Abstract
The regulatory role of redox-sensing regulator Rex was investigated in Streptomyces avermitilis. Eleven genes/operons were demonstrated to be directly regulated by Rex; these genes/operons are involved in aerobic metabolism, morphological differentiation, and secondary metabolism. Rex represses transcription of target genes/operons by binding to Rex operator (ROP) sequences in the promoter regions. NADH reduces DNA-binding activity of Rex to target promoters, while NAD+ competitively binds to Rex and modulates its DNA-binding activity. Rex plays an essential regulatory role in aerobic metabolism by controlling expression of the respiratory genes atpIBEFHAGDC, cydA1B1CD, nuoA1-N1, rex-hemAC1DB, hppA, and ndh2. Rex also regulates morphological differentiation by repressing expression of wblE, which encodes a putative WhiB-family transcriptional regulator. A rex-deletion mutant (Drex) showed higher avermectin production than the wild-type strain ATCC31267, and was more tolerant of oxygen limitation conditions in regard to avermectin production.
Streptomyces, a genus of filamentous Gram-positive soil-dwelling bacteria, are obligate aerobes1,2. During growth, they encounter a variety of environmental stresses resulting from the complex nature of soil. Growth in wet soil is particularly challenging because little or no oxygen is present. Streptomyces strains are widely used in industrial production of various antibiotics, and oxygen supply is a key parameter determining product yield during the antibiotic fermentation process3. It is important to understand how Streptomyces strains sense and respond to oxygen limitation.
Rex is a redox-sensing regulator widely distributed in Gram-positive bacteria4,5. NAD (H) plays a central role in redox metabolism. NAD+ is reduced to NADH by accepting electrons during substrate oxidation, and NADH is then reoxidized by the electron transport chain. Changes of oxygen status are reflected by the intracellular ratio of NADH to NAD+. When NADH/NAD+ ratio is low, Rex binds to target genes and represses transcription of genes involved in NAD+ regeneration. In contrast, high NADH/NAD+ ratio inhibits DNA-binding activity of Rex and derepresses transcription of its target genes4,6,7,8. Rex was first identified in Streptomyces coelicolor and shown to regulate expression of cytochrome bd terminal oxidase (cydABCD operon) and heme biosynthesis (rex-hemACD operon)4. In Bacillus subtilis, a facultative aerobe, Rex represses expression of cytochrome bd oxidase (cydABCD), NADH dehydrogenase (yjlC-ndh), NADH-linked fermentative lactate dehydrogenase (lctP-ldh), and a formate-nitrate transporter (ywcJ)6,9,10. In Staphylococcus aureus, Rex directly regulates at least 19 genes. It acts as a central regulator of anaerobic metabolism leading to anaerobic NAD+ regeneration, which includes lactate, formate, and ethanol fermentation (adh1, adhE, lctP, ldh1, pflBA) and nitrate respiration (narG, nirC, nirR)7. The binding sequence of Rex (Rex operator; ROP) is highly conserved in Gram-positive bacteria. Reported consensus sequence in S. coelicolor (5′-TGTGAACNNNTTCACA-3′)4, in B. subtilis (5′-WWTGTGAANTNNTNNNCAAW-3′; W represents either A or T)10, and in S. aureus (5′-TTGTGAAWWWWTTCACAA-3′)7 are very similar to the palindromic sequence in S. coelicolor.
Even though Rex was first characterized in S. coelicolor and its regulatory mechanism has been extensively studied, few target operons/genes of Rex in Streptomyces have been confirmed4, and the overall regulatory function of Rex in this genus remains to be elucidated. S. avermitilis is an important species used for industrial production of avermectins, a group of anthelmintic antibiotics widely used in the medical, veterinary, and agricultural fields11. We investigated the regulatory role of Rex in the expression of operons/genes involved in aerobic metabolism, morphology, and secondary metabolism of S. avermitilis. Our findings have potential application to novel genetic engineering strategies for high antibiotic-producing strains and hypoxia-tolerating strains of this genus.
Results
Expression of atpIBEFHAGDC, cydA1B1CD, nuoA1-N1, and rex-hemAC1DB is negatively regulated by Rex
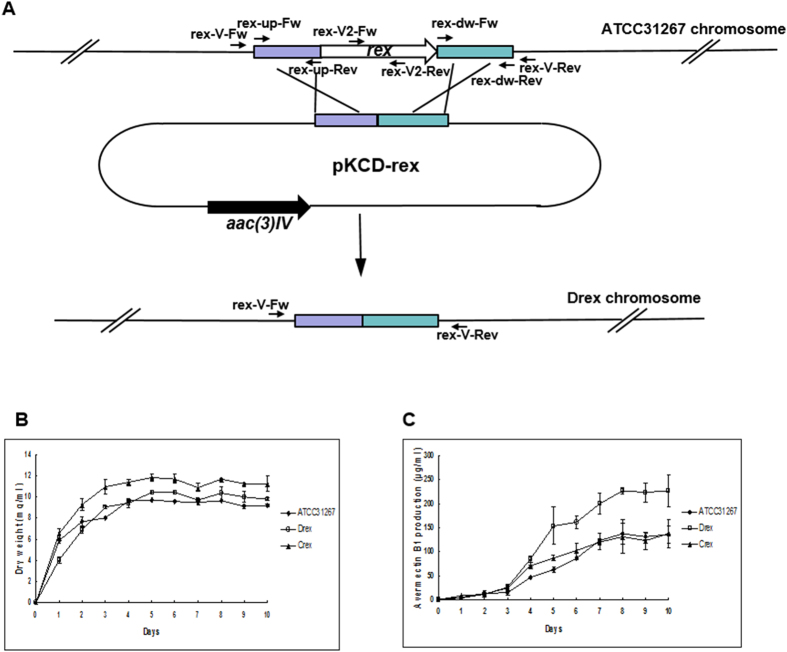
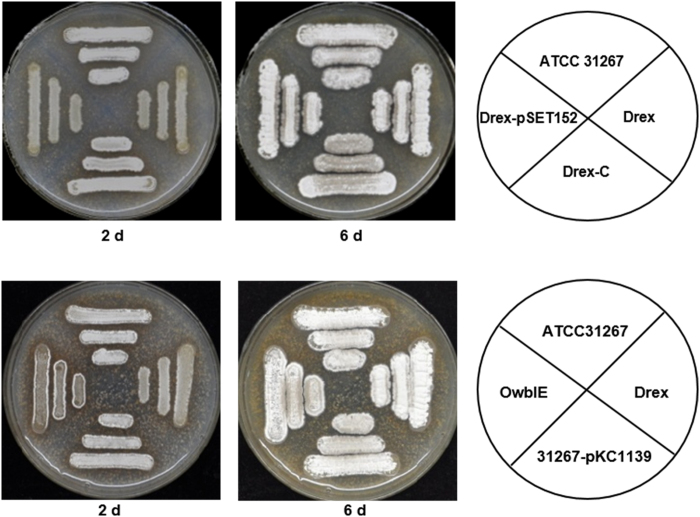
The rex gene is conserved within the genus Streptomyces and is cotranscribed with the heme synthesis genes hemACD4. To evaluate the regulatory role of Rex in S. avermitilis, we constructed a rex-deletion mutant (termed Drex) by homologous recombination in wild-type strain ATCC31267. rex deletion had no effect on growth in liquid fermentation medium (Fig. 1).
Figure 1. Construction and growth curve of rex-deletion mutant.
(A) Method (schematic) used for rex deletion. Long arrows: genes and their directions. Short black arrows: positions of primers used for cloning of exchange regions and confirmation of gene deletion (see M&M). Rectangles: exchange regions used for rex deletion. (B,C) Growth (B) and avermectin production (C) of rex-related mutant strains in liquid fermentation medium II. Values shown are mean ± SD from three replicate flasks.
The promoter regions of the operons atpIBEFHAGDC, cydA1B1CD, nuoA1-N1, and rex-hemAC1DB in S. avermitilis all contain a putative Rex-binding motif, 5′-TTGTGAANNNNTTCACAA-3′ (Table 1). nuoA1-N1 (SAV4837-4850) encodes putative NADH dehydrogenase I (complex I), cydA1B1CD (SAV4260-4258) encodes putative cytochrome bd-I oxidase (cytochrome bd complex), atpIBEFHAGDC (SAV2888-2880) encodes putative F-type proton-transporting ATPase, and hemAC1DB (SAV4739-4742) is cotranscribed with rex and encodes heme synthesis enzymes (Fig. S1). NADH dehydrogenase I, cytochrome bd-I oxidase, and F-type proton-transporting ATPase are essential components of the respiratory chain. Heme is most abundant in cytochromes, which are electron transfer proteins involved in the final reduction of oxygen during aerobic respiration. We performed qRT-PCR to determine whether expression of these genes involved in aerobic respiration is regulated by Rex.
Table 1. Putative Rex target genes.
| Gene | Function | Nucleotide positiona | PMW Scoreb | Sequence | ATG Distancec | |
|---|---|---|---|---|---|---|
| Start | End | |||||
| cydA1B1CD (SAV4260-4258) | putative cytochrome bd-I oxidase (cytochrome bd complex) | 5226395 | 5226412 | 1.00 | ATGTGAACGCGTTCACAA | 81 |
| rex-hemAC1DB (SAV4738-4742) | redox-sensing transcriptional repressor; heme biosynthetic enzymes | 5776959 | 5776976 | 1.00 | TTGTGCACGCGTTCACAA | 77 |
| atpIBEFHAGDC (SAV2888-2880) | putative F-type proton-transporting ATPase | 3533332 | 3533349 | 2.00 | TTGTGATACGGTTCACGA | 137 |
| wblE (SAV3016) | putative WhiB-family transcriptional regulator | 3771378 | 3771395 | 2.00 | ATGTGAACGCTTTCACGA | 43 |
| hppA (SAV4616) | putative inorganic H+ pyrophosphatase | 5632612 | 5632629 | 2.00 | TCGTGAATCAATTCACGA | 195 |
| nuoA1-N1 (SAV4837–4850) | putative NADH dehydrogenase I (complex I) | 5880239 | 5880256 | 2.00 | ATGTGAAGCAGGTCACAA | 147 |
| pbp3-4 (SAV3603-SAV3604) | putative penicillin-binding protein | 4460250 | 4460267 | 2.00 | TTCTGAACGTGTTCAGAA | 37 |
| SAV828 | putative rhamnosidase | 982811 | 982828 | 3.00 | CTGTGAATCGATTCACCT | 137 |
| echA7 (SAV2316) | putative enoyl-CoA hydratase | 2820643 | 2820660 | 3.00 | TCGTGACGACAGTCACAA | 66 |
| SAV2652 | putative regulatory protein | 3252402 | 3252419 | 3.00 | TTGTGCACCGCTTCACCC | 288 |
| ndh2 (SAV3529) | putative NADH dehydrogenase (complex I) | 4369478 | 4369495 | 3.00 | TTGTGAAGGGGCGCACGA | 119 |
| ectABCD (SAV6398-6395) | putative L-2,4-diaminobutyrate acetyltransferase, ectoine biosynthesis | 7673586 | 7673603 | 3.00 | TTGTGATCGACTCCACAT | 155 |
| SAV6368 | putative multiple sugar ABC transporter permease protein | 7638483 | 7638500 | 3.00 | TGGTGAAGCGCTTCGCGT | 81 |
| avaB- avaL2 (SAV2267-2268) | putative gamma-butyrolactone- dependent transcriptional regulator | 2766273 | 2766290 | 3.00 | TCGTGAACGAATTCTAAT | 29 |
| SAV3213 | putative nitroreductase family protein, NADH dehydrogenase/NAD(P)H nitroreductase | 4004553 | 4004570 | 3.00 | TGGTGATCGGCTTCACAG | 96 |
| SAV1351 | putative fatty acid-CoA racemase | 2820643 | 2820660 | 3.00 | TCGTGACGACAGTCACAA | 66 |
| tmk (SAV4622) | putative thymidylate kinase | 5643744 | 5643761 | 3.00 | GTGTGGAGGCGTCCACAA | 73 |
| folD2 (SAV543) | putative methylenetetrahydrofolate | 688370 | 688387 | 3.00 | TTGTGTGTGAGTTCAGAA | 231 |
| parA (SAV6508) | putative partitioning or sporulation protein | 7797460 | 7797477 | 3.00 | ATGTCGACTCATTCACAA | 114 |
| SAV7415-7416 | putative sugar isomerase putative simple sugar ABC transporter | 8846427 | 8846444 | 3.00 | TCGTGAAAGGTTTCAACT | 203 |
| SAV897 | putative alpha-amylase inhibitor | 1075479 | 1075496 | 3.00 | TTGCGAAAGTTGTCGCAA | 73 |
aGenomic position.
bNumber of mismatches with respect to the consensus. PWM, positive weight matrix.
cValues are distances (in nucleotides) to the predicted start codon of the downstream gene.
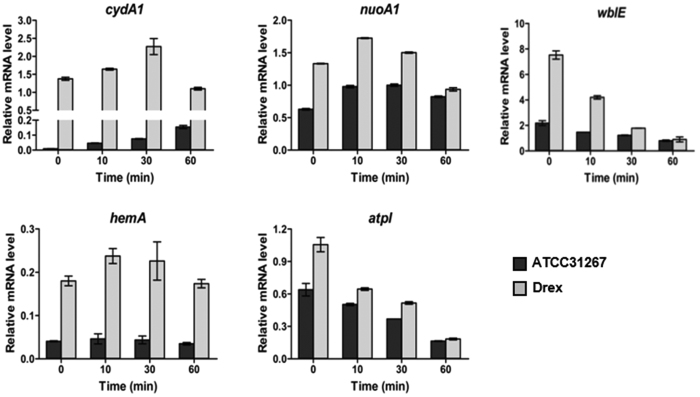
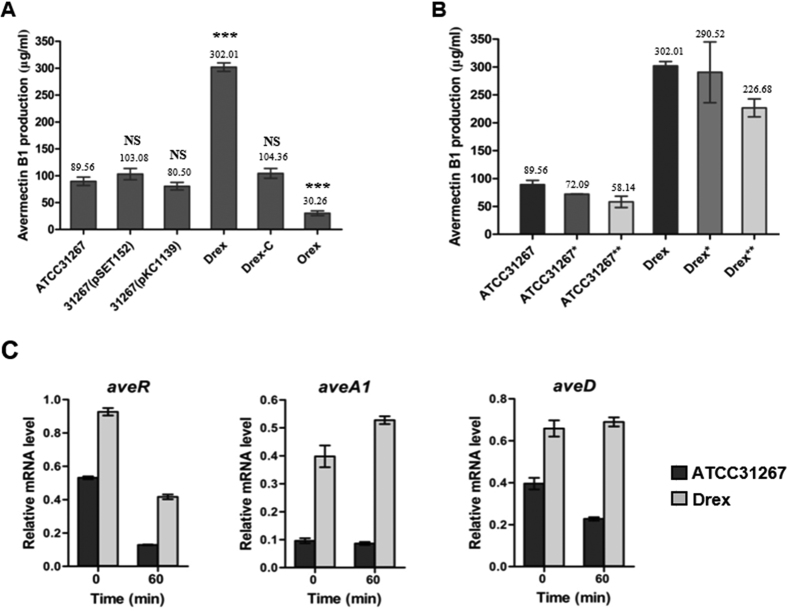
Expression of these genes differed greatly when cells were static-cultured following 3 days’ culture on a rotary shaker (250 rpm). Transcription level of cydA1 under oxygen limitation condition in ATCC31267 increased steadily during 60 min, whereas the level in Drex increased to a maximal value during the first 30 min, then gradually declined during the subsequent 30 min (Fig. 2). These findings suggest that induction of cydA1 under oxygen limitation condition is mediated by Rex. Expression of nuoA1 and hemA under oxygen limitation increased slightly in the first 10 min, then declined during the subsequent 50 min, in both ATCC31267 and Drex. In contrast, expression of atpI under oxygen limitation declined steadily during 60 min in both ATCC31267 and Drex (Fig. 2). Transcription levels of cydA1, nuoA1, hemA, and atpI were consistently higher for Drex than for ATCC31267 under equivalent treatments, confirming that these genes are negatively regulated by Rex.
Figure 2. RT-qPCR analysis of transcription levels of cydA1, nuoA1, hemA, atpI, and wblE in ATCC31267 and Drex.
RNA was prepared from cells grown in fermentation medium for 3 days on a rotary shaker (250 rpm) and then static-cultured for the indicated time. Quantitative data were normalized to hrdB (SAV2444) expression value. Values shown are mean ± SD from three replicates.
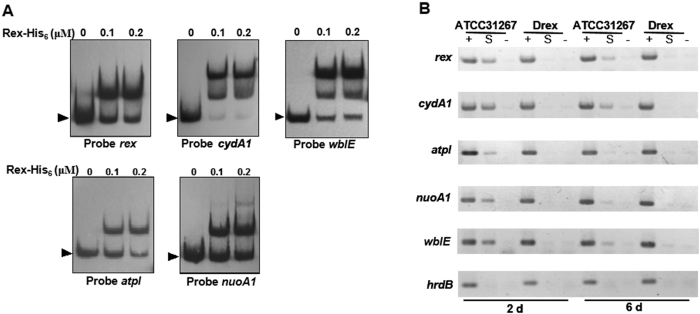
C-terminal His6-tagged Rex fusion protein was overexpressed in E. coli and purified for DNA binding analysis. EMSAs were performed to evaluate interactions between Rex and the promoters in vitro. Rex-His6 bound to the promoter regions of atpIBEFHAGDC, cydA1B1CD, nuoA1-N1, and rex-hemAC1DB operons (Fig. 3A). ChIP assays were performed to assess interactions in vivo. ATCC31267 and Drex cells were treated with formaldehyde at days 2 and 6 to cross-link Rex to its DNA targets. Cross-linked DNA was extracted, fragmented by sonication, and immunoprecipitated by anti-Rex antibodies for screening of Rex-bound DNA fragments. In comparison to control hrdB promoter, PCR products of rex, cydA1, atpI, and nuoA1 promoter regions were selectively enriched from immunoprecipitated DNA of ATCC31267, whereas no such PCR bands were amplified from immunoprecipitated DNA of Drex (Fig. 3B). Results of EMSAs and ChIP assays revealed that Rex binds specifically to the promoter regions of atpIBEFHAGDC, cydA1B1CD, nuoA1-N1, and rex-hemAC1DB operons.
Figure 3. Binding of Rex-His6 to promoter regions of rex, cydA1, atpI, nuoA1, and wblE.
(A) EMSAs using Rex-His6 protein at the indicated concentrations, and the probes indicated below the panels. Arrow: free probe. (B) ChIP assay analysis of Rex binding to promoter regions of rex, cydA1, atpI, nuoA1, and wblE in vivo. Rex-DNA complexes were immunoprecipitated by anti-Rex antibodies from formaldehyde-treated ATCC31267 and Drex cells. DNAs used for PCR were: total DNA prior to immunoprecipitation (positive control: lanes “+”), immunoprecipitated DNA (experimental sample: lanes “S”), and DNA without antibody (negative control: lanes “−”). hrdB promoter region was used as control.
Determination of Rex operator (ROP) sequences on promoter regions of atpIBEFHAGDC, cydA1B1CD, nuoA1-N1, and rex-hemAC1DB
Rex binding sequences in 5′-end fluorescein-labeled promoter regions of the above operons were determined by DNase I footprinting analysis. One protected region was detected in the rex promoter region in the presence of 1.2 or 2.4 μΜ Rex-His6. The region extends for 23 nucleotides from positions −39 to −17 relative to the transcriptional start site (TSS) of rex. A consecutive ROP site (5′-TTGTGCACGCGTTCACAA-3′) was found in the protected region; the site is located between −35 region and −10 region and encompasses −35 region (Fig. S2A). A 28-nt protected region (positions −3 to + 25 relative to TSS) was detected in the cydA1 promoter region. One ROP site (5′-ATGTGAACGCGTTCACAA-3′) was found in the protected region downstream from TSS. A half-site ROP (5′-TTGTGAA-3′) was also found in the protected region; it is located upstream from the ROP site and encompasses TSS (Fig. S2B). EMSA revealed two retarded bands between Rex and the cydA1 promoter region (Fig. 3A), suggesting that Rex can interact with the half-site ROP. A 29-nt protected region (positions −50 to −22 relative to TSS) containing a ROP site (5′-TTGTGATACGGTTCACGA-3′) was detected in the atpI promoter region (Fig. S2C). Rex-His6 protected a 27-nt region extending from positions −42 to −16 relative to TSS of nuoA1, which contains a ROP site (5′-TTGTGACCTGCTTCACAT-3′) (Fig. S2D). ROP in the nuoA1 and atpI promoter regions is located between −35 region and −10 region, and encompasses −35 region. These findings suggest that Rex blocks attachment of RNA polymerase to the promoters or inhibits the progress of RNA polymerase by binding to ROP in or downstream of the promoters of atpIBEFHAGDC, cydA1B1CD, nuoA1-N1, and rex-hemAC1DB, and blocks transcription of these operons.
DNA-binding activity of Rex is modulated by NADH/NAD+ ratio
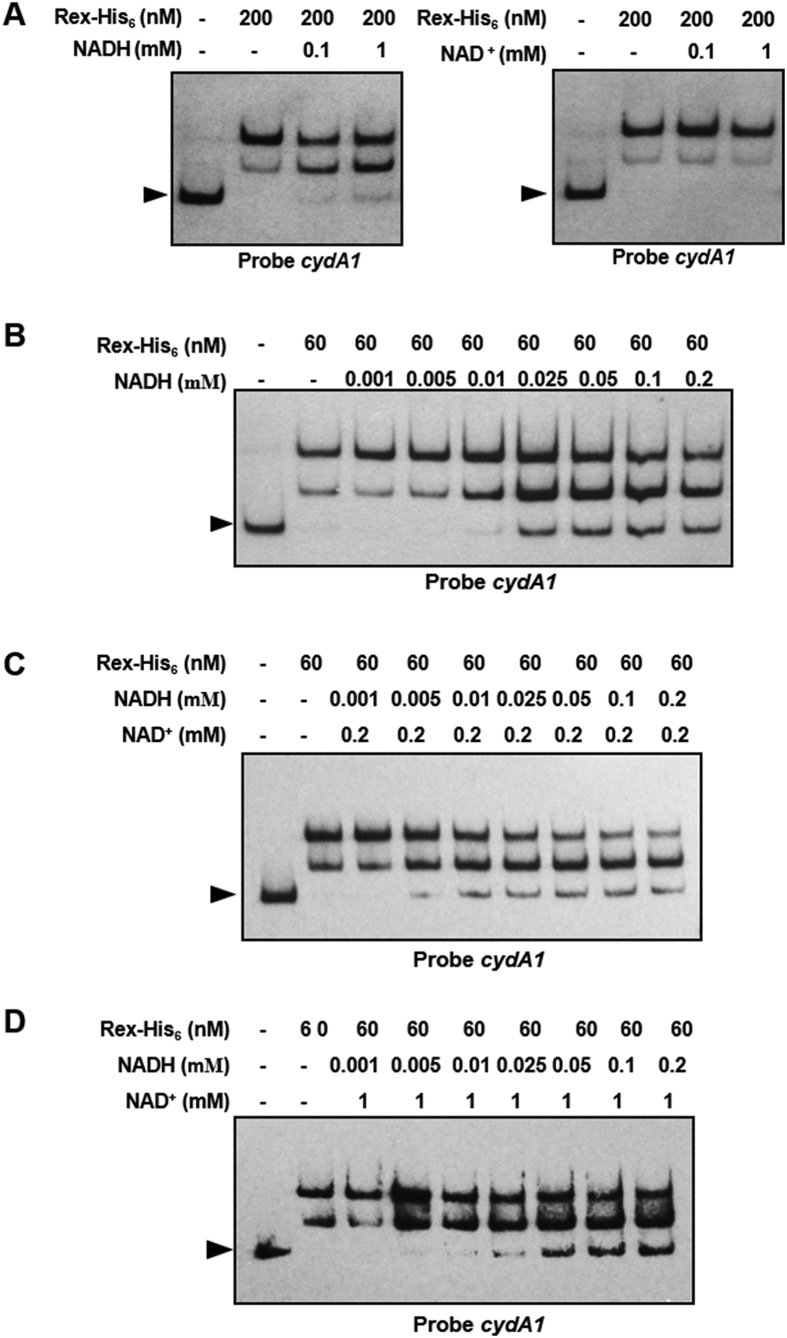
In S. coelicolor, NADH at concentrations <5 μM inhibits DNA-binding activity of Rex, whereas 1 mM NAD+ has no inhibitory effect. NAD+ competes with NADH for Rex binding4. In B. subtilis and S. aureus, NAD+ enhances binding of Rex to putative Rex-binding sites, while NADH competes with NAD+ for Rex binding and reduces Rex activity6,7. We examined the effects of NAD+ and NADH on DNA-binding activity of Rex to upstream regions of cydA1 in S. avermitilis. DNA-binding activity of Rex was reduced by addition of NADH, but not by NAD+ concentrations up to 1 mM (Fig. 4A,B; Fig. S3). NADH and NAD+ were added to EMSA binding buffer to assess the effect of NAD+/NADH ratio on DNA-binding activity of Rex in vitro. At NAD+ concentration 0.2 mM, 5 μM NADH was sufficient to dissociate the Rex-DNA complex (Fig. 4C). At NAD+ concentration 1 mM, dissociation of DNA-Rex complex required 25 μM NADH, suggesting that Rex-binding activity was recovered by addition of increasing amounts of NAD+ (Fig. 4D). These findings indicate that NAD+ and NADH bind competitively to Rex and modulate its DNA-binding activity. These findings also imply that Rex exploits the similar regulatory mechanism in Streptomyces.
Figure 4. DNA-binding activity of Rex is modulated by NADH/NAD+ ratio.
(A) EMSAs of cydA1 promoter region using Rex-His6 and 0.1 or 1 mM pyridine nucleotides. (B) EMSAs of cydA1 promoter region using Rex-His6 with various NADH concentrations. (C,D) Assay mixtures contained NADH at indicated concentration and 0.2 mM (C) or 1 mM (D) NAD+. Arrow: free probe.
Rex regulates morphological differentiation
In comparison to ATCC31267, Drex showed delayed morphogenesis on SFM agar at day 2, when aerial mycelium was initiated. Spore formation at day 6 did not differ notably between the two strains. Morphogenesis of the Drex complementation strain was similar to that of ATCC31267 (Fig. 5), indicating that the delayed morphogenesis was due solely to rex deletion.
Figure 5. Phenotypes of rex- and wblE-related mutant strains.
Growth of indicated strains on SFM agar for 2 and 6 days. WT, wild-type ATCC31267; Drex, rex-deletion mutant; Drex-C, complementation strain of Drex; Drex-pSET152 and 31267-pKC1139, empty plasmid-containing controls; OwblE, wblE overexpressing strain.
The promoter region of wblE in S. avermitilis contains a putative Rex-binding motif (Table 1). wblE encodes a putative WhiB-family transcriptional regulator, which may be involved in morphological differentiation12,13. qRT-PCR analysis revealed notable increases of wblE transcription level in Drex. Levels under oxygen limitation condition declined gradually during 60 min for both ATCC31267 and Drex, and were consistently higher for Drex than for ATCC31267 (Fig. 2). EMSAs showed that Rex-His6 bound to the wblE promoter region in vitro (Fig. 3A). In in vivo ChIP assays, PCR product of the wblE promoter region was selectively enriched from immunoprecipitated DNA of ATCC31267, whereas no such PCR band was amplified from immunoprecipitated DNA of Drex (Fig. 3B). These findings indicate that wblE is negatively regulated by Rex. Rex binding sequence in the wblE promoter region was determined by DNase I footprinting analysis. A 28-nt region protected by Rex-His6 was detected, extending from positions + 108 to + 135 relative to TSS of wblE (Fig. S4). The protected region contains a consecutive ROP site (5′-TCGTGAAAGCGTTCACAT-3′) and a half-site ROP (5′-TTCACAA-3′) located downstream of TSS. Rex may inhibit the progress of RNA polymerase by binding to ROP downstream of the wblE promoter, and thereby repress transcription.
To test the possibility that overexpression of wblE in Drex results in delayed morphogenesis, we attempted to delete wblE in S. avermitilis. However, this attempt was unsuccessful. wblE is evidently an essential gene in Streptomyces; an attempt to delete it in S. coelicolor was also unsuccessful13. When wblE was overexpressed in ATCC31267, the resulting strain had a phenotype similar to that of Drex (Fig. 5), suggesting that Rex regulates morphological differentiation through its effect on wblE expression.
Rex negatively regulates avermectin production
The overexpression of rex caused a decrease in avermectin production to 33% of ATCC31267 level. Drex had avermectin production ~3-fold higher than that of ATCC31267. The mycelial dry weight of Drex was similar to that of ATCC31267, indicating that the improved avermectin yield was not achieved by improved growth. In the Drex complementation strain, avermectin production was similar to that of ATCC31267 (Fig. 1; Fig. 6A). These findings indicate that rex negatively regulates avermectin production in S. avermitilis. We also measured avermectin production under oxygen limitation condition. In ATCC31267, lower agitation speed (230 rpm; control speed was 250 rpm) resulted in a 20% reduction of avermectin production, and static culture for 2 h on day 5 resulted in a 35% reduction. In Drex, avermectin production was reduced by 3.8% and 25%, respectively, under the above two conditions (Fig. 6B). Thus, rex deletion resulted in increased tolerance of S. avermitilis to oxygen limitation in regard to avermectin production.
Figure 6. Effect of rex deletion on avermectin production in S. avermitilis.
(A) Avermectin production in rex-related mutant strains. ATCC31267, wild-type; Drex, rex-deletion mutant; 31267 (pSET152) and 31267 (pKC1139), empty plasmid-containing controls; Drex-C, rex-deletion complementation strain 31267 (pSET-rex); Orex, rex overexpressing strain 31267 (pKC-rex). (B) Avermectin production in ATCC31267 and Drex with various oxygen limitation conditions. *Agitation speed 230 rpm (control, 250 rpm); **Static culture for 2 h at day 5 during fermentation (250 rpm). (C) RT-qPCR analysis of aveR, aveA1, and aveD transcription levels in ATCC31267 and Drex. RNA samples were the same ones used for experiments shown in Fig. 1. Quantitative data were normalized to hrdB expression value. Values shown are mean ± SD from three replicates. Statistical significance of differences was determined using Student’s t-test. ***P < 0.001; NS, not significant.
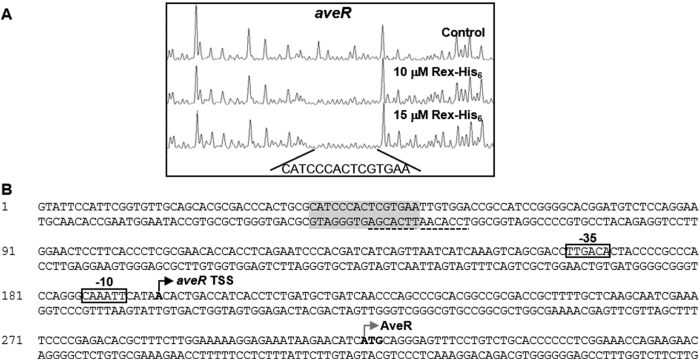
qRT-PCR analysis was performed to determine whether Rex regulates avermectin production at the transcriptional level. Drex showed significantly increased transcription levels of pathway-specific regulatory gene aveR and biosynthetic genes aveA1 and aveD, relative to ATCC31267. Oxygen limitation for 60 min reduced expression of these genes in ATCC31267; however, Drex showed lower fold repression, and a slight induction of aveA1 and aveD (Fig. 6C). EMSAs revealed that Rex-His6 did not bind to the aveR promoter region or the aveD-A1 intergenic region (Fig. S5). Although no retarded band was observed when aveR promoter region was probed with Rex-His6 protein, DNase I footprinting analysis showed one protected region extending for 15 nucleotides on the aveR coding strand in the presence of 10 or 15 μΜ Rex-His6 (Fig. 7). No consecutive ROP site was observed in the protected region; however, two adjacent half-site ROP (5′-TCGTGAA-3′ and 5′- TTGTGGA-3′) were found in the protected region and downstream region. Rex can evidently interact with the half-site ROP; however, because the interaction is weak and easily dissociated in vitro, EMSA did not reveal a clear shifted band.
Figure 7. Determination of Rex binding site on aveR promoter region by DNase I footprinting assay.
(A) Fluorograms correspond to control DNA fragment and to protected reactions (with 10 and 15 µM Rex-His6). (B) Nucleotide sequences of aveR promoter region. Shaded boxes: sequences protected from DNase I digestion. Dotted boxes: ROP. Dotted line: half-site ROP. Black bent arrows with boldface letters: TSSs. Boxes: presumed −35 and −10 elements of promoters. Gray bent arrows with boldface letters: translational start codons.
Confirmation of putative Rex target genes
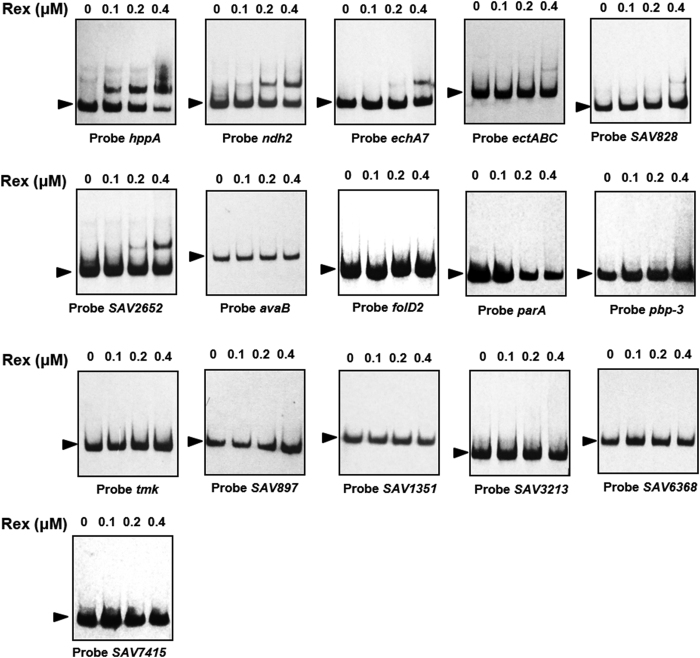
A genome-wide search of consensus motif 5′-TTGTGAANNNNTTCACAA-3′ using the genome sequence of ATCC31267 revealed the presence of 36 motifs up to 350 bp upstream of predicted genes: 2 motifs with one mismatch, 10 motifs with two mismatches, and 24 motifs with three mismatches. Our previous experiments showed that wblE, cydA1B1CD, rex-hemAC1DB, atpIBEFHAGDC, and nuoA1-N1 are directly controlled by Rex. To investigate whether Rex binds to promoter regions of other putative target genes, we selected 16 genes with predicted gene function for EMSAs (Table 1). Of these, Rex bound to the probes of hppA (encodes an inorganic H+ pyrophosphatase), ndh2 (encodes a NADH dehydrogenase [complex I]), echA7 (encodes an enoyl-CoA hydratase), ectABC (encodes ectoine biosynthesis enzymes), SAV828 (encodes a rhamnosidase), and SAV2652 (encodes a regulatory protein). Probes whose binding motif had one or two mismatches showed higher affinity than probes whose binding motif had three mismatches (Fig. 3A, Fig. 8). These findings demonstrated that 5′-TTGTGAANNNNTTCACAA-3′ is the consensus motif of Rex in S. avermitilis.
Figure 8. Binding of Rex-His6 to promoter regions of putative Rex targets.
Rex (μM): Rex-His6 concentrations. Arrow: free probe.
Discussion
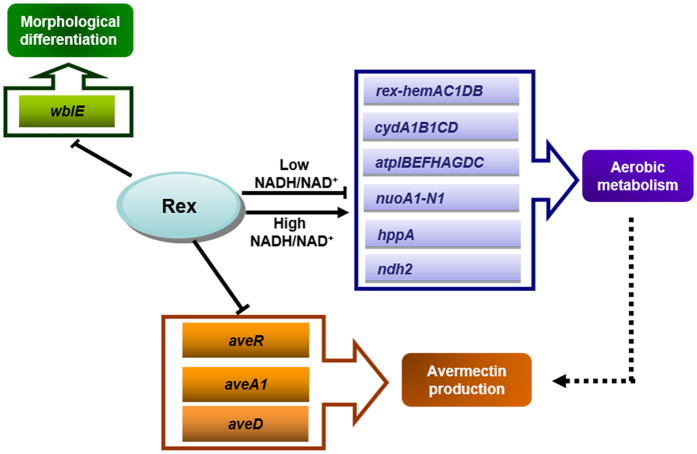
Results of this study show that Rex in S. avermitilis acts as a repressor of aerobic metabolism, morphological differentiation, and secondary metabolism (summarized schematically in Fig. 9). Results of EMSAs demonstrated that at least 11 genes/operons are directly regulated by Rex. Among these, atpIBEFHAGDC, cydA1B1CD, nuoA1-N1, and rex-hemAC1DB operons encode key components of the electron transfer chain and play crucial roles in aerobic metabolism14,15,16,17. hppA encodes a putative pyrophosphate-energized proton pump that converts energy from pyrophosphate hydrolysis into active H+ transport across the plasma membrane18. ndh2 encodes a NADH dehydrogenase involved in NAD+ regeneration19,20. echA7 encodes an enoyl-CoA hydratase that catalyzes the second step of the β-oxidation pathway of fatty acid metabolism21. SAV828 encodes a rhamnosidase that hydrolyzes L-rhamnose from L-rhamnoside22. Under oxygen limitation condition, the increase of intracellular NADH/NAD+ ratio in S. avermitilis dissociates binding of Rex from its target binding sites and derepresses its target genes/operons, and upregulation of cydA1B1CD, nuoA1-N1, rex-hemAC1DB, ndh2, and hppA increases oxygen utilization, NAD+ regeneration, and ATP synthesis (Fig. 9). On the other hand, expression of atpIBEFHAGDC in ATCC31267 and Drex is downregulated by oxygen limitation, suggesting that this operon is also directly controlled by regulators other than Rex. The F0F1-ATPase operon in Corynebacterium glutamicum is regulated by ECF σH 23. A sigH homolog is present in Streptomyces; whether it regulates atpIBEFHAGDC expression remains to be tested.
Figure 9. Regulatory network (schematic) whereby Rex controls major genes involved in aerobic metabolism, morphological differentiation, and secondary metabolism.
Solid arrows: direct induction. Solid lines with blunt end: repression. Dashed arrows: indirect induction.
WhiB-like family transcription factors are widely present in actinomycetes, but not found in other bacterial orders. WhiB was first identified as a small transcription factor-like protein essential for sporulation in S. coelicolor24. Genome sequencing revealed that Streptomyces species have multiple whiB-like genes (designated “wbl”). Eleven wbl genes (including whiB and whiD) have been identified in S. coelicolor13. Among these, wblA, whiB, and whiD are essential for sporulation, and WblA also negatively regulates antibiotic biosynthesis in Streptomyces13,25,26,27. Other wbl genes are not involved in morphological development, with the exception of wblE. Fowler-Goldsworthy et al.13 reported that wblE could not be deleted in various strains of S. coelicolor, and we made a similar observation in S. avermitilis. Thus, wblE appears to be essential in this genus. The homolog of wblE in Mycobacterium tuberculosis is whiB1, which encodes an essential transcription factor in response to nitric oxide exposure28. We demonstrated that wblE is directly negatively regulated by Rex, and that wblE overexpression results in delayed morphogenesis similar to that of Drex. Expression of wblE, like that of atpIBEFHAGDC, is downregulated by oxygen limitation in both ATCC31267 and Drex, suggesting that (i) wblE is jointly regulated by Rex and some other regulator, or (ii) wblE itself responds to low oxygen concentration via its own redox-sensitive [4Fe-4S] cluster. Under oxygen limitation condition, wblE expression in Streptomyces is downregulated, with consequent stimulation of sporulation and production of a large number of spores to maintain viability under conditions of little or no oxygen. Another Rex target, ectABC, encodes enzymes for biosynthesis of ectoine (a compatible solute) that serves as an osmolyte and promotes survival under osmotic or temperature stress29. By regulating ectABC transcription, Rex facilitates ectoine biosynthesis to enhance viability under these types of stress.
In Drex, expression of regulatory gene aveR and biosynthetic genes, and avermectin production, were notably increased. Although EMSA showed no clearly retarded band between aveR promoter region probe and Rex-His6, DNase I footprinting analysis revealed one 15-nt protected region consisting of two adjacent half-site ROP on the coding strand of aveR by Rex-His6. Thus, Rex may directly regulate aveR expression by interacting with the half-site ROP in the aveR promoter region. Expression of electron transfer chain components was enhanced in Drex, thus promoting aerobic respiration rate, ATP production, and secondary metabolism. The notable increase of atpIBEFHAGDC, cydA1B1CD, nuoA1-N1, and rex-hemAC1DB expression in Drex enhanced the tolerance of cells to oxygen limitation. The findings described here provide a basis for construction of new Streptomyces strains with high antibiotic production and hypoxia tolerance.
Materials and Methods
Bacterial strains and growth conditions
The S. avermitilis strains used were ATCC31267 (wild-type), Drex (rex-deletion strain), Drex-C (rex-deletion complementary strain harboring plasmid pSET-rex), and Orex (ATCC31267 harboring rex overexpressing plasmid pKC-rex). E. coli strains JM109 and BL21 (DE3) were used for routine cloning and protein expression, respectively. YMS medium and SFM medium were used for sporulation and phenotype studies30,31. Culture conditions for mycelial growth, protoplast preparation, and regeneration of S. avermitilis were as described previously30. Seed medium and fermentation medium FM-I were used for avermectin production and for RNA isolation, and soluble fermentation medium FM-II was used for ChIP analysis32.
Gene deletion, complementation, and overexpression
A rex (SAV4738) gene deletion mutant was generated through targeted gene deletion mediated by homologous recombination. A 566-bp fragment upstream of rex (position −460 to + 87 from start codon) was amplified by primers rex-up-Fw and rex-up-Rev, and a 579-bp fragment downstream of rex (position +539 to +1098) was amplified by primers rex-dw-Fw and rex-dw-Rev, using ATCC31267 genomic DNA as template (Table S1; Fig. 1). The two fragments, after recovery, were digested respectively by BamHI/HindIII and BamHI/EcoRI, and ligated together into EcoRI/HindIII-digested pKC113933 to produce rex-deletion vector pKCD-rex. pKCD-rex was introduced into ATCC31267 protoplasts. Double-crossover recombinant strains were selected as described previously34,35. The rex-deletion mutant (termed Drex) was confirmed by PCR using one pair of external primers (rex-V-Fw/rex-V-Rev) and one pair of internal primers (rex-V2-Fw/rex-V2-Rev) (Table S1; Fig. 1). Use of the external primers yielded a 1.3-kb band from Drex and a 1.8-kb band from ATCC31267. Use of the internal primers yielded a 225-bp band from ATCC31267 and no band from Drex (data not shown).
A 1038-bp DNA fragment carrying the rex ORF and its putative promoter was amplified by PCR using primers rex-E-Fw and rex-E-Rev (Suppl. Table 1), and then ligated into EcoRI/XbaI-digested pSET152 or pKC1139 to produce vector pSET-rex or pKC-rex. For complementation analysis of Drex, pSET-rex was transformed into Drex protoplasts. For overexpression of Rex, pKC-rex was introduced into ATCC31267 protoplasts.
RNA extraction and qRT-PCR analysis
RNA was isolated using Trizol reagent (Tiangen; China) from S. avermitilis mycelia grown in FM-I as described previously32. Transcription levels of various genes were determined by qRT-PCR using the primer pairs listed in Table S1. An RNA sample without prior reverse transcription was used as negative control to rule out chromosomal DNA contamination. hrdB gene (SAV2444) was used as internal control.
Chromatin Immunoprecipitation (ChIP) assay
ChIP assay was performed as described previously36. In brief, S. avermitilis cultures grown in FM-II for 2 or 6 days were fixed in cross-linking buffer (0.4 M sucrose, 10 mM Tris·Cl [pH 8.0], 1 mM EDTA) containing 1% formaldehyde for 20 min at 28 °C. ChIP was performed using anti-Rex antibody. After DNA extraction, pellets were washed with 70% ethanol and resuspended in 50 μl Tris·EDTA buffer. 1 μl DNA solution was subjected to PCR using the primer sets listed in Table S1.
Overexpression and purification of Rex-His6
The rex coding region was amplified by PCR using primers His-rex-Fw and His-rex-Rev. The purified fragment was cut with NcoI/HindIII and cloned into NcoI/HindIII-digested pET28a (+) to generate expression plasmid pET-rex. pET-rex was introduced into E. coli BL21 (DE3) for overexpression of C-terminal His6-tagged Rex. Rex-His6 was induced by 0.2 mM IPTG at 37 °C and purified from whole-cell lysate by Ni-NTA agarose chromatography (Bio-works; Sweden) according to the manufacturer’s instructions.
Electrophoretic mobility gel shift assays (EMSAs)
EMSAs were performed according to the manufacturer’s instructions (DIG Gel Shift Kit, 2nd Generation, Roche) as described previously35. DNA probes were obtained by PCR using the primers listed in Table S1, and labeled with Digoxigenin-11-ddUTP at the 3′ end using recombinant terminal transferase. DIG-labeled DNA probe was incubated with various quantities of Rex-His6 for 30 min at 25 °C in a total volume of 20 μl containing 1 μg poly[d(I-C)]. Electrophoresis (5.0% native polyacrylamide gel; 0.5 × TBE as running buffer) was performed to separate protein-bound probes from free probes. DNA was electroblotted onto a positively charged nylon membrane, and retarded and unbound bands were detected by chemiluminescence and recorded on X-ray film.
DNase I footprinting assays
A fluorescent labeling procedure was used for DNase I footprinting assays37. DNA fragments were obtained by PCR using FAM-labeled primers (Table S1), and purified from agarose gel. Labeled DNA fragments (400 ng) and various quantities of Rex-His6 were incubated in a 25-μl volume for 30 min at 25 °C. DNase I digestion was performed for 40 sec at 37 °C, and terminated by addition of 10 μl 0.2 M EDTA (pH 8.0). Samples were subjected to phenol/chloroform extraction, ethanol precipitation, and capillary electrophoresis. Electrophoregrams were analyzed using GeneMarker software v2.2.0.
Fermentation and HPLC analysis of avermectin production
Fermentation of S. avermitilis strains and estimation of avermectins yields by HPLC analysis were performed as described previously32.
Determination of transcriptional start sites
Transcriptional start sites (TSS) of rex and wblE were mapped by 5′-RACE using a 5′/3′ RACE Kit (2nd Generation, Roche). Total RNA was extracted from ATCC31267 grown in FM-I for 2 days. A gene-specific primer (sp1) was used to synthesize cDNA, and template RNA was degraded with RNase H. A homopolymeric A-tail was purified and added to the 3′-end of cDNA using terminal transferase. Tailed cDNA was PCR amplified through 35 cycles with a specific nested primer (sp2) and an oligo (dT)-anchor primer (Table S1). PCR products were electrophoresed, purified using a DNA agarose gel recovery kit (BioTek; China), and sequenced.
Prediction of Rex putative targets
To search for putative Rex target genes, Rex consensus motif 5′-TTGTGAANNNNTTCACAA-3′ was used to scan the intergenic regions of the S. avermitilis genome using Virtual Footprint software38.
Additional Information
How to cite this article: Liu, X. et al. Redox-sensing regulator Rex regulates aerobic metabolism, morphological differentiation, and avermectin production in Streptomyces avermitilis. Sci. Rep. 7, 44567; doi: 10.1038/srep44567 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study was supported by a grant (No. 31470190) from the National Natural Science Foundation of China. The authors are grateful to Dr. S. Anderson for English editing of the manuscript.
Footnotes
The authors declare no competing financial interests.
Author Contributions Designed the experiments: Z.C. Performed the experiments: X.L., Y.C., and M.L. Analyzed the data: X.L., Y.C., and M.L. Contributed reagents/materials/analysis tools: Y.W., Y.S., and J.L. Wrote the paper: Z.C., X.L., and Y.C.
References
- van Keulen G., Alderson J., White J. & Sawers R. G. The obligate aerobic actinomycete Streptomyces coelicolor A3(2) survives extended periods of anaerobic stress. Environmental Microbiology 9, 3143–3149, doi: 10.1111/j.1462-2920.2007.01433.x (2007). [DOI] [PubMed] [Google Scholar]
- Fischer M., Falke D. & Sawers R. G. A respiratory nitrate reductase active exclusively in resting spores of the obligate aerobe Streptomyces coelicolor A3(2). Molecular Microbiology 89, 1259–1273, doi: 10.1111/mmi.12344 (2013). [DOI] [PubMed] [Google Scholar]
- Dey E. S., Norrlow O. & Liu Y. Artificial carrier for oxygen supply in biological systems. Applied Microbiology and Biotechnology 64, 187–191, doi: 10.1007/s00253-003-1454-9 (2004). [DOI] [PubMed] [Google Scholar]
- Brekasis D. & Paget M. S. A novel sensor of NADH/NAD+ redox poise in Streptomyces coelicolor A3(2). The EMBO Journal 22, 4856–4865, doi: 10.1093/emboj/cdg453 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravcheev D. A. et al. Transcriptional regulation of central carbon and energy metabolism in bacteria by redox-responsive repressor Rex. Journal of Bacteriology 194, 1145–1157, doi: 10.1128/JB.06412-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyan S., Shiohira Y., Sato I., Takeuchi M. & Sato T. Regulatory loop between redox sensing of the NADH/NAD(+) ratio by Rex (YdiH) and oxidation of NADH by NADH dehydrogenase Ndh in Bacillus subtilis. Journal of Bacteriology 188, 7062–7071, doi: 10.1128/JB.00601-06 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagels M. et al. Redox sensing by a Rex-family repressor is involved in the regulation of anaerobic gene expression in Staphylococcus aureus. Molecular Microbiology 76, 1142–1161, doi: 10.1111/j.1365-2958.2010.07105.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitoun J. P., Liao S., Yao X., Xie G. G. & Wen Z. T. The redox-sensing regulator Rex modulates central carbon metabolism, stress tolerance response and biofilm formation by Streptococcus mutans. PloS One 7, e44766, doi: 10.1371/journal.pone.0044766 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J. T., Rogstam A. & von Wachenfeldt C. Coordinated patterns of cytochrome bd and lactate dehydrogenase expression in Bacillus subtilis. Microbiology 151, 3323–3335, doi: 10.1099/mic.0.28124-0 (2005). [DOI] [PubMed] [Google Scholar]
- Wang E. et al. Structure and functional properties of the Bacillus subtilis transcriptional repressor Rex. Molecular Microbiology 69, 466–478, doi: 10.1111/j.1365-2958.2008.06295.x (2008). [DOI] [PubMed] [Google Scholar]
- Ikeda H. & Omura S. Avermectin Biosynthesis. Chemical Reviews 97, 2591–2610 (1997). [DOI] [PubMed] [Google Scholar]
- Jakimowicz P. et al. Evidence that the Streptomyces developmental protein WhiD, a member of the WhiB family, binds a [4Fe-4S] cluster. The Journal of Biological Chemistry 280, 8309–8315, doi: 10.1074/jbc.M412622200 (2005). [DOI] [PubMed] [Google Scholar]
- Fowler-Goldsworthy K. et al. The actinobacteria-specific gene wblA controls major developmental transitions in Streptomyces coelicolor A3(2). Microbiology 157, 1312–1328, doi: 10.1099/mic.0.047555-0 (2011). [DOI] [PubMed] [Google Scholar]
- Hensel M., Lill H., Schmid R., Deckers-Hebestreit G. & Altendorf K. The ATP synthase (F1F0) of Streptomyces lividans: sequencing of the atp operon and phylogenetic considerations with subunit beta. Gene 152, 11–17 (1995). [DOI] [PubMed] [Google Scholar]
- Wikstrom M. & Hummer G. Stoichiometry of proton translocation by respiratory complex I and its mechanistic implications. Proceedings of the National Academy of Sciences of the United States of America 109, 4431–4436, doi: 10.1073/pnas.1120949109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C. P., Albrecht J. & Gunsalus R. P. Effect of microaerophilic cell growth conditions on expression of the aerobic (cyoABCDE and cydAB) and anaerobic (narGHJI, frdABCD, and dmsABC) respiratory pathway genes in Escherichia coli. Journal of Bacteriology 178, 1094–1098 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobius K. et al. Heme biosynthesis is coupled to electron transport chains for energy generation. Proceedings of the National Academy of Sciences of the United States of America 107, 10436–10441, doi: 10.1073/pnas.1000956107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono M., Mimura H., Nakanishi Y. & Maeshima M. Expression of functional Streptomyces coelicolor H+-pyrophosphatase and characterization of its molecular properties. Journal of Biochemistry 138, 183–191, doi: 10.1093/jb/mvi112 (2005). [DOI] [PubMed] [Google Scholar]
- Matsushita K., Ohnishi T. & Kaback H. R. NADH-ubiquinone oxidoreductases of the Escherichia coli aerobic respiratory chain. Biochemistry 26, 7732–7737 (1987). [DOI] [PubMed] [Google Scholar]
- Bjorklof K., Zickermann V. & Finel M. Purification of the 45 kDa, membrane bound NADH dehydrogenase of Escherichia coli (NDH-2) and analysis of its interaction with ubiquinone analogues. FEBS Letters 467, 105–110 (2000). [DOI] [PubMed] [Google Scholar]
- Agnihotri G. & Liu H. W. Enoyl-CoA hydratase. reaction, mechanism, and inhibition. Bioorganic & Medicinal Chemistry 11, 9–20 (2003). [DOI] [PubMed] [Google Scholar]
- Ichinose H., Fujimoto Z. & Kaneko S. Characterization of an alpha-L-rhamnosidase from Streptomyces avermitilis. Bioscience, Biotechnology, and Biochemistry 77, 213–216, doi: 10.1271/bbb.120735 (2013). [DOI] [PubMed] [Google Scholar]
- Barriuso-Iglesias M., Barreiro C., Sola-Landa A. & Martin J. F. Transcriptional control of the F0F1-ATP synthase operon of Corynebacterium glutamicum: SigmaH factor binds to its promoter and regulates its expression at different pH values. Microbial Biotechnology 6, 178–188, doi: 10.1111/1751-7915.12022 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. K. & Chater K. F. The Streptomyces coelicolor whiB gene encodes a small transcription factor-like protein dispensable for growth but essential for sporulation. Molecular & General Genetics: MGG 232, 351–358 (1992). [DOI] [PubMed] [Google Scholar]
- Kang S. H. et al. Interspecies DNA microarray analysis identifies WblA as a pleiotropic down-regulator of antibiotic biosynthesis in Streptomyces. Journal of Bacteriology 189, 4315–4319, doi: 10.1128/JB.01789-06 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh J. H., Kim S. H., Lee H. N., Lee S. Y. & Kim E. S. Isolation and genetic manipulation of the antibiotic down-regulatory gene, wblA ortholog for doxorubicin-producing Streptomyces strain improvement. Applied Microbiology and Biotechnology 86, 1145–1153, doi: 10.1007/s00253-009-2391-z (2010). [DOI] [PubMed] [Google Scholar]
- Molle V., Palframan W. J., Findlay K. C. & Buttner M. J. WhiD and WhiB, homologous proteins required for different stages of sporulation in Streptomyces coelicolor A3(2). Journal of Bacteriology 182, 1286–1295 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. J. et al. Mycobacterium tuberculosis WhiB1 is an essential DNA-binding protein with a nitric oxide-sensitive iron-sulfur cluster. The Biochemical Journal 432, 417–427, doi: 10.1042/BJ20101440 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann A. U. & Bremer E. Osmotically regulated synthesis of the compatible solute ectoine in Bacillus pasteurii and related Bacillus spp. Applied and Environmental Microbiology 68, 772–783 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macneil D. J. & Klapko L. M. Transformation of Streptomyces avermitilis by plasmid DNA. Journal of Industrial Microbiology 2, 209–218, doi: Doi 10.1007/Bf01569542 (1987). [DOI] [Google Scholar]
- Kieser T., Bibb M. J., Buttner M. J., Chater K. F. & Hopwood D. A. Practical Streptomyces Genetics. (2000). [Google Scholar]
- Jiang L. et al. Inactivation of the extracytoplasmic function sigma factor Sig6 stimulates avermectin production in Streptomyces avermitilis. Biotechnology Letters 33, 1955–1961, doi: 10.1007/s10529-011-0673-x (2011). [DOI] [PubMed] [Google Scholar]
- Bierman M. et al. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116, 43–49, doi: Doi 10.1016/0378-1119(92)90627-2 (1992). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. Deletion analysis of oligomycin PKS genes (olmA) in Streptomyces avermitilis. Chinese Science Bulletin 49, 350, doi: 10.1360/03wc0469 (2004). [DOI] [Google Scholar]
- Liu Y. et al. Characterization of SAV7471, a TetR-family transcriptional regulator involved in the regulation of coenzyme A metabolism in Streptomyces avermitilis. Journal of Bacteriology 195, 4365–4372, doi: 10.1128/JB.00716-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J. et al. The pathway-specific regulator AveR from Streptomyces avermitilis positively regulates avermectin production while it negatively affects oligomycin biosynthesis. Molecular Genetics and Genomics: MGG 283, 123–133, doi: 10.1007/s00438-009-0502-2 (2010). [DOI] [PubMed] [Google Scholar]
- Zianni M., Tessanne K., Merighi M., Laguna R. & Tabita F. R. Identification of the DNA bases of a DNase I footprint by the use of dye primer sequencing on an automated capillary DNA analysis instrument. Journal of Biomolecular Techniques: JBT 17, 103–113 (2006). [PMC free article] [PubMed] [Google Scholar]
- Munch R. et al. Virtual Footprint and PRODORIC: an integrative framework for regulon prediction in prokaryotes. Bioinformatics 21, 4187–4189, doi: 10.1093/bioinformatics/bti635 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.