Abstract
Background:
The current analysis was performed to evaluate the impact of PIK3CA hotspot mutations on everolimus efficacy in BOLERO-2 participants, using cell-free DNA (cfDNA) from plasma samples collected at the time of patient randomisation.
Methods:
PIK3CA H1047R, E545K, and E542K mutations in plasma-derived cfDNA were analysed by droplet digital PCR (ddPCR). Median PFS was estimated for patient subgroups defined by PIK3CA mutations in each treatment arm.
Results:
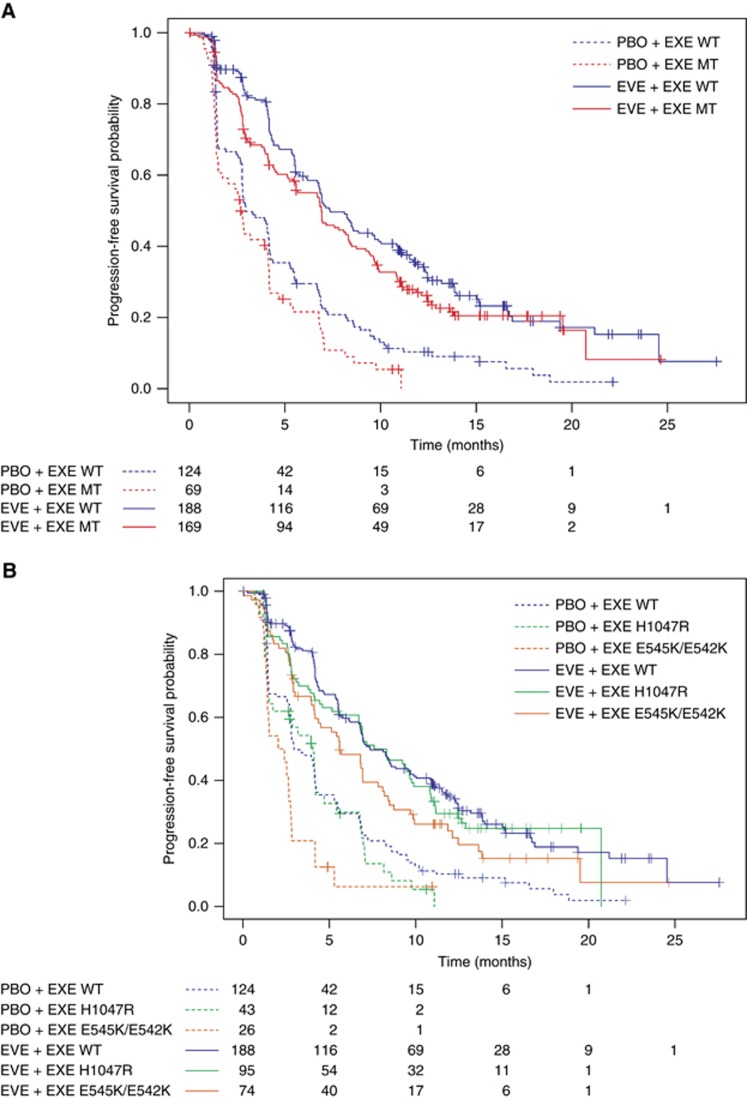
Among 550 patients included in cfDNA analysis, median PFS in everolimus vs placebo arms was similar in patients with tumours that had wild-type or mutant PIK3CA (hazard ratio (HR), 0.43 and 0.37, respectively). Everolimus also prolonged median PFS in patients with PIK3CA H1047R (HR, 0.37) and E545K/E542K mutations (HR=0.30) with a similar magnitude.
Conclusions:
Mutation analysis of plasma-derived cfDNA by ddPCR suggests that PFS benefit of everolimus was maintained irrespective of PIK3CA genotypes, consistent with the previous analysis of archival tumour DNA by next-generation sequencing.
Keywords: BOLERO-2, PIK3CA, cfDNA, everolimus, HR+ breast cancer
Aberrant signalling through the PI3K/AKT/mTOR pathway has been implicated in tumorigenesis, progression, and therapeutic resistance in various cancers (Rodon et al, 2013; Thorpe et al, 2015). PIK3CA mutations result in constitutive activation of p110α, which in turn activates AKT1 and its downstream target mTOR. Somatic PIK3CA mutations are frequent in human breast cancer, occurring in up to 45% of luminal A tumours (Cancer Genome Atlas Network, 2012); the majority are missense mutations clustering in the helical (E524K and E545K) and catalytic (H1047R) domains (Kalinsky et al, 2009).
In the phase 3 BOLERO-2 trial, everolimus plus exemestane prolonged the progression-free survival (PFS) vs placebo plus exemestane (median PFS, 7.8 vs 3.2 months; hazard ratio (HR), 0.45; P<0.0001) in patients with hormone receptor positive (HR+), human epidermal growth factor receptor-2 negative (HER2−) metastatic breast cancer (MBC) progressing after a non-steroidal aromatase inhibitor (NSAI) (Yardley et al, 2013). An exploratory analysis of predominantly primary tumour samples from roughly 40% of the BOLERO-2 trial population suggested that the PFS benefit from everolimus was largely maintained irrespective of PIK3CA genotype (Hortobagyi et al, 2016). However, given that differences between primary and MBC may arise, we performed an analysis of PIK3CA hotspot (HS) mutations by droplet digital PCR (ddPCR) using cell-free DNA (cfDNA) from plasma samples collected at the time of patient randomisation and evaluated their impact on everolimus efficacy in 76% of the BOLERO-2 participants.
Materials and methods
Patients and cfDNA analysis
BOLERO-2 study design has been described previously (Baselga et al, 2012). Briefly, patients with HR+, HER2− MBC recurring/progressing on/after NSAI were randomised 2 : 1 to everolimus plus exemestane or placebo plus exemestane. Plasma collection, cfDNA extraction, and quantification and analysis by ddPCR for detection were performed as described previously (Chandarlapaty et al, 2016). PIK3CA mutations in cfDNA were detected by ddPCR in singleplex assays using inventoried assays for PIK3CA H1047R (dHsaCP2000077), E545K (dHsaCP2000075), and E542K (dHsaCP2000073) (BioRad, Hercules, CA, USA) on a BioRad QX200 Droplet Digital PCR System. Each assay run included mutation positive template, wild-type template, and no template controls. The fractional abundance for the PIK3CA mutation was calculated from the number of FAM-positive events (mutation positive) over total positive events (both FAM and HEX positive for mutation and wild type, respectively) using QuantaSoft Version 1.7.4.0917 (BioRad). Mutation analyses were performed on anonymised samples.
Written informed consent was obtained from all patients included in the study. The study was undertaken in accordance with the Good Clinical Practice guidelines and the Declaration of Helsinki. The study protocol was approved by an independent ethics committee or institutional review board at each site.
Statistical analysis
Median PFS was estimated by Kaplan–Meier method in patient subgroups defined by PIK3CA mutations in each treatment arm. Hazard ratios with 95% confidence intervals (CIs) were calculated using Cox proportional hazards model for treatment vs placebo arm in the wild-type and mutant subgroups. Multivariate analysis was performed to adjust for the potential effects of prior hormonal therapy, visceral disease, and Eastern Cooperative Oncology Group status.
Results
PIK3CA mutation frequency
Of the 724 patients in BOLERO-2, 550 patients (76%) underwent PIK3CA cfDNA analysis. The baseline characteristics and clinical outcomes were similar between the cfDNA and overall population (Supplementary Table 1). PIK3CA HS mutations were identified in 238 patients (43.3%); the most prevalent was H1047R (25.1%), followed by E545K (11.1%) and E542K (7.1%). Four patients had multiple HS mutations; three, of which, were dual helical mutations. Prevalence of total and domain-specific PIK3CA mutations was higher in the everolimus arm (47.3%) than the placebo arm (35.8% Table 1).
Table 1. PIK3CA mutation prevalence – overall and by treatment arm.
|
Overall |
PBO+EXE |
EVE+EXE |
||||
|---|---|---|---|---|---|---|
| PIK3CA genotype | N | % Genotype | N | % Genotype | N | % Genotype |
| WT | 312 | 56.7% | 124 | 64.2% | 188 | 52.7% |
| MT | 238 | 43.3% | 69 | 35.8% | 169 | 47.3% |
| H1047R | 138 | 25.1% | 43 | 22.3% | 95 | 26.6% |
| E545K | 61 | 11.1% | 26 | 13.5% | 74 | 20.7% |
| E542K | 39 | 7.1% | ||||
| Multiple | (4)a | 0.7% | ||||
Abbreviations: EVE=everolimus; EXE=exemestane; MT=mutant; PBO=placebo; WT=wild type.
Samples with multiple mutations were categorised by allele with highest mutant fraction.
Concordance between mutations detected in cfDNA and archival tumour samples
Plasma-derived cfDNA samples were available in 247 of the 302 patients who underwent mutation analysis on archival tumour samples (198 primary; 49 metastatic) by next-generation sequencing (NGS) (Hortobagyi et al, 2016). The overall concordance in PIK3CA mutation status between archival tumour and cfDNA sample pairs was 70.4%, with a higher concordance (81.6%) for metastatic lesions. For concordant samples, the cfDNA mutant fractional abundance was generally higher than in the discordant samples where mutations were detected only by ddPCR in cfDNA (Supplementary Figure 1).
Correlation between PIK3CA genotypes and everolimus efficacy
Clinical benefit with everolimus as measured by PFS was similar in patients with tumours that had wild-type or mutant PIK3CA (HR, 0.43 (95% CI, 0.34–0.56) and 0.37 (95% CI, 0.27–0.51), respectively) (Table 2; Figure 1A). A multivariate analysis yielded similar results (HRs for PFS: PIK3CA wild-type HR, 0.39 (95% CI, 0.30–0.51); PIK3CA-mutant HR, 0.35 (95% CI, 0.25–0.49)) (Supplementary Table 3).
Table 2. PFS benefit in patient subgroups defined by overall and domain-specific mutation status of PIK3CA.
| PIK3CA status | Treatment | n | Events | Median PFS, months (95% CI) | Hazard ratio (95% CI) |
|---|---|---|---|---|---|
| WT | EVE+EXE | 188 | 132 | 7.36 (6.77–9.69) | 0.43 (0.34–0.56) |
| PBO+EXE | 124 | 111 | 2.96 (2.76–4.17) | ||
| MT | EVE+EXE | 169 | 123 | 6.90 (5.55–8.31) | 0.37 (0.27–0.51) |
| PBO+EXE | 69 | 60 | 2.69 (1.51–4.11) | ||
| H1047R | EVE+EXE | 95 | 65 | 7.59 (5.59–9.76) | 0.37 (0.24–0.56) |
| PBO+EXE | 43 | 38 | 4.04 (1.51–4.70) | ||
| E545K/E542K | EVE+EXE PBO+EXE | 74 26 | 58 22 | 5.59 (4.14–7.82) 2.22 (1.38–2.76) | 0.30 (0.18–0.51) |
Abbreviations: CI=confidence interval; EVE=everolimus; EXE=exemestane; MT=mutant; PBO=placebo; PFS=progression-free survival; WT=wild type.
Figure 1.
Kaplan-Meier Plot of Progression-free Survival by PIK3CA mutation status. (A) PIK3CA wild-type vs hotspot mutations. (B) PIK3CA wild-type vs domain-specific hotspot mutations.
To examine the role of domain-specific mutations in PIK3CA on everolimus efficacy, patients were categorised by mutation site, H1047R in the catalytic domain, and E545K and E542K in the helical domain. Everolimus prolonged median PFS vs placebo in patients with PIK3CA H1047R mutation (HR, 0.37; 95% CI, 0.24–0.56) and E545K/E542K mutations (HR, 0.30; 95% CI, 0.18–0.51) with a similar magnitude (Table 2; Figure 1B). These data suggest that PFS benefit of everolimus was maintained irrespective of PIK3CA genotypes.
In the placebo arm, patients with E545K/E542K mutation had shorter PFS and overall survival than those with wild-type PIK3CA (Supplementary Table 2), suggesting that PIK3CA mutations in the helical domain might play a role in resistance to hormone therapy. However, this analysis by mutation site involved small numbers and was highly exploratory.
Discussion
The advent of cfDNA analyses has facilitated the analysis of large clinical trial populations through minimally invasive technologies. We recently determined the feasibility for detection of ESR1 mutations in cfDNA by ddPCR on stored plasma samples from the BOLERO-2 clinical trial (Chandarlapaty et al, 2016). Notably, collection of these plasma samples began in mid-2009 at trial enrolment in the absence of a plan for subsequent cfDNA analysis. Thus, data from large, mature clinical trials can be extended through the use of this emerging technology. In this report, we assessed the impact of three most prevalent PIK3CA-activating mutations on everolimus efficacy in >75% of the BOLERO-2 trial population, thus enabling a robust mutation analysis that demonstrates everolimus efficacy regardless of PIK3CA genotypes.
In the BOLERO-2 analysis, patients with tumours harbouring HS mutations in both helical and catalytic domains of PIK3CA benefit from the addition of everolimus. Interestingly, in the placebo arm, there is a suggestion of inferior clinical outcome in the small group harbouring E545K/E542K mutations. This is in contrast to a favourable prognosis associated with these mutations in early-stage cancer (Kalinsky et al, 2009; Sabine et al, 2014), no effect on prognosis in patients with MBC undergoing first-line hormonal therapy with tamoxifen and an improved outcome in patients with MBC with first-line aromatase inhibitor therapy (Ramirez-Ardila et al, 2013). From a biologic perspective, mutation site-specific differences are noted for interacting proteins (Zhao and Vogt, 2008; Hao et al, 2013), oncogenic potency and behaviour (Bader et al, 2006; Pang et al, 2009), and the phosphoproteome (Wu et al, 2014; Zahari et al, 2015) that may be clinically important and deserve further analysis in clinical studies and preclinical modelling.
Approximately, 70% concordance for PIK3CA genotype by PIK3CA analysis of archival tumour DNA by NGS and contemporaneous plasma-derived cfDNA by ddPCR is similar to that reported for BEAMING of cfDNA and sequencing of archival tumour (Higgins et al, 2012). There are several potential biologic and technical reasons for this degree of discordance. ddPCR of plasma cfDNA is more sensitive for mutation detection than tumour sequencing (Guttery et al, 2015; Schiavon et al, 2015; Chu et al, 2016) with mutant allele fraction (mAF) <1–3% not detected by NGS (Guttery et al, 2015). When both concurrent biopsy and plasma collection are performed and the material is analysed by the same highly sensitive methodology, concordance is quite high (Higgins et al, 2012).
In breast cancer, PIK3CA mutation is thought to be truncal, identified in DCIS, primary breast cancer, and selected for in metastatic tumour (Kalinsky et al, 2011). However, compelling evidence from tumour genome sequencing highlights that reliance on a single analysis of the primary tumour may not be suitable for guiding therapy in patients with metastatic disease. Genomic heterogeneity in multifocal primary tumours identifies PIK3CA mutations occurring in ∼44% of patients, but in only half is the mutation identified in all tumours (Desmedt et al, 2015). Even in metastatic disease, emergence of a PIK3CA E545K mutation during progression has been reported by serial cfDNA analysis (Guttery et al, 2015). Conversely, absence of an H1047R mutation in hepatic metastasis and cfDNA is reported, when present in the primary tumour at a high mAF of 40% (Butler et al, 2015). In larger data sets of paired samples, both the loss and gain of PIK3CA mutation in metastatic progression is observed (Dupont Jensen et al, 2011; Arthur et al, 2014; Markou et al, 2014). Thus, although selection for a PIK3CA-activating mutation is expected, more dominant subclonal populations may give rise to the metastatic tumour.
Conclusions
Mutational analysis of plasma-derived cfDNA by ddPCR suggests that PFS benefit of everolimus was maintained irrespective of PIK3CA genotypes, consistent with the previous analysis of archival tumour DNA by NGS. These results suggest that PIK3CA genotype is not a predictive determinant for everolimus benefit.
Acknowledgments
We thank the patients who participated in the BOLERO-2 study and their families. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Amol Hosing (Novartis Healthcare Pvt Ltd) for providing medical editorial assistance with this manuscript.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
Presented as poster at the 2016 American Society of Clinical Oncology (ASCO) Annual Meeting; 3–7 June 2016; Chicago, IL, USA.
MEM: Grant and personal fees from Novartis; patent related to biomarkers for response to PI3K inhibitors pending. GNH: personal fees from Merck, Eli Lilly, Peregrine Pharmaceuticals, Novartis, and Celgene as consultant; personal fees from Novartis, Bayer, Metastat, Pfizer, Antigen Express, and Galena Biopharma as scientific/advisory committee member. SC: Grant from Novartis and Eli Lilly; personal fees from Foresite Capital, Chugai Pharmaceuticals, Oncotheryeon, and Astra Zeneca. DC, WH, PP, and FR are Novartis employees. The remaining authors declare no conflict of interest.
Supplementary Material
References
- Arthur LM, Turnbull AK, Renshaw L, Keys J, Thomas JS, Wilson TR, Lackner MR, Sims AH, Dixon JM (2014) Changes in PIK3CA mutation status are not associated with recurrence, metastatic disease or progression in endocrine-treated breast cancer. Breast Cancer Res Treat 147(1): 211–219. [DOI] [PubMed] [Google Scholar]
- Bader AG, Kang S, Vogt PK (2006) Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proc Natl Acad Sci USA 103(5): 1475–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366(6): 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TM, Johnson-Camacho K, Peto M, Wang NJ, Macey TA, Korkola JE, Koppie TM, Corless CL, Gray JW, Spellman PT (2015) Exome sequencing of cell-free DNA from metastatic cancer patients identifies clinically actionable mutations distinct from primary disease. PLoS One 10(8): e0136407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network (2012) Comprehensive molecular portraits of human breast tumours. Nature 490(7418): 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandarlapaty S, Chen D, He W, Sung P, Samoila A, You D, Bhatt T, Patel P, Voi M, Gnant M, Hortobagyi G, Baselga J, Moynahan ME (2016) Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: a secondary analysis of the BOLERO-2 clinical trial. JAMA Oncol 2: 1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D, Paoletti C, Gersch C, VanDenBerg DA, Zabransky DJ, Cochran RL, Wong HY, Toro PV, Cidado J, Croessmann S, Erlanger B, Cravero K, Kyker-Snowman K, Button B, Parsons HA, Dalton WB, Gillani R, Medford A, Aung K, Tokudome N, Chinnaiyan AM, Schott A, Robinson D, Jacks KS, Lauring J, Hurley PJ, Hayes DF, Rae JM, Park BH (2016) ESR1 mutations in circulating plasma tumor DNA from metastatic breast cancer patients. Clin Cancer Res 22(4): 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt C, Fumagalli D, Pietri E, Zoppoli G, Brown D, Nik-Zainal S, Gundem G, Rothe F, Majjaj S, Garuti A, Carminati E, Loi S, Van Brussel T, Boeckx B, Maetens M, Mudie L, Vincent D, Kheddoumi N, Serra L, Massa I, Ballestrero A, Amadori D, Salgado R, de Wind A, Lambrechts D, Piccart M, Larsimont D, Campbell PJ, Sotiriou C (2015) Uncovering the genomic heterogeneity of multifocal breast cancer. J Pathol 236(4): 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont Jensen J, Laenkholm AV, Knoop A, Ewertz M, Bandaru R, Liu W, Hackl W, Barrett JC, Gardner H (2011) PIK3CA mutations may be discordant between primary and corresponding metastatic disease in breast cancer. Clin Cancer Res 17(4): 667–677. [DOI] [PubMed] [Google Scholar]
- Guttery DS, Page K, Hills A, Woodley L, Marchese SD, Rghebi B, Hastings RK, Luo J, Pringle JH, Stebbing J, Coombes RC, Ali S, Shaw JA (2015) Noninvasive detection of activating estrogen receptor 1 (ESR1) mutations in estrogen receptor-positive metastatic breast cancer. Clin Chem 61(7): 974–982. [DOI] [PubMed] [Google Scholar]
- Hao Y, Wang C, Cao B, Hirsch BM, Song J, Markowitz SD, Ewing RM, Sedwick D, Liu L, Zheng W, Wang Z (2013) Gain of interaction with IRS1 by p110alpha-helical domain mutants is crucial for their oncogenic functions. Cancer Cell 23(5): 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins MJ, Jelovac D, Barnathan E, Blair B, Slater S, Powers P, Zorzi J, Jeter SC, Oliver GR, Fetting J, Emens L, Riley C, Stearns V, Diehl F, Angenendt P, Huang P, Cope L, Argani P, Murphy KM, Bachman KE, Greshock J, Wolff AC, Park BH (2012) Detection of tumor PIK3CA status in metastatic breast cancer using peripheral blood. Clin Cancer Res 18(12): 3462–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortobagyi GN, Chen D, Piccart M, Rugo HS, Burris HA 3rd, Pritchard KI, Campone M, Noguchi S, Perez AT, Deleu I, Shtivelband M, Masuda N, Dakhil S, Anderson I, Robinson DM, He W, Garg A, McDonald ER 3rd, Bitter H, Huang A, Taran T, Bachelot T, Lebrun F, Lebwohl D, Baselga J (2016) Correlative analysis of genetic alterations and everolimus benefit in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from BOLERO-2. J Clin Oncol 34(5): 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinsky K, Heguy A, Bhanot UK, Patil S, Moynahan ME (2011) PIK3CA mutations rarely demonstrate genotypic intratumoral heterogeneity and are selected for in breast cancer progression. Breast Cancer Res Treat 129(2): 635–643. [DOI] [PubMed] [Google Scholar]
- Kalinsky K, Jacks LM, Heguy A, Patil S, Drobnjak M, Bhanot UK, Hedvat CV, Traina TA, Solit D, Gerald W, Moynahan ME (2009) PIK3CA mutation associates with improved outcome in breast cancer. Clin Cancer Res 15(16): 5049–5059. [DOI] [PubMed] [Google Scholar]
- Markou A, Farkona S, Schiza C, Efstathiou T, Kounelis S, Malamos N, Georgoulias V, Lianidou E (2014) PIK3CA mutational status in circulating tumor cells can change during disease recurrence or progression in patients with breast cancer. Clin Cancer Res 20(22): 5823–5834. [DOI] [PubMed] [Google Scholar]
- Pang H, Flinn R, Patsialou A, Wyckoff J, Roussos ET, Wu H, Pozzuto M, Goswami S, Condeelis JS, Bresnick AR, Segall JE, Backer JM (2009) Differential enhancement of breast cancer cell motility and metastasis by helical and kinase domain mutations of class IA phosphoinositide 3-kinase. Cancer Res 69(23): 8868–8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ardila DE, Helmijr JC, Look MP, Lurkin I, Ruigrok-Ritstier K, van Laere S, Dirix L, Sweep FC, Span PN, Linn SC, Foekens JA, Sleijfer S, Berns EM, Jansen MP (2013) Hotspot mutations in PIK3CA associate with first-line treatment outcome for aromatase inhibitors but not for tamoxifen. Breast Cancer Res Treat 139(1): 39–49. [DOI] [PubMed] [Google Scholar]
- Rodon J, Dienstmann R, Serra V, Tabernero J (2013) Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol 10(3): 143–153. [DOI] [PubMed] [Google Scholar]
- Sabine VS, Crozier C, Brookes CL, Drake C, Piper T, van de Velde CJ, Hasenburg A, Kieback DG, Markopoulos C, Dirix L, Seynaeve C, Rea DW, Bartlett JM (2014) Mutational analysis of PI3K/AKT signaling pathway in tamoxifen exemestane adjuvant multinational pathology study. J Clin Oncol 32(27): 2951–2958. [DOI] [PubMed] [Google Scholar]
- Schiavon G, Hrebien S, Garcia-Murillas I, Cutts RJ, Pearson A, Tarazona N, Fenwick K, Kozarewa I, Lopez-Knowles E, Ribas R, Nerurkar A, Osin P, Chandarlapaty S, Martin LA, Dowsett M, Smith IE, Turner NC (2015) Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med 7(313): 313ra182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe LM, Yuzugullu H, Zhao JJ (2015) PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer 15(1): 7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Renuse S, Sahasrabuddhe NA, Zahari MS, Chaerkady R, Kim MS, Nirujogi RS, Mohseni M, Kumar P, Raju R, Zhong J, Yang J, Neiswinger J, Jeong JS, Newman R, Powers MA, Somani BL, Gabrielson E, Sukumar S, Stearns V, Qian J, Zhu H, Vogelstein B, Park BH, Pandey A (2014) Activation of diverse signalling pathways by oncogenic PIK3CA mutations. Nat Commun 5: 4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley DA, Noguchi S, Pritchard KI, Burris HA 3rd, Baselga J, Gnant M, Hortobagyi GN, Campone M, Pistilli B, Piccart M, Melichar B, Petrakova K, Arena FP, Erdkamp F, Harb WA, Feng W, Cahana A, Taran T, Lebwohl D, Rugo HS (2013) Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther 30(10): 870–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahari MS, Wu X, Blair BG, Pinto SM, Nirujogi RS, Jelinek CA, Malhotra R, Kim MS, Park BH, Pandey A (2015) Activating mutations in PIK3CA lead to widespread modulation of the tyrosine phosphoproteome. J Proteome Res 14(9): 3882–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Vogt PK (2008) Helical domain and kinase domain mutations in p110alpha of phosphatidylinositol 3-kinase induce gain of function by different mechanisms. Proc Natl Acad Sci USA 105(7): 2652–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.