. L., Whaley, S. G., Qui, M. & Krishnan, K. (2003) BMC Cancer 3, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi, K. & Loo, G. (2004) Biochem. Pharmacol. 67, 315-324. [DOI] [PubMed] [Google Scholar]
- 18.He, L., Mo, H., Hadisusilo, S., Qureshi, A. A. & Elson, C. E. (1997) J. Nutr. 127, 668-674. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann, T. (1983) J. Immunol. Methods 65, 55-63. [DOI] [PubMed] [Google Scholar]
- 20.Herrmann, M., Lorenz, H. M., Voll, R., Grunke, M., Woith, W. & Kalden, J. R. (1994) Nucleic Acids Res. 22, 5506-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, X., Marani, M., Mannucci, R., Kinsey, B., Andriani, F., Nicoletti, I., Denner, L. & Marcelli, M. (2001) Cancer Res. 61, 1699-1706. [PubMed] [Google Scholar]
- 22.Fyrst, H., Herr, D. R., Harris, G. L. & Saba, J. D. (2004) J. Lipid Res. 45, 54-62. [DOI] [PubMed] [Google Scholar]
- 23.Bose, R. & Kolesnick, R. (2000) Methods Enzymol. 322, 373-378. [DOI] [PubMed] [Google Scholar]
- 24.Avis, I., Hong, S. H., Martinez, A., Moody, T., Choi, Y. H., Trepel, J., Das, R., Jett, M. & Mulshine, J. L. (2001) FASEB J. 15, 2007-2009. [DOI] [PubMed] [Google Scholar]
- 25.Sun, Y., Tang, X. M., Half, E., Kuo, M. T. & Sinicrope, F. A. (2002) Cancer Res. 62, 6323-6328. [PubMed] [Google Scholar]
- 26.Tsujii, M., Kawano, S., Tsuji, S., Sawaoka, H., Hori, M. & DuBois, R. N. (1998) Cell 93, 705-716. [DOI] [PubMed] [Google Scholar]
- 27.Jones, K. H., Liu, J. J., Roehm, J. S., Eckel, J. J., Eckel, T. T., Stickrath, C. R., Triola, C. A., Jiang, Z., Bartoli, G. M. & Cornwell, D. G. (2002) Lipids 37, 173-184. [DOI] [PubMed] [Google Scholar]
- 28.Calviello, G., Di Nicuolo, F., Piccioni, E., Marcocci, M. E., Serini, S., Maggiano, N., Jones, K. H., Cornwell, D. G. & Palozza, P. (2003) Carcinogenesis 24, 427-433. [DOI] [PubMed] [Google Scholar]
- 29.Coffey, R. N., Watson, R. W., Hegarty, N. J., O'Neill, A., Gibbons, N., Brady, H. R. & Fitzpatrick, J. M. (2000) Cancer 88, 2092-2104. [DOI] [PubMed] [Google Scholar]
- 30.McLaughlin, P. J. & Weihrauch, J. L. (1979) J. Am. Diet. Assoc. 75, 647-665. [PubMed] [Google Scholar]
- 31.Kolesnick, R. (2002) J. Clin. Invest. 110, 3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogretmen, B. & Hannun, Y. A. (2004) Nat. Rev. Cancer 4, 604-616. [DOI] [PubMed] [Google Scholar]
- 33.Radin, N. S. (2001) Eur. J. Biochem. 268, 193-204. [DOI] [PubMed] [Google Scholar]
- 34.Cuvillier, O., Edsall, L. & Spiegel, S. (2000) J. Biol. Chem. 275, 15691-15700. [DOI] [PubMed] [Google Scholar]
- 35.Solomon, J. C., Sharma, K., Wei, L. X., Fujita, T. & Shi, Y. F. (2003) Cell Death Differ. 10, 193-202. [DOI] [PubMed] [Google Scholar]
- 36.Uchida, Y., Nardo, A. D., Collins, V., Elias, P. M. & Holleran, W. M. (2003) J. Invest. Dermatol. 120, 662-669. [DOI] [PubMed] [Google Scholar]
- 37.Bose, R., Verheij, M., Haimovitz-Friedman, A., Scotto, K., Fuks, Z. & Kolesnick, R. (1995) Cell 82, 405-414. [DOI] [PubMed] [Google Scholar]
- 38.Watts, J. D., Gu, M., Patterson, S. D., Aebersold, R. & Polverino, A. J. (1999) Cell Death Differ. 6, 105-114. [DOI] [PubMed] [Google Scholar]
- 39.Watts, J. D., Gu, M., Polverino, A. J., Patterson, S. D. & Aebersold, R. (1997) Proc. Natl. Acad. Sci. USA 94, 7292-7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tserng, K. Y. & Griffin, R. L. (2004) Biochem. J. 380, 715-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reiss, U., Oskouian, B., Zhou, J., Gupta, V., Sooriyakumaran, P., Kelly, S., Wang, E., Merrill, A. H., Jr., & Saba, J. D. (2004) J. Biol. Chem. 279, 1281-1290. [DOI] [PubMed] [Google Scholar]
- 42.Kroesen, B. J., Pettus, B., Luberto, C., Busman, M., Sietsma, H., de Leij, L. & Hannun, Y. A. (2001) J. Biol. Chem. 276, 13606-13614. [DOI] [PubMed] [Google Scholar]
- 43.Eto, M., Bennouna, J., Hunter, O. C., Hershberger, P. A., Kanto, T., Johnson, C. S., Lotze, M. T. & Amoscato, A. A. (2003) Prostate 57, 66-79. [DOI] [PubMed] [Google Scholar]
- 44.Yang, Y., Zhao, S. & Song, J. (2004) Int. J. Biochem. Cell Biol. 36, 223-234. [DOI] [PubMed] [Google Scholar]
- 45.Wang, H., Charles, A. G., Frankel, A. J. & Cabot, M. C. (2003) Urology 61, 1047-1052. [DOI] [PubMed] [Google Scholar]
- 46.Okabe, M., Oji, M., Ikeda, I., Tachibana, H. & Yamada, K. (2002) Biosci. Biotechnol. Biochem. 66, 1768-1771. [DOI] [PubMed] [Google Scholar]
- 47.Burton, G. W., Traber, M. G., Acuff, R. V., Walters, D. N., Kayden, H., Hughes, L. & Ingold, K. U. (1998) Am. J. Clin. Nutr. 67, 669-684. [DOI] [PubMed] [Google Scholar]
- 48.Handelman, G. J., Machlin, L. J., Fitch, K., Weiter, J. J. & Dratz, E. A. (1985) J. Nutr. 115, 807-813. [DOI] [PubMed] [Google Scholar]