ABSTRACT
We examined whether older individuals experience greater levels of hyperthermia and cardiovascular strain during an extreme heat exposure compared to young adults. During a 3-hour extreme heat exposure (44°C, 30% relative humidity), we compared body heat storage, core temperature (rectal, visceral) and cardiovascular (heart rate, cardiac output, mean arterial pressure, limb blood flow) responses of young adults (n = 30, 19–28 years) against those of older adults (n = 30, 55–73 years). Direct calorimetry measured whole-body evaporative and dry heat exchange. Body heat storage was calculated as the temporal summation of heat production (indirect calorimetry) and whole-body heat loss (direct calorimetry) over the exposure period. While both groups gained a similar amount of heat in the first hour, the older adults showed an attenuated increase in evaporative heat loss (p < 0.033) in the first 30-min. Thereafter, the older adults were unable to compensate for a greater rate of heat gain (11 ± 1 ; p < 0.05) with a corresponding increase in evaporative heat loss. Older adults stored more heat (358 ± 173 kJ) relative to their younger (202 ± 92 kJ; p < 0.001) counterparts at the end of the exposure leading to greater elevations in rectal (p = 0.043) and visceral (p = 0.05) temperatures, albeit not clinically significant (rise < 0.5°C). Older adults experienced a reduction in calf blood flow (p < 0.01) with heat stress, yet no differences in cardiac output, blood pressure or heart rate. We conclude, in healthy habitually active individuals, despite no clinically observable cardiovascular or temperature changes, older adults experience greater heat gain and decreased limb perfusion in response to 3-hour heat exposure.
KEYWORDS: aging, calorimetry, climate change, extreme heat events, heat stress
Introduction
Climate change has resulted in a rise in average ambient temperatures paralleled by a rise in the number and intensity of extreme heat events (EHE). Experts predict that global warming, if left unchecked, will have catastrophic effects on human health1 especially in older adults.2 Epidemiological evidence demonstrates a 2–5% increase in all-cause mortality for a 1°C increase in average daily temperature during hot temperature periods.3 With our global population rapidly aging,4 we can expect a dramatic increase in the risk of heat-related morbidity and mortality during EHE.5,6 As recent evidence demonstrates, the impact can be catastrophic. Extreme heat events claimed an estimated 70,000 lives in 2003 in Europe7 and 55,000 in 2010 in Russia,8,9 the majority of these being older adults and those with chronic health conditions. Moreover, these EHEs will place an insurmountable strain on health care systems worldwide, causing the greatest increase in public sector health care costs relative to all natural weather hazards.10
At present there is an alarming scarcity of guidance about how to respond to EHE and to address heat-related health risks.11 In the absence of any adaptation of the population, heat-related mortality in developed countries is projected to increase dramatically over the next few decades.6 International evidence suggests that appropriate public health programs can dramatically limit EHE-related mortality.12-14 Unfortunately, the most recent meta-analysis on the topic reported that knowledge on the effects of heat exposure on older adults remains incomplete15 and, as demonstrated by nation-wide EHE plan evaluations,14 this limits the ability of health professionals to make evidence-based clinical decisions during EHE.
Thus, the primary aim of this study was to examine the thermoregulatory and cardiovascular adjustments in healthy habitually active older individuals during an acute 3-hour exposure to extreme heat conditions (44°C, 30% relative humidity); conditions which are associated with a high risk of heat-related morbidity and mortality.3 In this context, we addressed 2 fundamental questions:
Question 1: Do older individuals (55–73 years) experience greater levels of hyperthermia during exposure to extreme heat compared to young (19–28 years) adults? To address this question, we compared the amount of heat stored in the body between the younger and older adults during the 3-hour extreme heat exposure. To ensure the highest quality of research evidence, measurements were performed using whole-body direct calorimetry, the gold standard for accurately measuring heat exchange between the body and the environment. Previous studies show that older adults are less responsive to heat exposure and take longer to respond to ambient air temperature changes than their younger counterparts.16 This is paralleled by a decrease in sweat production17,18 and skin blood flow18,19 in response to increases in core temperature; a response which occurs independent of the level of heat acclimation.18 Therefore, we evaluated the hypothesis that older adults would experience higher levels of hyperthermia as defined by a greater body heat storage compared to young adults.
Question 2: Do older adults experience greater levels of cardiovascular strain during heat exposure compared to young adults? To address this question we compared the cardiovascular responses (i.e., heart rate, cardiac output, blood pressure and limb blood flow) of young adults against those of older individuals during the 3-hour passive heat exposure. Large-scale epidemiological studies report that many heat-induced deaths are of cardiovascular origin, yet there is no increase in cardiovascular-related hospital admissions during EHE.20,21 This has led to the conclusion that deaths from cardiovascular events during EHE in older individuals occur rapidly before the patient is admitted to a hospital20-22 and that the first hours of heat exposure have a major impact on cardiovascular mortality in this vulnerable population.23,24 Therefore, we hypothesized that older individuals would experience greater levels of cardiovascular strain compared to young adults.
Methods
Ethical approval
This study was approved by the University of Ottawa Health Sciences and Science Research Ethics Board and was conducted according to the principles expressed in the Declaration of Helsinki. Volunteers provided written informed consent before participating in the study.
Participants
Habitually active and healthy young (n = 30, 19–28 years) and older (n = 30, 55–73 years) adults matched for body mass and surface area participated in the study (Table 1).
Table 1.
Participant characteristics.
| YOUNG (n = 30) | OLDER (n = 30) | |
|---|---|---|
| Males (#) | 23 | 24 |
| Females (#) | 7 | 6 |
| Age (years) | 23 ± 3 | 62 ± 6* |
| Height (m) | 1.72 ± 0.08 | 1.74 ± 0.08 |
| Weight (kg) | 74.3 ± 14.3 | 79.7 ± 16.3 |
| Body mass index (kg·m−2) | 24.9 ± 4.3 | 26.2 ± 4.1 |
| Body fat (%) | 20.4 ± 7.5 | 25.6 ± 7.1* |
| Body surface area (m2) | 1.87 ± 0.19 | 1.94 ± 0.22 |
Values: mean ± SD. Note:
= comparison against YOUNG statistically significant at p < 0.05; No significant between-group chi-square differences in the distribution of sexes (p > 0.05); YOUNG = adults 19–28 y of age; OLDER = adults 55–73 y of age.
Experimental protocol
Participants volunteered for one preliminary session and one experimental session involving a 3-hour passive heat exposure seated in a semi-recumbent position inside the Snellen whole-body direct calorimeter in very hot ambient conditions (air temperature of 44°C and 30% relative humidity). All trials were performed in the late fall and winter months.
During the preliminary session, we used standardized techniques to assess participant age, body height (Detecto, model 2391, Webb City, MO, USA), weight (model CBU150X, Mettler Toledo Inc., Mississauga, ON, Canada), and body fat percentage. Participants' levels of physical activity were assessed using quantitative (3 month) and 7 day physical activity recall questionnaires.25
For the experimental session, participants were encouraged to arrive well-hydrated as no fluid replacements were provided during the experiment. Upon arrival at the laboratory, hydration status of the participant was assessed. Only those volunteers with urine specific gravity values of <1.020 (i.e., hydrated26-28; model TS400, Reichter Inc., Depew, NY, USA) were permitted to participate in the experimental trial. Following verification of hydration status, participants were instrumented while they remained resting (∼45 min) in an upright seated position outside of the calorimeter in a non-heat stressed environment (∼26°C). All participants wore light pairs of athletic shorts and sandals (females also wore a sports bra). At the end of the instrumentation period, the participants remained resting for an additional ∼15 min after which baseline thermoregulatory and cardiovascular measurements were performed. The participants were then transferred to the calorimeter (transition time ∼5 min) where they underwent the 3-hour heat exposure while seated in the upright position. At the end of the 3-hour exposure, the calorimeter door was opened and the final cardiovascular measurements were performed. For some cardiovascular measurements (i.e., cardiac output, limb blood flow), we were only able to perform them before and after the 3-hour exposure due to the nature of direct calorimetry requiring an individual to be completely sealed inside the chamber for the entire duration to the study. Given that the whole-body calorimeter is housed within a large temperature controlled chamber which is used to supply the conditioned air to the calorimeter, ambient temperature conditions for the final cardiovascular measurements remain unchanged (i.e., 44°C, 30% relative humidity).
Measurements
We used a whole-body direct calorimeter to record whole-body heat exchange continuously and assess body heat storage via the subtraction of heat dissipation (evaporative heat loss and dry heat exchange) from heat production. The reader is referred to a full technical description of the fundamental principles and performance characteristics of the Snellen calorimeter.29 In parallel, we used standardized techniques26-28 to continuously record: [1] rectal temperature via a thermocouple (Mon-a-therm General Purpose Temperature Probe, Mallinckrodt Medical, St. Louis, MO, USA) inserted ≥12 cm past the anal sphincter; [2] visceral temperature using a telemetric pill (VitalSense; Mini Mitter Company Inc., USA); [3] skin temperature on the upper back, chest, thigh, and calf using thermocouples (Concept Engineering, Old Saybrook, CT, USA); [4] heart rate (RS400, Polar, Kempele, Finland); [5] the physiological heat stress index30; and [6] local sweat rate at the forearm, chest, upper back, and thigh via a ventilated capsule. We also recorded thermal sensation using the ASHRAE 7-point scale (0 = Neutral to 7 = Very, Very Hot) every 30-minutes.
Prior to and following the 3-hour heat exposure we measured cardiovascular responses outside the direct calorimeter. Specifically, we assessed: [1] resting heart rate (RS400, Polar, Kempele, Finland); [2] cardiac output via the inert gas (mixture consisting of 5% blood soluble N2O, 1% blood insoluble SF6, and 94% of O2) rebreathing technique as established by Innocor (Innovision, Odense, Denmark); [3] arterial blood pressure via manual auscultation with a validated mercury sphygmomanometer (Baumanometer Standby Model, WA Baum Co, Copiague, NY, USA); and [4] limb blood flow (forearm and calf) by venous occlusion plethysmography (Hokanson AI6, D.E. Hokanson, Inc., Bellevue, WA, USA). Moreover, stroke volume was calculated as cardiac output/heart rate, while total peripheral resistance was calculated as mean arterial pressure/cardiac output.
Statistical analysis
Data were checked for normality using Kolmogorov-Smirnov tests and all variables were found to be normally distributed. To test our first hypothesis, a repeated-measures analysis of variance was utilized to assess the differences between the 2 groups (i.e., Young, Older) in terms of whole-body and local thermoregulatory responses as well as perceived heat stress throughout (i.e., minutes 0–30, 31–60, 61–90, 91–120, 121–150, and 151–180) the 3-hour heat exposure. To test the second hypothesis, the same analysis was utilised for pre and post cardiovascular responses in both groups. In all analyses, the level of statistical significance was set at p < 0.05, while post-hoc tests incorporating a Bonferroni adjustment were used for pairwise comparisons between groups and time points. All statistical analyses were conducted using SPSS 24 (IBM, Armonk, NY, USA) statistical software.
Results
Whole-body heat exchange
The rates of metabolic heat production, total heat loss, evaporative heat loss, dry heat exchange, as well as the change in body heat content are presented in Table 2. Metabolic heat production increased throughout the heat exposure (p < 0.001) and responses were similar between the 2 groups (p = 0.798). The rate of whole-body heat loss; as defined by the combined changes in the rates of evaporative heat loss and dry heat exchange, increased (p < 0.001) for both groups over time. The rate of whole-body heat loss was lower in the older compared to the younger adults during the first hour of heat exposure (p = 0.006) which was primarily due to an attenuated increase in evaporative heat loss (p < 0.001) and greater rate of dry heat gain (p < 0.001). This difference persisted for the duration of the heat exposure such that the older adults maintained a reduced rate of evaporative heat loss despite a 11 ± 1 W greater rate of dry heat gain (p = 0.05). As a result, older adults stored more heat relative to their younger counterparts throughout the 3-hour exposure; storing 1.8-fold more heat by the end of the exposure (p < 0.001, Fig. 1 and Table 2).
Table 2.
Whole-body calorimetry data during the 3-hour extreme heat exposure in young and older adults.
| Time (min) |
|||||||
|---|---|---|---|---|---|---|---|
| Group | 0–30 | 31–60 | 61–90 | 91–120 | 121–150 | 151–180 | |
| M-W, W | YOUNG | 102 ± 22 | 105 ± 24 | 106 ± 22 | 108 ± 22 | 108 ± 21 | 108 ± 22 |
| OLDER | 105 ± 23 | 107 ± 22 | 108 ± 23 | 108 ± 23 | 110 ± 25 | 109 ± 25 | |
| THL, W | YOUNG | 44 ± 28 | 79 ± 23 | 92 ± 24 | 99 ± 25 | 104 ± 23 | 109 ± 24 |
| OLDER | 20 ± 32* | 61 ± 26* | 82 ± 23 | 90 ± 24 | 97 ± 25 | 97 ± 24 | |
| EHL, W | YOUNG | 151 ± 29 | 168 ± 26 | 172 ± 26 | 173 ± 26 | 174 ± 25 | 176 ± 27 |
| OLDER | 134 ± 31* | 160 ± 33 | 172 ± 34 | 176 ± 36 | 179 ± 37 | 175 ± 36 | |
| DHL, W | YOUNG | −107 ± 18 | −89 ± 15 | −80 ± 16 | −75 ± 16 | −71 ± 15 | −68 ± 15 |
| OLDER | −114 ± 27 | −98 ± 24 | −90 ± 22* | −85 ± 23* | −82 ± 25* | −78 ± 23* | |
| Hb, kJ | YOUNG | 105 ± 33 | 48 ± 24 | 25 ± 17 | 16 ± 19 | 8 ± 15 | 0 ± 13 |
| OLDER | 154 ± 53* | 82 ± 40* | 45 ± 33* | 33 ± 28* | 24 ± 28* | 21 ± 27* | |
Values: (mean ± SD). Note:
= comparison against YOUNG significant at p < 0.05; YOUNG = adults 19–28 y of age; OLDER = adults 55–73 y of age. M-W = rate of metabolic heat production; THL = rate of total heat loss (combined rates of evaporative heat loss and dry heat exchange); EHL = rate of evaporative heat loss; DHL = rate of dry heat exchange; Hb= change in body heat storage.
Figure 1.
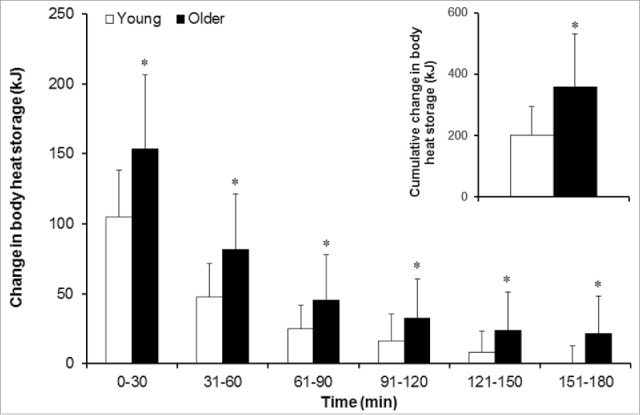
Body heat storage (mean ± SD) during the 3-hour extreme heat exposure in young and older adults. Note: * = comparison against the young group for the same time point statistically significant at p < 0.05; the white bars indicate the young group (19–28 y of age) and the black bars indicate the older group (55–73 y of age). Inset figure shows older adults stored 1.8-times more heat over the 3-hour exposure.
Tissue temperatures and local heat loss responses
Core (rectal and visceral) and mean skin temperatures, local sweat rates, thermal sensation, as well as the physiological heat strain index are presented in Table 3. After 30 minutes of heat exposure, rectal temperature was significantly greater in the older adults albeit the relative increase from baseline was similar (0.4–0.5°C) between groups over the 3-hour heat exposure (p < 0.001). No differences were observed in visceral temperature with the exception that responses were greater at the end of the exposure only in older relative to the younger adults (p = 0.05). Due to technical problems, visceral temperature measurements were not recorded for 8 young and 10 older participants. Local sweat rate at all 4 sites (forearm, upper back, chest, and thigh) increased throughout the heat exposure in both groups (p < 0.001), however, only thigh sweat rate increased to greater levels in the young relative to the older adults (p = 0.006). While both groups reported a greater thermal sensation over time (p < 0.001), the older adults reported significantly increased thermal sensation (indicating feeling hotter) throughout the exposure (p < 0.001). The physiological heat stress index increased during the heat exposure in both groups (p < 0.001), but the increase observed in the older adults was larger than that observed in the younger adults (p = 0.009).
Table 3.
Rectal temperature and other indicators of thermal strain during the heat exposure in young and older adults.
| Time (min) |
|||||||
|---|---|---|---|---|---|---|---|
| Group | 0–30 | 31–60 | 61–90 | 91–120 | 121–150 | 151–180 | |
| Rectal temperature | YOUNG | 37.2 ± 0.2 | 37.3 ± 0.2 | 37.4 ± 0.2 | 37.5 ± 0.2 | 37.6 ± 0.2 | 37.6 ± 0.2 |
| (°C) | OLDER | 37.3 ± 0.3 | 37.5 ± 0.3* | 37.6 ± 0.3* | 37.7 ± 0.3* | 37.7 ± 0.3* | 37.8 ± 0.3* |
| Visceral temperature | YOUNG | 37.3 ± 0.3 | 37.4 ± 0.3 | 37.5 ± 0.3 | 37.6 ± 0.3 | 37.6 ± 0.2 | 37.7 ± 0.2 |
| (°C) | OLDER | 37.3 ± 0.4 | 37.5 ± 0.4 | 37.6 ± 0.3 | 37.7 ± 0.4 | 37.8 ± 0.3 | 37.9 ± 0.3* |
| Mean skin temperature | YOUNG | 36.0 ± 0.3 | 36.1 ± 0.3 | 36.2 ± 0.3 | 36.3 ± 0.3 | 36.3 ± 0.3 | 36.4 ± 0.3 |
| (°C) | OLDER | 36.0 ± 0.4 | 36.1 ± 0.4 | 36.2 ± 0.3 | 36.2 ± 0.3 | 36.2 ± 0.3 | 36.2 ± 0.4† |
| Forearm sweat rate | YOUNG | 0.60 ± 0.16 | 0.62 ± 0.16 | 0.65 ± 0.17 | 0.69 ± 0.19 | 0.71 ± 0.21 | 0.72 ± 0.23 |
| (mg·min−1·cm−2) | OLDER | 0.63 ± 0.22 | 0.66 ± 0.24 | 0.69 ± 0.24 | 0.71 ± 0.26 | 0.73 ± 0.25 | 0.71 ± 0.25 |
| Upper back sweat rate | YOUNG | 0.57 ± 0.14 | 0.60 ± 0.16 | 0.63 ± 0.18 | 0.65 ± 0.18 | 0.67 ± 0.20 | 0.68 ± 0.22 |
| (mg·min−1·cm−2) | OLDER | 0.60 ± 0.22 | 0.60 ± 0.21 | 0.63 ± 0.23 | 0.65 ± 0.24 | 0.67 ± 0.27 | 0.67 ± 0.26 |
| Chest sweat rate | YOUNG | 0.48 ± 0.20 | 0.49 ± 0.21 | 0.50 ± 0.22 | 0.53 ± 0.25 | 0.53 ± 0.18 | 0.54 ± 0.20 |
| (mg·min−1·cm−2) | OLDER | 0.51 ± 0.17 | 0.52 ± 0.20 | 0.55 ± 0.21 | 0.57 ± 0.22 | 0.59 ± 0.21 | 0.60 ± 0.22 |
| Thigh sweat rate | YOUNG | 0.60 ± 0.14 | 0.63 ± 0.15 | 0.65 ± 0.15 | 0.68 ± 0.17 | 0.69 ± 0.18 | 0.70 ± 0.19 |
| (mg·min−1·cm−2) | OLDER | 0.50 ± 0.14* | 0.49 ± 0.14* | 0.49 ± 0.14* | 0.50 ± 0.14* | 0.50 ± 0.15* | 0.51 ± 0.15* |
| Thermal sensation | YOUNG | 2.4 ± 0.9 | 2.6 ± 0.9 | 2.7 ± 0.9 | 2.8 ± 1.0 | 2.8 ± 1.0 | 2.9 ± 1.0 |
| OLDER | 3.2 ± 1.4* | 3.6 ± 1.3* | 3.8 ± 1.4* | 4.1 ± 1.6* | 4.3 ± 1.7* | 4.3 ± 1.7* | |
| Physiological heat strain | YOUNG | 0.10 ± 0.21 | 0.53 ± 0.44 | 0.85 ± 0.51 | 1.26 ± 0.61 | 1.46 ± 0.70 | 1.70 ± 0.75 |
| index | OLDER | 0.11 ± 0.33 | 0.73 ± 0.64 | 1.31 ± 0.83* | 1.75 ± 0.92* | 2.00 ± 0.97* | 2.26 ± 1.02* |
Values: (mean ± SD). Note:
= comparison against YOUNG significant at p < 0.05. YOUNG = adults 19–28 y of age; OLDER = adults 55–73 y of age.
Cardiovascular responses
The cardiovascular responses for the young and older adults are presented in Table 4. An increase in heart rate, forearm blood flow, and calf blood flow was measured in both groups (all p < 0.001). In contrast, mean arterial pressure, total peripheral resistance and cardiac output remained unchanged (all p > 0.116), while a reduction in stroke volume (p < 0.001) was measured. Mean arterial pressure was higher in the older adults compared to the younger adults at baseline only (p = 0.003). Heart rate, stroke volume, and cardiac output were lower while total peripheral resistance was greater in the older adults at baseline and at the end of the 3-hour exposure relative to their younger counterparts (all p < 0.05). As such, no differences in the proportional rise of heart rate, cardiac output, blood pressure and total peripheral resistance were observed in younger vs. older adults; however, older adults demonstrated an attenuated increase in calf blood flow compared to their younger counterparts (p < 0.001).
Table 4.
Cardiovascular responses at baseline and following the heat exposure in young and older adults.
| Time (min) |
|||
|---|---|---|---|
| Group | Baseline | Post | |
| Resting heart rate | YOUNG | 78 ± 16 | 98 ± 16 |
| (beats·min−1) | OLDER | 72 ± 13* | 90 ± 14* |
| Cardiac output | YOUNG | 5.7 ± 0.9 | 5.7 ± 0.9 |
| (L·min−1) | OLDER | 4.3 ± 1.2* | 4.2 ± 1.0* |
| Stroke volume | YOUNG | 75.9 ± 17.5 | 60.0 ± 13.0 |
| (ml) | OLDER | 62.5 ± 20.0* | 48.0 ± 13.5* |
| Mean arterial pressure | YOUNG | 88.5 ± 7.1 | 90.6 ± 9.5 |
| (mmHg) | OLDER | 93.9 ± 7.0* | 95.1 ± 10.5 |
| Total peripheral resistance | YOUNG | 14.3 ± 3.0 | 16.4 ± 2.5 |
| (mmHg· L·min−1) | OLDER | 23.7 ± 8.0* | 25.5 ± 10.5* |
| Forearm blood flow | YOUNG | 2.8 ± 0.6 | 6.3 ± 2.8 |
| (ml·100 ml tissue−1·min−1) | OLDER | 2.6 ± 1.1 | 5.3 ± 2.2 |
| Calf blood flow | YOUNG | 2.4 ± 0.5 | 5.0 ± 1.6 |
| (ml·100 ml tissue−1·min−1) | OLDER | 1.8 ± 0.7* | 3.4 ± 1.3* |
Note:
= comparison against YOUNG significant at p < 0.05; YOUNG = adults 19–28 y of age; OLDER = adults 55–73 y of age.
Discussion
We show that healthy habitually active older adults stored 1.8-fold more heat as compared to their younger counterparts during a 3-hour passive extreme heat exposure. This was paralleled by a greater level of hyperthermia as defined by a more pronounced body core temperature (rectal and visceral) response measured at the end of the exposure, although the relative increase from the baseline resting temperature response was not different between groups (0.4–0.5°C). While a greater level of thermal strain was observed in the older adults, the degree of heat strain imposed in this experiment was not paralleled by marked age-related differences in cardiovascular adjustments.
Epidemiological evidence shows that the first hours of heat exposure have the largest effect on the mortality rates of older individuals.20-24 We found that young adults achieved heat balance (indicating that they ceased storing any additional heat) within the first 2 hours of the 3-hour extreme heat exposure (rate of body heat storage: 0.0 ± 12.9 W). This was not the case for older adults who continued to store heat during this same period with an elevated rate of heat storage of 21.5 ± 26.8 W. As our study demonstrates, older adults had an impaired ability to increase whole-body sweating as evidenced by a reduced rate of whole-body evaporative heat loss measured in the first 30-min. While evaporative heat loss was not significantly different between groups thereafter, older adults continued to gain more heat as evidenced by a significantly greater rate of dry heat gain that was maintained for the duration of the exposure. This was however not offset by a concomitant increase in evaporative heat loss such that the older adults continued to store heat even in the final 30-min as noted above. As a consequence, the older adults stored more heat relative to their younger counterparts throughout the exposure. Previous studies investigating exercise in hot environments also reported an attenuated whole-body evaporative heat loss (and therefore whole-body sweat production) in healthy habitually active older adults.31-33 In this study, we show that the attenuated thermoregulatory adjustments of older adults to an exercise-induced heat stress is present also in resting conditions during an acute 3-hour extreme heat exposure.
While we measured a markedly greater increase in the amount of heat stored in the body in older adults, this was paralleled by only a modest change in body core temperature of 0.4–0.5°C across both groups; an increase in core temperature equivalent to a non-pathological sign of heat stress according to current guidelines.34 When the calculated mean body temperature (considered a proxy measure of body heat storage35) responses were compared, thermometry was shown to underestimate the change in mean body temperature relative to direct calorimetry at the end of the 3-hour exposure for both groups, albeit the disparity was considerably more pronounced in older adults (Young: 37.51°C vs 37.87°C; Older: 37.64°C vs 38.41°C) (Fig. 2). Recent studies employing whole-body direct calorimetry have reported similar differences, challenging the validity of body core temperature measurement for estimating whole-body thermal strain.31,32,36 Measurements of body core temperature, such as those associated with rectal or visceral temperature, only indicate regional changes in heat content and their use for estimating thermal strain across the entire body has been challenged35-37; a response paralleling the observed disparity between whole-body and local sweating responses.26,28,32,35 The variation in the measured increase in mean body temperature observed in the present study is likely the result of differences in the exchange of heat between body compartments (i.e., visceral organs, muscle, skin, others). Regional variations in tissue blood flow, which are more evident in older adults,38,39 combined with group differences in thermoregulatory adjustments measured in the present study, can lead to differences in heat transfer/distribution between internal tissues.35,36,40 In turn, as demonstrated by our study findings, this can limit the validity of core temperature measurement for representing whole-body hyperthermia in older adults. While core temperature is the currently recommended34 clinical assessment practice for addressing heat-related health risks, additional studies are required to assess its effectiveness in enabling the diagnosis and assessment of heat-related illnesses for different levels of heat stress and or exposure times.
Figure 2.
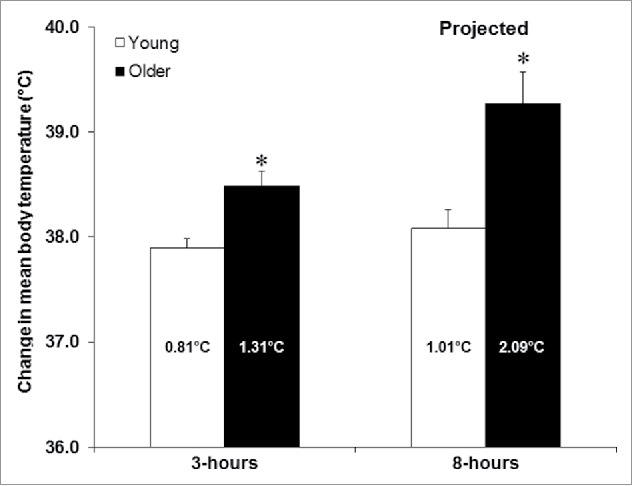
The change in mean body temperature (mean ± SD) at the end of the 3-hour extreme heat exposure and the projected change in mean body temperature after an extended 8-hour exposure in young and older adults. Note: the relative change in mean body temperature for each group is presented in the bar; * = comparison against the young group statistically significant at p < 0.05; the white bars indicate the young group (19–28 y of age) and the black bars indicate the older group (55–73 y of age). Mean body temperature at the end of the 3-hour exposure was calculated as the cumulative change in body heat storage (kJ) divided by body mass (kg) and the average specific heat capacity of the body (i.e., 3.47 kJ·kg−1·°C−1). The projected theoretical change in mean body temperature after 8 h of heat exposure was estimated based on the difference measured during the last hour of the heat exposure.
A novel finding from this study was that age did not compromise the cardiovascular adjustments observed during a 3-hour extreme heat exposure. It is important to note, however, that we studied highly-screened habitually active older individuals with no underlying cardiovascular condition to examine age-related differences in cardiovascular adjustments to a heat stress. A previous study reported that, compared to young individuals, older adults (70 years) exposed to a passive heat stress condition while in a supine position demonstrated an attenuated increase in cardiac output in conjunction with impaired redistribution of blood flow from the splanchnic and renal circulations.38 Nevertheless, the aforementioned study exposed individuals to extreme heat stress using a full-body heating suit with a plastic coverall (to reduce the evaporation of sweat) resulting in rapid increases in core temperature near 38.5°C and skin temperatures ∼40°C (levels equivalent to the limits of the individuals thermal tolerance) within just 64 ± 7 min. The marked increases in core temperature were associated with greater increases (above baseline resting) in heart rate (Young: ∼100%, Older: ∼80%) and forearm blood flow (Young: 473%, Older: 273%) for the young versus the older adults respectively. As shown in the present study, a 3-hour heat exposure to 44°C in the upright seated posture did not cause such elevated levels of hyperthermia and the corresponding adjustments in heart rate (Young: 25% vs Older: 25%) and forearm blood flow (Young: 130%, Older: 103%) were less pronounced in both groups. Of note, we showed that the relative increase in forearm (non-significant trend) and calf blood flow (Young: 108%, Older: 88%, p < 0.006) was attenuated in the older relative to the young adults which was consistent with our observation of a greater rate of heat gain observed in the older adults. Our findings parallel those by Sagawa et al.41 who exposed 6 older (66 years) adults to 40°C for up to 130 minutes while also seated in the upright position. Forearm blood flow was significantly reduced after 95 min in the older adults (cardiovascular responses were only measured in the first 95 min) and no differences in the relative changes in heart rate, cardiac output, mean arterial pressure, and total peripheral resistance were observed. This was paralleled by a greater, albeit clinically non-significant core temperature response (Young: 37.03°C; Elderly: 37.31°C) and this difference remained intact at the end of the 130 min exposure.
It is abundantly clear that cardiovascular mortality is markedly increased during EHE.20-22 Knowing whether older adults experience increased cardiovascular strain during the initial hours of heat exposure is crucial for clinical care, as large-scale epidemiological studies show that deaths from cardiovascular disease during EHE in older individuals occur rapidly before the patient is admitted to a hospital20-22 and that the first hours of heat exposure have a large effect on the mortality rate of this vulnerable population.23,24 Our results show that the cardiovascular adjustments to the degree of heat stress that we imposed in healthy habitually active older adults caused by passive heat exposure are adequate. However, as reduced limb perfusion was already being demonstrated in older subjects, increased heat stress severity or duration, greater age and/or the presence of underlying clinical or subclinical cardiovascular health conditions will undoubtedly lead to further reductions in cardiovascular adjustments with potentially catastrophic sequelae. This is in line with epidemiological data demonstrating that cardiovascular disease is not a primary reason for hospital admission during EHE but, as a pre-existing condition, it is a risk factor for hospitalization and mortality.42,43
We showed that the physiological heat stress index, an aggregate of rectal temperature and heart rate [calculated as: 5(current rectal temperature – resting rectal temperature)·(39.5 – resting rectal temperature)−1 + 5(current heart rate – resting heart rate)·(180 – resting heart rate)−1],30 was significantly greater in the older adults as compared to their younger counterparts over the final 2-hours of the exposure, principally due to altered temperature. The difference between groups (difference in index of ∼0.5) remained intact for the duration of the exposure. In parallel, we showed that the rate of body heat storage as assessed by direct calorimetry was maintained at a near constant elevated rate over the last hour; a response which resulted in the older adults storing 1.8-times more heat than their younger counterparts. Taken together, the physiological heat stress index may provide important information that could assist in identifying those adults at greater risk of experiencing potentially dangerous levels of hyperthermia. Further studies are warranted to examine the applicability of this index under different heat stress conditions and for different population groups.
Perspective
The present study examined the heat stress response in healthy habitually active older adults during a short 3-hour extreme heat exposure. Therefore, it is unclear if this age-related attenuation in the body's ability to dissipate heat would remain intact for an extended exposure period (i.e., >3 hours). Based on the assumption that the rate of body heat storage would remain unchanged from the rate measured over the last hour of the exposure, we estimated that increases in mean body temperature could exceed 39.0°C after an 8-hour exposure (Fig. 2). While such a hypothetical scenario is unlikely (i.e., without some behavioral and or physiological adjustments), it does reveal that even small differences in the body's ability to dissipate heat can lead to dangerous levels of hyperthermia if left unchecked. Such a situation may however only occur if vulnerable individuals are unable to respond to heat warnings and take preventative measures (e.g. use of air conditioners, fans, relocating to a cooling center, etc.) to reduce their exposure to the heat stress condition thereby mitigating their risk of experiencing a heat-related injury or death. Finally, as noted above, our older participants were healthy habitually active individuals. Aging-associated diseases such as type 2 diabetes, hypertension, cardiovascular disease and others may lead to more pronounced attenuations in the body's ability to dissipate heat2 especially under a state of dehydration which may occur with prolonged heat exposure and or medication use.2 Future studies are therefore warranted to determine the extent to which these health conditions may change the temperature threshold at which marked impairments in heat dissipation occur (i.e., exposure-response relationship for temperature). Such knowledge will help facilitate the development of guidelines and policies related to the management of vulnerable populations in extreme heat conditions.
In conclusion, we showed that adults aged 55–73 y experience greater levels of thermal strain despite no observable cardiovascular strain and minimal changes in core temperature during a passive 3-hour extreme heat exposure as compared to young adults aged 19–28 y. This increased heat strain is due to an attenuation of whole-body evaporative heat loss due to reduced sweating during the early stages of the exposure and an inability to maintain a sufficiently elevated rate to counterbalance the effects of a greater rate of heat gain from the environment over the mid- and late-stages of the exposure. The potential for marked heat strain without observable clinically significant differences in cardiovascular and temperature responses in older adults needs to be factored in the evaluation of patients being treated for heat stress.
Abbreviations
- EHE
Extreme Heat Events
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are indebted to the study participants and to Ms. Joanie Larose for the success of this project. The authors would like to thank Mr. Michael Sabino of Can-Trol Environmental Systems Limited (Markham, ON, Canada) for his support.
Funding
This study was funded by the Canadian Institutes of Health Research (Grant number 286363, held by Glen P. Kenny). The funding agency had no role in the design, conduct or interpretation of results. G. P. Kenny was supported by a University of Ottawa Research Chair Award. R.J. Sigal was supported by a Health Senior Scholar award from Alberta Innovates-Health Solutions. Mr. Martin P. Poirier was supported by the Natural Sciences and Engineering Research Council of Canada Alexander Graham Bell Graduate Scholarship (CGS-D). S. Dervis was supported by a MITACS Accelerate PhD Internship Award. B. Friesen was supported by an Ontario Graduate Scholarship.
Authors contributions
G.P.K conceived and designed the experiment. M.P., S.D., and B.J.F. contributed to data collection. A.D.F. and M.P. performed data analysis. G.P.K., A.D.F., G.S.M., M.P.P., P.B., S.D., B.J.F., J.M. R.J.S., and A.J.S. interpreted the experimental results. A.D.F., G.S.M. and M.P. completed the statistical analysis. G.P.K. and A.D.F. drafted the manuscript. G.P.K., M.P.P., G.S.M., P.B., S.D., B.J.F., J.M. R.J.S., A.J.S, and A.D.F. edited and revised the manuscript. All authors provided critical revisions of the manuscript, they approved its final version, and are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- [1].Luber G, Hess J. Climate change and human health in the United States. J Environ Health 2007; 70:43-4, 46; PMID:18189039 [PubMed] [Google Scholar]
- [2].Kenny GP, Yardley J, Brown C, Sigal RJ, Jay O. Heat stress in older individuals and patients with common chronic diseases. CMAJ 13 2010; 182:1053-60; http://dx.doi.org/ 10.1503/cmaj.081050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yu W, Mengersen K, Wang X, Ye X, Guo Y, Pan X, Tong S. Daily average temperature and mortality among the elderly: a meta-analysis and systematic review of epidemiological evidence. Int J Biometeorol 2012; 56:569-81; PMID:21975970; http://dx.doi.org/ 10.1007/s00484-011-0497-3 [DOI] [PubMed] [Google Scholar]
- [4].United Nations, World Population Aging; ST/ESA/SER.A/348, Department of Economic and Social Affairs, Population Division, 2013. [Google Scholar]
- [5].Li T, Horton RM, Bader DA, Zhou M, Liang X, Ban J, Sun Q, Kinney PL. Aging will amplify the heat-related mortality risk under a changing climate: Projection for the elderly in Beijing, China. Sci Rep 2016; 6:28161; PMID:27320724; http://dx.doi.org/ 10.1038/srep28161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hajat S, Vardoulakis S, Heaviside C, Eggen B. Climate change effects on human health: projections of temperature-related mortality for the UK during the 2020s, 2050s and 2080s. J Epidemiol Community Health 2014; 68:641-8; PMID:24493740; http://dx.doi.org/ 10.1136/jech-2013-202449 [DOI] [PubMed] [Google Scholar]
- [7].Robine JM, Cheung SL, Le Roy S, Van Oyen H, Griffiths C, Michel JP, Herrmann FR. Death toll exceeded 70,000 in Europe during the summer of 2003. C R Biol 2008; 331(2):171-8; PMID:18241810; http://dx.doi.org/ 10.1016/j.crvi.2007.12.001 [DOI] [PubMed] [Google Scholar]
- [8].Osborn A. Moscow smog and nationwide heat wave claim thousands of lives. Bmj 2010; 341:c4360; PMID:20699322; http://dx.doi.org/ 10.1136/bmj.c4360 [DOI] [PubMed] [Google Scholar]
- [9].Hoag H. Russian summer tops ‘universal’ heatwave index. 2014; http://www.nature.com/news/russian-summer-tops-universal-heatwave-index-1.16250. Accessed August9, 2016, 2016. [Google Scholar]
- [10].Knowlton K, Rotkin-Ellman M, Geballe L, Max W, Solomon GM. Six climate change-related events in the United States accounted for about $14 billion in lost lives and health costs. Health Aff (Millwood) 2011; 30:2167-76; PMID:22068410 [DOI] [PubMed] [Google Scholar]
- [11].Health professionals: be prepared for heatwaves. Lancet 2015; 386:219. [DOI] [PubMed] [Google Scholar]
- [12].World Health Organisation - Europe Heat-health action plans: guidance. Copenhagen, Denmark: World Health Organisation; 2008. [Google Scholar]
- [13].Fouillet A, Rey G, Wagner V, Laaidi K, Empereur-Bissonnet P, Le Tertre A, Frayssinet P, Bessemoulin P, Laurent F, De Crouy-Chanel P, et al.. Has the impact of heat waves on mortality changed in France since the European heat wave of summer 2003? A study of the 2006 heat wave. Int J Epidemiol 2008; 37:309-17; PMID:18194962; http://dx.doi.org/ 10.1093/ije/dym253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Boyson C, Taylor S, Page L. The national heatwave plan - a brief evaluation of issues for frontline health staff. PLoS Curr 2014; 6. pii: 6; PMID:24475366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Benmarhnia T, Deguen S, Kaufman JS, Smargiassi A. Review Article: Vulnerability to heat-related mortality: A systematic review, meta-analysis, and meta-regression analysis. Epidemiology 2015; 26:781-93; PMID:26332052; http://dx.doi.org/ 10.1097/EDE.0000000000000375 [DOI] [PubMed] [Google Scholar]
- [16].Dufour A, Candas V. Ageing and thermal responses during passive heat exposure: sweating and sensory aspects. Eur J Appl Physiol 2007; 100:19-26; PMID:17242944; http://dx.doi.org/ 10.1007/s00421-007-0396-9 [DOI] [PubMed] [Google Scholar]
- [17].Kenney WL, Fowler SR. Methylcholine-activated eccrine sweat gland density and output as a function of age. J Appl Physiol (1985) 1988; 65:1082-6; PMID:3182477 [DOI] [PubMed] [Google Scholar]
- [18].Armstrong CG, Kenney WL. Effects of age and acclimation on responses to passive heat exposure. J Appl Physiol (1985) 1993; 75:2162-7; PMID:8307874 [DOI] [PubMed] [Google Scholar]
- [19].Kenney WL, Morgan AL, Farquhar WB, Brooks EM, Pierzga JM, Derr JA. Decreased active vasodilator sensitivity in aged skin. Am J Physiol Regul Integr Comp Physiol 1997; 272:H1609-14. [DOI] [PubMed] [Google Scholar]
- [20].Michelozzi P, Accetta G, De Sario M, D'Ippoliti D, Marino C, Baccini M, Biggeri A, Anderson HR, Katsouyanni K, Ballester F, et al.. High temperature and hospitalizations for cardiovascular and respiratory causes in 12 European cities. Am J Respir Crit Care Med 2009; 179:383-9; PMID:19060232; http://dx.doi.org/ 10.1164/rccm.200802-217OC [DOI] [PubMed] [Google Scholar]
- [21].Linares C, Diaz J. Impact of high temperatures on hospital admissions: comparative analysis with previous studies about mortality (Madrid). Eur J Public Health 2008; 18:317-22; PMID:18045814; http://dx.doi.org/ 10.1093/eurpub/ckm108 [DOI] [PubMed] [Google Scholar]
- [22].Mastrangelo G, Fedeli U, Visentin C, Milan G, Fadda E, Spolaore P. Pattern and determinants of hospitalization during heat waves: an ecologic study. BMC Public Health 2007; 7:200; PMID:17688689; http://dx.doi.org/ 10.1186/1471-2458-7-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Basu R, Malig B. High ambient temperature and mortality in California: exploring the roles of age, disease, and mortality displacement. Environ Res 2011; 111:1286-92; PMID:21981982 [DOI] [PubMed] [Google Scholar]
- [24].Huynen MM, Martens P, Schram D, Weijenberg MP, Kunst AE. The impact of heat waves and cold spells on mortality rates in the Dutch population. Environ Health Perspect 2001; 109:463-70; PMID:11401757; http://dx.doi.org/ 10.1289/ehp.01109463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kohl HW, Blair SN, Paffenbarger RS Jr., Macera CA, Kronenfeld JJ. A mail survey of physical activity habits as related to measured physical fitness. Am J Epidemiol 1988; 127:1228-39; PMID:3369421 [DOI] [PubMed] [Google Scholar]
- [26].Stapleton JM, Larose J, Simpson C, Flouris AD, Sigal RJ, Kenny GP. Do older adults experience greater thermal strain during heat waves? Appl Physiol Nutr Metab 2014; 39:292-8; PMID:24552369; http://dx.doi.org/ 10.1139/apnm-2013-0317 [DOI] [PubMed] [Google Scholar]
- [27].Larose J, Boulay P, Sigal RJ, Wright HE, Kenny GP. Age-related decrements in heat dissipation during physical activity occur as early as the age of 40. PloS One 2013; 8:e83148; PMID:24349447; http://dx.doi.org/ 10.1371/journal.pone.0083148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kenny GP, Stapleton JM, Yardley JE, Boulay P, Sigal RJ. Older adults with type 2 diabetes store more heat during exercise. Med Sci Sports Exerc 2013; 45:1906-14; PMID:23542894; http://dx.doi.org/ 10.1249/MSS.0b013e3182940836 [DOI] [PubMed] [Google Scholar]
- [29].Reardon FD, Leppik KE, Wegmann R, Webb P, Ducharme MB, Kenny GP. The Snellen human calorimeter revisited, re-engineered and upgraded: design and performance characteristics. Med Biol Eng Comput 2006; 44:721-8; PMID:16937214; http://dx.doi.org/ 10.1007/s11517-006-0086-5 [DOI] [PubMed] [Google Scholar]
- [30].Moran DS, Shitzer A, Pandolf KB. A physiological strain index to evaluate heat stress. Am J Physiol Regul Integr Comp Physiol 1998; 275:R129-34. [DOI] [PubMed] [Google Scholar]
- [31].Stapleton JM, Poirier MP, Flouris AD, Boulay P, Sigal RJ, Malcolm J, Kenny GP. At what level of heat load are age-related impairments in the ability to dissipate heat evident in females? PloS One 2015; 10:e0119079; PMID:25790024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stapleton JM, Poirier MP, Flouris AD, Boulay P, Sigal RJ, Malcolm J, Kenny GP. Aging impairs heat loss, but when does it matter? J Appl Physiol (1985) 2015; 118:299-309; PMID:25505030; http://dx.doi.org/ 10.1152/japplphysiol.00722.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Anderson RK, Kenney WL. Effect of age on heat-activated sweat gland density and flow during exercise in dry heat. J Appl Physiol (1985) 1987; 63:1089-94; PMID:3654456 [DOI] [PubMed] [Google Scholar]
- [34].World Meteorological Organization, World Health Organization Heatwaves and health: guidance on warning-system development. Geneva, Switzerland: World Meteorological Organization; 2015. [Google Scholar]
- [35].Kenny GP, Jay O. Thermometry, calorimetry, and mean body temperature during heat stress. Compr Physiol 2013; 3:1689-719; PMID:24265242; http://dx.doi.org/ 10.1002/cphy.c130011 [DOI] [PubMed] [Google Scholar]
- [36].Jay O, Gariepy LM, Reardon FD, Webb P, Ducharme MB, Ramsay T, Kenny GP. A three-compartment thermometry model for the improved estimation of changes in body heat content. Am J Physiol Regul Integr Comp Physiol 2007; 292:R167-75; PMID:16931653; http://dx.doi.org/ 10.1152/ajpregu.00338.2006 [DOI] [PubMed] [Google Scholar]
- [37].Sawka MN, Castellani JW. How hot is the human body? J Appl Physiol (1985) 2007; 103:419-20; PMID:17556501; http://dx.doi.org/ 10.1152/japplphysiol.00592.2007 [DOI] [PubMed] [Google Scholar]
- [38].Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol (1985) 1998; 84:1323-32; PMID:9516200 [DOI] [PubMed] [Google Scholar]
- [39].Martin HL, Loomis JL, Kenney WL. Maximal skin vascular conductance in subjects aged 5–85 yr. J Appl Physiol (1985) 1995; 79:297-301; PMID:7559234 [DOI] [PubMed] [Google Scholar]
- [40].Kenny GP, Jay O, Zaleski WM, Reardon ML, Sigal RJ, Journeay WS, Reardon FD. Postexercise hypotension causes a prolonged perturbation in esophageal and active muscle temperature recovery. Am J Physiol Regul Integr Comp Physiol 2006; 291:R580-8; PMID:16513764; http://dx.doi.org/ 10.1152/ajpregu.00918.2005 [DOI] [PubMed] [Google Scholar]
- [41].Sagawa S, Shiraki K, Yousef MK, Miki K. Sweating and cardiovascular responses of aged men to heat exposure. J Gerontol January 1988; 43:M1-8; PMID:3335747; http://dx.doi.org/ 10.1093/geronj/43.1.M1 [DOI] [PubMed] [Google Scholar]
- [42].Semenza JC, McCullough JE, Flanders WD, McGeehin MA, Lumpkin JR. Excess hospital admissions during the July 1995 heat wave in Chicago. Am J Prev Med 1999; 16:269-77; PMID:10493281; http://dx.doi.org/ 10.1016/S0749-3797(99)00025-2 [DOI] [PubMed] [Google Scholar]
- [43].Green RS, Basu R, Malig B, Broadwin R, Kim JJ, Ostro B. The effect of temperature on hospital admissions in nine California counties. I Int J Public Health 2010; 55:113-21; PMID:19771392; http://dx.doi.org/ 10.1007/s00038-009-0076-0 [DOI] [PubMed] [Google Scholar]


