ABSTRACT
The family of Transient Receptor Potential (TRP) ion channels is constituted by 7 subfamilies among which are those that respond to temperature, the thermoTRPs. These channels are versatile molecules of a polymodal nature that have been shown to be modulated in various fashions by molecules of a lipidic nature. Some of these molecules interact directly with the channels on specific regions of their structures and some of these promote changes in membrane fluidity or modify their gating properties in response to their agonists. Here, we have discussed how some of these lipids regulate the activity of thermoTRPs and included some of the available evidence for the molecular mechanisms underlying their effects on these channels.
KEYWORDS: cholesterol, lysophosphatidic acid, lysophosphatidylcholine, monounsaturated fatty acids, oleic acid, PIP2, TRP channels, thermoTRP
Introduction
Transient Receptor Potential (TRP) ion channels were first discovered in the fruit fly, Drosophila. In this organism, it was shown that the trp mutation led to the transient receptor potential phenotype1 and the gene responsible for this phenomenon was cloned by Montel and collaborators in 1989.2 Since then, the field of study of TRP channels has actively generated an extensive amount of work that has led to the elucidation of the roles of these proteins in cellular processes.
TRP channels are present from simple unicellular algae to vertebrates and involved in a wide variety of physiological functions that include taste, smell, vision, touch, audition, temperature detection, pain and itch sensations, among others.3 The TRP family of ion channels is subdivided into 7 subfamilies: TRPC (canonical), TRPA (ankyrin), TRPM (melastatin- related), TRPV (vanilloid), TRPN (no mechanoreceptor potential C), TRPP (polycystin) and TRPML (mucolipin).3
By taking advantage of the polymodal nature of several of the members of the TRP family of ion channels, organisms are able to respond to a plethora of endogenous and exogenous signals, some of these are potentially noxious.
Among TRP channels, there are those which are intrinsically sensitive to temperature, the thermoTRPs. These proteins allow organisms to detect a broad range of changes in temperature, from cold to hot and allow Ca2+ influx through the membranes of sensory neurons, leading to depolarization and action potential transmission. Neurons that allow the detection of sensory stimuli are located in the dorsal root ganglia (DRG) and in the cranial nerve ganglia of which trigeminal ganglia (TG) are part. Nociceptive neurons permit the detection of noxious chemical, mechanical and thermal signals.
Among the thermoTRPs are TRPA1, TRPM2-5, TRPM8 and TRPV1-44 and several of these channels have been associated with pathophysiological processes.5
TRP channels are not only polymodal in their activation in response to different stimuli, but their function can be modulated by molecules of diverse origins. This review will focus on the most recent advances on the study of the role of some molecules of a lipid nature on the function of thermoTRPs. The roles of arachidonic acid, diacylglycerol and polyunsaturated fatty acids (PUFAs) have been studied extensively and thus, we would like to refer the reader to a recent review on this subject.6 Thus, here we will specifically discuss how thermoTRPs are regulated by phospholipids, lysophospholipids, monounsaturated fatty acids and cholesterol.
Phosphatidylinositol 4,5-bisphosphate (PIP2)
PIP2 is an acidic phospholipid (Fig. 1) which is found in the plasma membranes of cells,7 albeit less abundantly than phosphatidylinositol monophosphate. PIP2 is cleaved by phospholipase C (PLC), resulting in the generation of inositol 3-phosphate (IP3) and diacylglycerol (DAG).6 PIP2 has been shown to regulate the function of several transporters and ion channels, including thermoTRPs, as will be discussed below.
Figure 1.
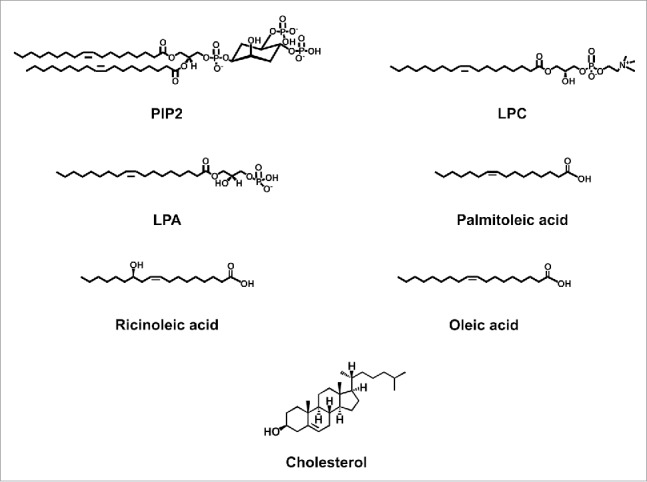
Chemical structures of lipid molecules that regulate the function of thermoTRPs.
TRPV subfamily
The TRPV1 channel is mainly expressed in small-diameter neurons from DRG and TG, although it is also found in the central nervous system and in cells from the liver, smooth muscle and immune system.8 Activation of TRPV1 by temperatures near 42°C as well as by pungent compounds such as capsaicin, resiniferatoxin and allicin,8,9 makes this channel an important target in the pathway of pain generation. PIP2 has been reported to modulate the activity of TRPV1 in an intricate fashion. In whole-cell experiments, PIP2 hydrolysis (e.g. depletion of PIP2) by PLC leads to the release of the channel from PIP2 inhibition and to its sensitization by capsaicin.10 In this study, Prescott and Julius describe a region with a stretch of positively-charged residues in the C-terminus of TRPV1 which is putatively responsible for interaction of the channel with this molecule that they describe as an inhibitor of the activity of TRPV1.10 On the other hand, several groups have described positive modulation of TRPV1 by PIP2. Stein and collaborators showed that application of PIP2 to excised inside-out membrane patches from mice DRG neurons is necessary for full activation of TRPV1 by capsaicin,11 a result that is in agreement with experiments performed by Liu et al., in TRPV1-expressing HEK293 cells, where synthesis of PIP2 is required for recovery of TRPV1 from desensitization.12 Interestingly, Lukacs and colleagues have proposed dual effects for PIP2 on TRPV1. In this scenario and in accordance with the results by Liu and collaborators,12 these authors propose that capsaicin-induced signaling desensitizes TRPV1 through the activation of PLC which results in the depletion of PIP2 in the membrane.13 The complex role of phosphoinositides on TRPV1 (and on other TRP channels) function has recently been discussed by Tibor Rohacs.14 Finally, the Gordon laboratory has shown that membrane asymmetry is a factor that must be considered in studying the apparent affinity of PIPs for TRP-channel regulation. Recent work from this group includes experiments where a water-soluble analog of PIP2 was applied to the inner and outer faces of the channel. In this case, PIP2 functions as an inhibitor when present in both the extracellular and intracellular leaflets of the membrane. Moreover, patch-fluorometry experiments using a fluorescent PIP2 analog, show that incorporation of this molecule to the extracellular leaflet of the membrane is necessary for the inhibitory effects of PIP2 on TRPV1 channel activation. They also conclude that dose-response curves for activation of TRPV1 have underestimated the selectivity of PIP2 for this channel15 and that PIP2 incorporation to the intracellular plasma membrane leaflet leads to potentiation of capsaicin-activated currents.16 Moreover, in another study by the Gordon group, it was shown that the TRPV1 channel displays specificity for regulation of capsaicin activation by PIP2 since phosphatidylinositol 4-phosphate (PI(4)P) does not support activation of the channel by this vanilloid compound.17
With respect to the molecular interactions of PIP2 with TRPV1, it has been proposed that this molecule binds to the C-terminus of the channel. However, the exact sites of interaction remain controversial. Prescott and Julius as well as Cao et al., have shown that the negatively-charged phosphate group of PIP2 interacts with a stretch of positively-charged amino acids (777-820) in the C-terminus.10,18 Other groups have proposed that PIP2 interacts with a more proximal region of the C-terminus.19 Specifically, it has been shown that the important amino acid residues for PIP2 interaction with TRPV1 include R701 and K71019 and/or R721.20,21
By utilizing a combination of mutagenesis, molecular modeling and electrophysiological techniques, Poblete and colleagues identified a PIP2 binding site located between the TRP box and the S4-S5 linker.22 These authors showed that PIP2 interacts with the amino acid residues R575 and R579 in the S4-S5 linker and with K694 in the TRP box. They also confirmed that mutations in these sites reduce PIP2 binding affinity.22 Moreover, in silico mutations of residues R575A, R579A, and K694A showed that the reduction in PIP2 binding affinity is due to a delocalization of PIP2 in the binding pocket. Molecular dynamics simulations performed by this group, suggested that PIP2 binding induces conformational rearrangements of the structure formed by S6 and the TRP domain,22 leading to the opening of the lower TRPV1 channel gate.23
With respect to the role of PIP2-regulation of TRPV1 activation by heat, Prescott and Julius also reported that this molecule is involved in the thermal sensitivity of TRPV1. These authors performed experiments using the 777-820 TRPV1 deletion-mutant TRPV1 channel and showed that this channel displayed a marked thermal threshold shift toward lower temperatures.10 They concluded that these effects where due to the alteration of interaction sites for PIP2 in the TRPV1 channel.10
Finally, Wright and collaborators found that PIP5K1C+/− mice, where PIP2 production is expected to be lower since lipid kinase PIP5K1C is an enzyme that catalyzes the generation of PIP2 by phosphorylating position 5 on the inositol ring of PtdIns(4)P), exhibited reduced sensitivity to heat.24 In contrast, Cao and collaborators performed experiments were they incorporated purified TRPV1 into artificial lipid vesicles and showed that, under conditions where phosphoinositides were added, the sensitivity of the channel to heat was decreased.25 Thus, the role of PIP2 on thermal sensitivity related to TRPV1 activation needs further clarification.
Although the focus of this review is not to detail the role of thermoTRP regulation by lipids on pathologies, it is interesting to note that, a link between PIP2 metabolism dysregulation and Alzheimer disease (AD) has been recently described and its worth mentioning here. In its early stages, AD is partly characterized by the accumulation of amyloid-β oligomeric peptides (Aβ) and synaptic modifications are associated to amnesic changes. These oligomers affect the function of several types of proteins including receptors that impact on neurotransmission and that can lead to changes in Ca2+ influx into the neurons. There are reports that directly link Aβ peptides to the disruption of PIP2 metabolism,26 thus it is not unwarranted that abnormal regulation of the activity of some thermoTRPs in neurons of the central nervous system (CNS) could contribute to the development and progression of this complex pathology, specially under a scenario where PIP2 metabolism is altered. TRPM2, a reactive oxygen species, (ROS)-sensitive channel, has been involved in oxidative stress-induced cell death27 and TRPV1 activation has been linked to neurodegeneration.6
In the CNS, the TRPV1 channel has been shown to be involved in synaptic plasticity, memory and learning since this channel is expressed in the hippocampus.28 Additionally, Mori and colleagues have shown that TRPV1 plays a role in cortical excitability by regulating glutamatergic synaptic transmission.29 In a recent study, Eslamizade et al., 2015 have suggested that Aβ could influence excitability of these neurons through modification of the activity of TRPV1.30 They conclude that dysregulation of PIP2 metabolism by Aβ could be partly responsible for neuronal death through changes in TRPV1 function.
PIP2 regulates the apparent affinity of the TRPM2 channel to Ca2+31 and, to further complicate this scenario, it has been suggested that the amyloid protein can be directly incorporated into neuronal membranes and form Ca2+-permeable ion channels (amyloid channels32), which could also contribute to increases in intracellular Ca2+ and which could further activate TRPM2 and cause neurotoxicity.
The TRPV2 channel is found in DRG and TG neurons and is activated in response to temperatures near 52°C and to the synthetic ligands 2-aminoethoxyldiphenyl borate (2-APB) and probenicid.33 For this channel it has been shown that, when the PIP2 membrane-concentration is decreased, the channel is desensitized in the presence of Ca2+ (in whole-cell recordings;34) and if PIP2 is applied to the inside-out excised patches, currents through TRPV2 can be recovered.35
The TRPV3 channel is expressed in DRG, TG and spinal cord neurons as well as in cells from the skin, tongue and brain.36 It is activated by temperatures that range between 31-39°C and by compounds such as camphor, thymol, carvacrol36 and by the endogenous lipid farnesyl pyrophosphate or FPP.37 This channel has been shown to be positively regulated by the depletion of PIP2.38 Using excised inside-out membrane patches of cells expressing TRPV3, it was demonstrated that the application of an anti-PIP2 antibody produced activation of the channel. Interestingly, higher concentrations of PIP2 applied to the intracellular face of the channel lead to decreased activity of TRPV3 and, in contrast, the application of a hydrolysis product of PIP2, phosphatidylinositol 3-phosphate (PI(3)P), resulted in an increase in the activity of the channel. Moreover, a direct interaction of PIP2 with basic residues located in the C-terminus, specifically the TRP domain, of TRPV3 was also demonstrated.38
TRPV4 is a channel activated by temperatures that range from 27–42°C, by changes in osmotic pressure and by molecules of a lipid nature which are downstream of the activity of PLC,39 among others. In this case, binding of PIP2 to the N-terminus of TRPV4 was shown to be important for activation of the channel by heat and hypotonic stimuli. By performing single-channel measurements, Garcia-Elias and colleagues showed that PIP2 is required for activation of TRPV4. Under their experimental conditions, activation of TRPV4 at 24°C was not possible independently of the presence of PIP2 while at 38°C, the open probability of the channel increased notably with the presence of this molecule at the intracellular side of the channel. These authors also performed experiments where positive charges in positions 121-125 of the N-terminus (where PIP2 may interact with TRPV4), were neutralized and showed that these are necessary for activation of the channel by heat (and hypotonicity) but not by 4α-phorbol 12,13-didecanoate. Taking advantage of fluorescence experiments where FRET (Förster Resonance Energy Transfer) was measured using TRPV4 channels coupled to fluorescent proteins, they were able to demonstrate that the N-terminal domains exist in a more compact conformation in the absence of PIP2 and, in contrast, when PIP2 was applied, the N-terminal domains expanded, leading to positive regulation of channel function.40
As discussed by Steinberg et al., PIP2 binding to TRP channels may favor reconfiguration of their structural determinants in order to promote channel activation.41 Nonetheless, it is evident that, structurally, the regulation of TRPV4 activation by PIP2 seems to involve a different mechanism to that described for TRPV1, for example, since PIP2 binds to different regions in these channels.
In contrast to what is discussed above, another group found that the binding of PIP2 to the ankyrin repeat domains (ARDs) of TRPV4, as revealed by a crystal structure of the ARD in complex with the head group of PIP2, negatively regulates TRPV4's activity.42 In light of these contrasting results, further work will be required to determine the role of PIP2 on the activity of the TRPV4 channel.
TRPA1 channels
The TRPA1 channel, is expressed by nociceptive neurons of peripheral ganglia as well as in the tissue of the inner ear.43,44 It responds to noxious cold temperatures (<18°C) and to pungent compounds such as icilin, allicin, isothiocyanates and cinnamaldehyde.45 With respect to the response of TRPA1 to PIP2, it has been shown that activation of PLC leads to a decrease in this phosphoinositide and releases TRPA1 from the PIP2-induced inhibition, consistent with a negative regulatory role for PIP2 on this channel, as found by another research group in HeLa cells expressing TRPA1.46 In contrast, another study found that the presence of a PIP2 scavenger, neomycin, promoted a faster desensitization of whole-cell TRPA1 currents in Chinese hamster ovary (CHO) cells expressing this channel.47 These authors also demonstrated that desensitization was slower in the presence of intracellular PIP2, arguing for a positive role in the regulation of TRPA1 by this molecule.
It is important to note that several types of thermoTRPs are simultaneously expressed in primary DRG cultures. Specifically, TRPA1 and TRPV1 are co-expressed by small-diameter DRG neurons. This suggests that the activity of one of these channels could regulate the activity of the other. For example, it has been shown that desensitization of TRPA1 in sensory neurons can be caused by its agonist mustard oil through a pathway that is independent of Ca2+ or by a mechanism by which TRPV1 activation by capsaicin leads to the entry of Ca2+ into the cell and promotes Ca2+-dependent depletion of PIP2.48
TRPM channels
The TRPM2 channel is expressed in the brain, spleen, heart, bone marrow, liver, leukocytes and lung49 and activated by temperatures near 37°C,50 by adenosine diphosphate ribose (ADPR), intracellular Ca2+,49,51 among other stimuli. This channel participates in glucose-induced insulin secretion, neuronal apoptosis, phagocyte activation and oxidative stress.50 The TRPM2 channel exhibits rundown of its currents in excised membrane patches. Tóth and Csanády showed that PIP2 binds to the TRPM2 channel with high affinity, since trace concentrations of PIP2 are able to sustain maximal activity of the channel, but that PIP2 does not promote channel activation per se. Moreover, these authors also showed that PIP2 regulates the apparent affinity of the channel to Ca2+ (as well as to ADPR), without changing its sensitivity to this cation.31
The TRPM3 channel is expressed in somatosensory neurons, in cells from the pancreas and kidney and in the brain.52 This channel is responsive to pregnenolone sulfate and to temperatures near 40°C, among other stimuli, and its activation results in pain responses in mice.53
At first, it was shown that PIP2 binds to the N-terminus of the TRPM3 where calmodulin also binds,54 but no function was described for this interaction. In 2015, Tóth and collaborators showed that application of PIP2 and ATP to the intracellular face of TRPM3 (in membrane excised patches) promotes recovery of the channel from desensitization. In this case, ATP functions as a stimulator of phosphatidylinositol kinases which allows for continued synthesis of PIPs in the plasma membrane to ensure channel activity.55
Uchida and collaborators showed that PIP2 is necessary for PS-induced opening of TRPM3 channels incorporated into planar lipid bilayers.56 In their experiments, although addition of PIP2 resulted in a weak activation of the channel by temperature, it could not promote a fully open channel. The authors also concluded that, under their experimental conditions, the TRPM3 channel did not exhibit a strong intrinsic sensitivity to temperature, as the one observed in other thermoTRPs.56
The TRPM4 and TRPM5 ion channels are also thermoTRPs which are activated at temperatures that range from 15-35°C and are directly gated by intracellular Ca2+ ions.57 TRPM4 can be activated by decavanadate and ATP58 and is expressed by cells in the liver, kidney, spleen, skeletal muscle, colon, heart, etc., where it mediates membrane depolarization.57 On the other hand, TRPM5 is expressed by taste receptor cells (TRCs)57 where it participates in the detection of bitter, sweet and umami tastes.59 TRPM4 depends on PIP2 for recovery from rundown but this molecule does not gate this channel.60 In a similar fashion, Nilius and collaborators also showed that PIP2 is an important positive regulator of TRPM4 and that the pleckstrin homology domain in the C-terminus of this channel is important for the actions of this molecule.61
With regard to the molecular interactions of PIP2 with the TRMP4 channel it has been shown that the TRP domain is not fundamental for the effects of this molecule62 and that interactions occur with basic amino acid residues in the pleckstrin homology domain61 rather than with negatively-charged residues.
With respect to TRPM5, Liu and Liman showed that the TRPM5 is a cation channel activated by micromolar concentrations of Ca2+ and that after activation, it undergoes fast Ca2+-dependent desensitization, which can be partially reversed by PIP2. These authors also showed that PIP2 alters TRPM5 activity by enhancing sensitivity of the channel to Ca2+.63 These authors proposed a model where, during desensitization, Ca2+ induces an allosteric change in the TRPM5 protein that lowers its affinity for PIP2 which, in turn, further promotes this conformational state of the channel.63 This model is interesting as this channel is a taste transducing molecule and, the data obtained by these authors, allows them to implicate regulation of TRPM5 by Ca2+ and PIP2 in sensory activation in the taste system.
Finally, the TRPM8 channel, a protein activated by menthol and that responds to temperatures below 25°C and is expressed in sensory neurons as well as in smooth muscle and the prostate,64 is another thermoTRP whose gating depends on PIP2. In contrast to TRPM4 and TRPM5 channels, TRPM8 channels can be opened in the presence of PIP2.65 It is well known that TRPM8 suffers rundown when the membrane is excised from the cell. This rundown can be prevented by the application of intracellular PIP2 and high concentrations (20 mM) of this phosphoinositide can promote currents through TRPM8.65
Rohacs et al., have described for TRPM8 that PIP2 putatively interacts with the TRP domain in the C-terminus of the channel. These authors performed experiments using mutations that neutralized positive charges in the proximal C-terminus of TRPM8 (K995Q, R998Q and R1008Q) and demonstrated that the mutant channels exhibited decreased sensitivity to PIP2.66
For the TRPM8 channel, contradictory results have been reported regarding for the role of PIP2 on its thermal sensitivity. Daniels and colleagues have shown that PIP2 depletion does not alter the thermal threshold of TRPM867 while Liu and Qin have shown that rundown of responses to cold, possibly due to PIP2- and other soluble factors-depletion, was slower than that observed with menthol. Based on the different effects that PIP2 has on TRPV1 and TRPM8, these authors interestingly conclude that PIP2 may act as “bimodal switch” in the control of heat and cold sensitivity in the nociceptors where they are co-expressed.65
Lysophospholipids and thermoTRPs
Lysophospholipids are molecules with several described bioactive properties that possess a single carbon chain and a polar head group.68 These membrane-derived molecules are subdivided into 2 large groups that include those that possess a sphingoid base backbone (lysosphingolipids) and those that contain a glycerol backbone (lysoglycerophospholipids). Diverse chemical and enzymatic pathways have been described for the synthesis of lysophospholipids. These include complete chemical synthesis of lysophospholipids from glycerol or derivatives, enzymatic transformation of natural glycerophospholipids using specific enzymes such as phospholipases A1, A2 (PLA2) and D as well as the coupling of enzymatic processes with chemical transformations.69 Most of the literature points to a role of these molecules on ion channels through the activation of GPCRs68 or even through the modification of the curvature of the membrane, which can influence ion channel activity.70 Next, we will describe some of the literature concerning the modulation of thermoTRPs by lysophospholipids. We will make special emphasis on lysophosphatidylcholine (LPC) and lysophosphatidic acid (LPA), a product that is synthesized from LPC through the removal of the choline group by lysophospholipase D or autotaxin.
LPC and regulation of the activity of thermoTRPs
LPC is the best-studied and most abundant lysophospholipid in nature and it is involved in the regulation of gene transcription, monocyte chemotaxis, mitogenesis, smooth muscle relaxation and platelet activation and it accumulates in tissues during ischemia and in the plasma, when inflammatory arthritis is present (reviewed in ref. 69). LPC is released from the liver as a product of hydrolysis by PLA2 or produced by the enzyme lecithin/cholesterol acyl transferase in the plasma. Importantly, LPC is converted by lysophospholipase D to LPA(reviewed in ref. 69).
The role of LPC (Fig. 1) in regulating the function of thermoTRPs has not been sufficiently explored; some of the scarce published evidence will be discussed in this section. LPC has been shown to activate TRPM8 channels in heterologous expression systems. By performing single-channel experiments, Vanden Abeele and collaborators showed that LPC increases the open probability of TRPM8 at 37°C,71 a temperature at which this channel exhibits poor activity. The mechanism by which LPC exerts its effects on TRPM8 are not yet understood but they possibly involve changes in the mechanical properties of the plasma membrane an this phenomenon has been reported by other authors.72 In a study performed by Gentry and collaborators, the authors found that the inhibition of a subtype of PLA2, iPLA2 with bromenol lactone, blocked the development of cold hypersensitivity in animals administered with intraplantar icilin.73 In contrast, inhibition of iPLA2 did not affect menthol-induced hypersensitivity. These results showed that LPC can induce cold hypersensitivity and that activity of TRPM8 in vivo, and the response to pain in the presence of noxious cold, is supported and modulated by the activation of iPLA2. Furthermore, by performing studies on genetically modified mice, the authors also demonstrated that the effects of icilin and LPC were mediated by TRPM8 but not by TRPA1.73
In fact, contrary to what has been observed for LPC, which can induce cold hypersensitivity through TRPM8,73 this lysophospholipid is thought to reduce hypersensitivity to cold through TRPA1, although the mechanisms for this phenomenon are not yet clear.73
The literature to date is also scarce as for the role of LPC on TRPV1 function. Nonetheless, it has been suggested that TRPV1 activation is partly responsible for LPC-induced monocyte migration, where Ca2+ influx through non-selective cation channels has been proposed to be important.74 However, the molecular mechanisms by which LPC produces its effects on monocyte function are no understood.
With respect to the role of LPC on TRPV1s temperature sensitivity, Cao and collaborators found that incorporating LPC into liposome patches expressing this channel did not affect the temperature activation of TRPV1.18
LPC is an endogenous activator of the TRPV2 channel that induces Ca2+ influx through this protein. By activating Gq/Go-protein and phosphatidylinositol-3,4 kinase signaling, LPC causes translocation of TRPV2 to the plasma membrane and leads to migration in cells of the prostate cancer cell line PC3.75 LPC has also been shown to cause cytotoxicity in osteoblast-like forming cells by promoting a fast and transient influx of Ca2+. In a study by Fallah and collaborators, it was suggested that this occurs through the activation of TRPV2 in these cells and it has been hypothesized that this can be a cause for osteoporosis in patients with atherosclerosis.76
In the nervous system, expression and physiological roles for TRPV2 in the glia have been explored by Shibasaki and collaborators. These authors assessed the functional expression of TRPV2 in astrocytes and determined that, when this channel was activated by LPC, large inward currents were generated. This response was greatly diminished in astrocytes transfected with a dominant-negative form of TRPV2 and thus they suggested that astrocytic TRPV2 might regulate neuronal electrical activity in response to lipid metabolism.77
A link between LPC and AD has also been suggested. Sheikh and colleagues have shown that LPC can increase the oligomer formation process of the Aβ which promotes an increase in ROS. Moreover, ROS production is also further enhanced by LPC. If not through direct effects of LPC on thermoTRP channels, this lysophospholipid can alter pathways (such as the one related to Aβ formation) which, in turn, impacts on PIP2 metabolism that directly regulates the activities of of some thermoTRP channels.78
LPA and thermoTRPs
LPA is constituted by an 18-carbon acyl chain, with a single unsaturation and a charged phosphate group79 (Fig. 1), which is produced from LPC (see above). LPA has been shown to function as an important intermediate in transmembrane signal transduction processes mediated by platelet activating factor and in the stimulation of cell proliferation. It thus regulates the development and function of the nervous, immune, cardiovascular and reproductive systems (reviewed ref. 69).
An important regulator of axonal growth during the development of the brain is intracellular Ca2+ signaling. In this sense, Ca2+ influx through TRP channels can potentially be important for the development of the brain and for other functions in this organ. The TRPM2 channel is abundantly expressed in the embryonic brain and pharmacological inhibition of these channels or it´s knockdown results in an increased axonal growth. For example, it has been observed that neurons from the brains of Trpm2−/− mice have longer neurites than neurons from the brains of wild-type mice.80 Conversely, overexpression of this channel inhibits axonal growth. In vitro, administration of ADPR, which activates TRPM2, to PC12 cells leads to suppressed axonal growth.80 It has also been found that TRPM2 participates in the neuronal retraction induced by cerebrospinal fluid-rich LPA, pointing to a role of LPA on neuronal development through actions on TRPM2 channels.
A role for LPA on TRPV1 channels was described by Pan and collaborators in 2010. These authors used a bone cancer model (where LPA levels are increased) generated by injecting mammary gland carcinoma cells into the rat tibia. After bone cancer pain was developed, they studied TRPV1 expression levels as well as capsaicin-evoked currents and found these were up- regulated in DRG neurons. They also suggested that capsaicin-induced currents were potentiated by LPA. By performing biochemical assays, the authors also concluded that LPA potentiates TRPV1 currents through a protein kinase C epsilon-dependent pathway and proposed this as a novel peripheral mechanism responsible for the induction of bone cancer pain.81
Our research group also performed experiments to determine the role of LPA on the function of TRPV1 channels. LPA has been long proposed to participate in neuropathic pain and has been suggested to do so by interacting with specific GPCRs in the membranes of neurons and TRPV1 has also been extensively linked to this type of pain. With this in mind, we first determined that in an animal model where the lipid phosphatase 3, which breaks down LPA, was knocked-out in the peripheral nervous system, mice displayed exacerbated responses to heat and capsaicin-injection. We also found that injection of LPA itself to the paws of wild-type mice produced pain-related behavior, which was ablated in Trpv1−/− animals. We then sought to determine the molecular mechanism underlying these effects. We tested for activation of TRPV1 channels expressed in a heterologous expression system (HEK293) cells and found that channels could be activated by LPA in a dose-dependent fashion. By inhibiting GPCR- related signaling pathways, we found that LPA did not act through these receptors to modulate TRPV1 function.
Site-directed mutagenesis as well as biochemical experiments revealed that LPA could directly bind to the TRPV1 channel. Our study revealed that the charged phosphate group of LPA could interact with a positively charged residue in the proximal carboxyl terminus of the channel (K710) and lead to the opening of TRPV1.82 We thus concluded that LPA could directly interact with TRPV1 to produce pain.
Monounsaturated fatty acids and thermoTRPs
The following section will discuss the less studied role of monounsaturated fatty acids (MUFAs) on the physiology of these channels.
MUFAs are molecules that have one double bond in their acyl chain and the rest of the carbon atoms are single-bonded. Common MUFAs are: palmitoleic, myristoleic, ricinoleic, elaidic, petroselinic, erucic, nervonic and oleic acids. Very few of these have been shown to exert changes in thermoTRPs functions, as discussed below.
Palmitoleic acid
Palmitoleic acid (Fig. 1) is a common constituent of glycerides in the human adipose tissue and specially found in the liver. It is present in a variety of plant and animal oils such as macadamia oil.
The TRPV1 channel has been shown to play important roles in neurogenic inflammation and acute pancreatitis. TRPV1 is activated by ethanol and a recent study has shown that, in a mouse model, administration of ethanol and palmitoleic acid (POA), which is a non-oxidative metabolite of ethanol, causes acute pancreatitis, a phenomenon that is not observed neither in Trpv1−/− mice nor in wild-type mice injected with ethanol or POA separately.83 The mechanism by which POA and ethanol together cause acute pancreatitis in this animal model is not yet completely understood.
Ricinoleic acid
Pro- and anti-inflammatory effects of ricinoleic acid (RA, Fig. 1), a fatty acid commonly found in castor oil, have been investigated in an experimental model of blepharitis (inflammation of the eyelids). Using guinea pigs, Vieira et al., used topical treatment with RA or capsaicin and reported that this caused eyelid reddening and edema. These authors also used dissociated rat DRG neurons and observed that RA significantly inhibited the inward currents induced by capsaicin and that it failed to induce currents when applied by itself,84 similar to what we observed in HEK293 cells expressing TRPV1.85 The authors thus concluded that RA does not induce TRPV1-dependent inward currents in DRG neurons and that, it in itself, lacks algesic properties in vivo and that it rather acts by decreasing calcitonin gene-related peptide release induced by capsaicin after a 24 hr treatment in DRG neurons.84
Oleic acid
Oleic acid (OA), a naturally occurring long-chain monounsaturated fatty acid that resembles LPA both in acyl chain composition and in that it is negatively charged. It is commonly found in oils of plant origin as well as in animal cells.86
With respect to the role of OA in the regulation TRP-channel function, in 2007, Matta and collaborators demonstrated, using 2-electrode voltage-clamp experiments in TRPV1-expressing Xenopus oocytes that relatively high concentrations of OA (50 μM) could achieve small antagonistic effects upon capsaicin activation of this channel.87
Most recently, we have shown that OA can significantly inhibit responses of heterologously- or natively-expressed TRPV1 channels in excised membrane patches of cells expressing these proteins. In our study, we performed experiments where we applied OA to both the intracellular and extracellular faces of the channel and this resulted in an almost complete inhibition of the channel's response to capsaicin. Moreover, we found that activation of TRPV1 by intracellular acid pH, LPA and temperature was also inhibited. By closely examining the molecular mechanism underlying the inhibition of TRPV1 by OA we found, in single-channel experiments, that OA produced a decrease in the open probability of the channel and that this occurred through the stabilization of a closed state of TRPV1. Using a combination of site-directed mutagenesis, biochemical and electrophysiological techniques, we found that OA interacts with the vanilloid binding pocket (VBP) of TRPV1.88 By comparing the sequences of the chick TRPV1 channel (CkTRPV1) that is insensitive to capsaicin with that of the rat TRPV1 channel, we found that a residue in the VBP, threonine in position 550 (T550), which corresponds to an alanine in position A558 of the CkTRPV1 channel, importantly contributes to the binding of OA in TRPV1. This is consistent with the observation that in the CkTRPV1, OA inhibits only a fraction of the pH-induced TRPV1 currents and that a mutant CkTRPV1 in which A558 is substituted for a threonine (as in the rat TRPV1 channel), now renders the chick channels sensitive to OA. We further extended our study and showed that injection of OA into mice paws and necks, could inhibit pain and itch responses elicited by capsaicin and by histamine or cyclic phosphatidic acid (cPA), a novel itch-inducer which we also described in this study.88
Another compound similar to oleic acid is nitrooleic acid, an oxidized lipid which is generated during inflamamation-induced nitrative stress. This compound activates TRPA1 and TRPV1 channels in DRG neurons,89 possibly by a mechanism that involves covalent modification of cysteine residues.
Cholesterol
Cholesterol is formed by a rigid structure region with 4 hydrocarbon rings, a hydroxyl group at position 3 and a side chain at carbon 17 (Fig. 1). Cholesterol is an important component of membrane structure90 and a molecule that actively modulates ion channel function.
Cholesterol and TRPM channels
The TRPM3 channel is not only important for noxious heat perception (∼40°C)53 but it also plays pivotal roles in the vascular system where its activation promotes contraction and suppresses cytokine secretion from vascular smooth muscle cells.91 In these cells, it has been shown that the depletion of cholesterol by methyl-β-cyclodextrin (MβCD) results in TRPM3 activation in the absence of its agonists and that cholesterol abolishes TRPM3 activation by pregnenolone sulfate.91 From this results it was concluded that endogenous cholesterol maintains TRPM3 in a partially inhibited state and that conditions were there is cholesterol overload, e.g., as in atherosclerothic processes, TRPM3 function could be completely inhibited in the cardiovascular system, resulting in failed cell contraction and secretion of proinflammatory cytokines.91 The mechanisms by which cholesterol inhibits TRPM3 activity remains unclear and further efforts must be directed to understand them.
As for the TRPM8 channel, it has been shown that it is also sensitive to cholesterol content in the plasma membrane. In HEK293 cells and DRG neurons, segregation of TRPM8 into lipid rafts92 is important for channel function since lipid raft disruption through the depletion of cholesterol enhances TRPM8 activation by menthol. It has also been shown that when TRPM8 is located outside of cholesterol-rich micro domains, the thermal threshold for activation of TRPM8 is modified, consistent with the observation that depletion of cholesterol shifts the temperature activation curve of TRPM8 toward warm temperatures.92 Another report suggests that cholesterol reduction stabilizes TRPM8 in the plasma membrane, increasing the number of channels in the membrane without modifying biophysical properties such as its open probability or unitary conductance.93
Regulation of TRPV channels by cholesterol
Since the TRPV1 ion channel is widely linked to pain and other disorders, the discovery of molecules that influence TRPV1 activity has received considerable attention and contrasting evidence as to the effects of this molecule on TRPV1 function have been described.
The role of cholesterol on TRPV1 regulation was initially resolved in DRG neurons by performing experiments where the plasma membrane of these cells was depleted of cholesterol. In these experiments it was observed that capsaicin-dependent TRPV1 currents were significantly reduced as compared to untreated cells.94 By analyzing the level of immunoreactivity to TRPV1 in DRG neurons, it was concluded that cholesterol depletion resulted in a reduction of the number of ion channels in these cells.94
In another study, our group evaluated the effects of cholesterol on TRPV1 function in excised membranes patches of HEK293 cells transiently expressing rat TRPV1 channels. In these experiments, we incubated the membrane patches with a MβCD:cholesterol mix and found that, under these experimental conditions, there was a significant reduction in the magnitude of TRPV1 currents, as compared to control cells.95 This inhibition of TRPV1 activity by cholesterol did not produce changes in the open probability or the conductance of the channel and the reduction of TRPV1 currents by cholesterol was attributed to a decrease on the number of functional ion channels in the membrane patch, as analyzed by noise analysis and single channel experiments.95
By performing site-directed mutagenesis and biochemical assays, we showed that cholesterol interacted with a CRAC (cholesterol recognition amino acid consensus)-like motif in the S5 transmembranal region of TRPV1.95 The functional characterization of the rat TRPV1 CRAC motif revealed that mutations of the arginine (R579Q) or phenylalanine (F582Q) residues yield channels where cholesterol inhibitory effects are less pronounced. Furthermore, when we replaced a leucine residue for isoleucine (L585I), the inhibitory effects of cholesterol on rat TRPV1 activation by capsaicin, were abolished.
Docking simulations that we have now performed using the recently published structure of TRPV1 (Fig. 2) suggest that, the leucine at position 585, as well as other hydrophobic residues in the S5, promote the presence of a cavity into which the aliphatic tail of cholesterol can introduce itself, thus stabilizing the lipid-protein interaction. Furthermore, we propose that the hydroxyl group of cholesterol interacts electrostatically with the arginine residue at position 579 and the phenylalanine at position 582 allows for cholesterol stacking (Fig. 2).
Figure 2.
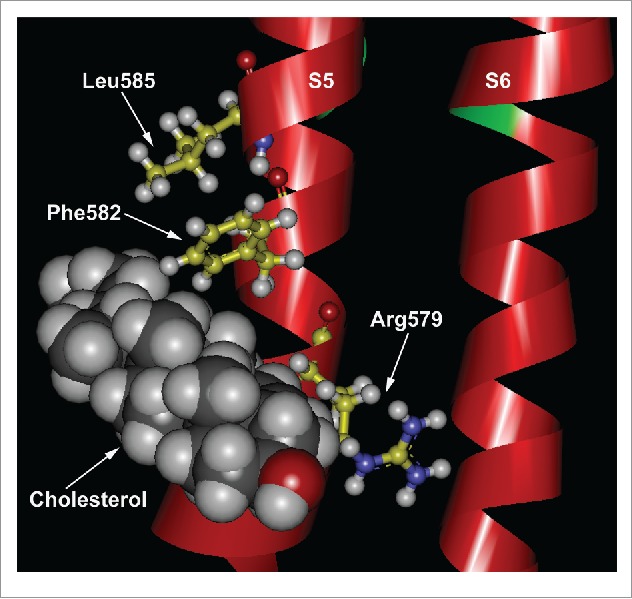
Colesterol interacts with a CRAC motif in the TRPV1 channel. Docking simulation of cholesterol's interactions with the CRAC motif in the S5 transmembrane segment of TRPV1. The cryo-EM structure of TRPV1 in the closed state (PDB 3J5P, chain A) was used and constructed using UCSD-Chimera.
It is also interesting to note that, in this study, we also observed that there were species-specific interactions of cholesterol with TRPV1. While the rat TRPV1 was efficiently inhibited by cholesterol, we observed that the human TRPV1 channel was only partially inhibited by cholesterol. We attributed these differences to the presence of a valine at residue 585 rather than a leucine and our experimental data confirmed this hypothesis since a mutant construct of the human TRPV1 channel where V585 was replaced by L585, rendered the human TRPV1 channels sensitive to cholesterol.95
Another study suggested that cholesterol depletion from the plasma membrane modifies the permeability properties of TRPV1 channels96 by decreasing the size of the ion conducting pathway of TRPV1. Thus, TRPV1 ion channels expressed in cells with high cholesterol content should allow for the flow of large cations. In our study,95 we did not perform experiments to explore the relationship between cholesterol and pore dilation. Nonetheless, a possible explanation for the fact that we did not observe this phenomenon is the different recording conditions. While Jansson and collaborators used whole-cell recordings, we used excised membrane patches. Moreover, our experiments were performed using symmetrical Na+ as a permeant ion, precluding the observation of a change in the reversal potential, as suggested for pore dilation.96 It is also possible that in whole-recording experiments pore dilation can be observed due to existent interactions between the channel and/or the cytoskeleton or a soluble modulatory factor.
For the TRPV3 ion channel, cholesterol and farnesyl pyrophosphate or FPP, an intermediary of the same metabolic mevalonate pathway, act as positive endogenous regulators of TRPV3 function.97
Enrichment of membranes expressing TRPV3 with cholesterol modified the sensitivity of this channel to its agonists (2-APB, carvacrol and thymol), resulting in a leftward shift of the dose response curves to these. Other experiments showed that cholesterol enrichment increases heat-activated TRPV3 current densities.97
Although the TRPV4 has a CRAC-like motif in its sequence, no functional relevance for this feature has been demonstrated.98 However, TRPV4 co-localizes with caveolin-1, indicating that TRPV4 is present in caveolae, and this is highly dependent upon cholesterol content since the treatment with MβCD disrupts localization of TRPV4 in the plasma membrane channel.98 Nevertheless, neither the functional relevance nor the molecular mechanism underlying this phenomenon have been clarified as of yet.
As discussed here, thermoTRPs are regulated by several lipids and Table 1 complies the effects of the molecules discussed in this review.
Table 1.
Summary of tissue distribution, physiological relevance and lipid regulation of thermoTRP channels.
| Channel | Tissue distribution | Physiological relevance | Positive lipid regulation | Negative lipid regulation | |
|---|---|---|---|---|---|
| TRPA1 (<18°C) | Peripheral sensory neurons, inner ear, ovary, spleen, testis43,44 | Noxious chemical and cold sensation; mechanical and inflammatory thermal hyperalgesia43,45 | PIP247 LPC73 | ||
| TRPM2 (∼37°C) | Fetal and adult brain, placenta, spleen, heart, bone marrow, liver, leukocytes, lung49 | Heat sensation, glucose-induced insulin secretion;50 neuronal apoptosis;80 phagocyte activation; oxidative stress.27 | PIP231 | LPA80 | |
| TRPM3 (∼40°C) | Somatosensory neurons, pancreas, kidney, brain, pituitary52 | Response to steroids (pregnenolone sulfate); noxious heat53 | PIP255 | Cholesterol92,93 | |
| TRPM4 (15–35°C) | Liver, kidney, spleen, skeletal muscle, colon, heart, brain, vascular endothelium, prostate, testis57 | Temperature sensation57 | PIP261 | ||
| TRPM5 (15–35°C) | Taste receptor cells, intestine, liver, lung57 | Detection of bitter, sweet, umami tastes; temperature sensation57 | PIP263 | ||
| TRPM8 (<25°C) | Peripheral sensory neurons, smooth muscle, prostate, liver64 | Noxious cold and chemical sensation64 | PIP265,67 LPC71-73 | Cholesterol92, 93 | |
| TRPV1 (∼42°C) | Spinal cord, brain, peripheral sensory neurons8 | Noxious chemical and heat sensation; inflammatory thermal hyperalgesia8 | PIP211-15,17 LPC74 LPA82 | PIP210,13-15Cholesterol95 POA83 RA84 OA88 | |
| TRPV2 (∼52°C) | Spleen, lung, spinal cord, brain, peripheral sensory neurons34 | Noxious heat sensation27 | PIP227,28 | LPC69-71 | |
| TRPV3 (31–39°C) | Peripheral sensory spinal cord neurons, skin, tongue, brain29 | Temperature and chemical sensation29 | PIP231 Cholesterol17 | ||
| TRPV4 (22–42°C) | Brain, liver, fat, kidney, heart, salivary gland, testis, trachea32 | Pressure sensing (DRG), central nervous system osmotic sensing, nociception, warm temperature sensing32 | PIP233,34 Cholesterol91 | PIP235 |
The temperatures at which each channel is activated are shown below each channel name. Some lipids are shown as positive and/or negative regulators of channel function due to contrasting experimental results from different research groups. Positive and negative regulation of ion channels include mechanisms where the lipids interact directly with the proteins, membrane trafficking, or signaling pathways affecting the activity of the channels.
Final remarks
During the last decade, the field of study of TRP channels has experienced and explosion on research related to clarify the roles of lipids on the function of these proteins. In some cases, the information available has reported contrasting results and further studies are needed to fully understand how these molecules promote changes in channel function. Moreover, although the regulation of TRP channels by some lipids has been demonstrated to have clear effects on the physiology/pathophysiology of an organism (as is the case of LPA that can produce pain through the activation of the TRPV1 channel), in many cases it has not been clearly established how this regulation impacts the function of the channels in all the tissues. Thus, we will have to direct new efforts toward understanding how complex regulation of TRP channels by these molecules affects physiological processes.
Abbreviations
- 2-APB
2-aminoethoxyldiphenyl borate
- ADPR
Adenosine Diphosphate Ribose
- ARD
Ankyrin Repeat Domain
- C-terminus
Carboxyl terminus
- CkTRPV1
Chick TRPV1 channel
- CRAC
Cholesterol Recognition Amino Acid Consensus
- DAG
Diacylglycerol
- DRG
Dorsal Root Ganglia
- FPP
farnesyl pyrophosphate
- GPCR
G protein-Coupled Receptor
- HEK293
Human embryonic kidney cells 293
- IP3
Inositol 3-phosphate
- LPA
Lysophosphatidic acid
- LPC
Lysophosphaditylcholine
- MβCD
Methyl-Beta-Cyclodextrin
- MUFA
Monounsaturated fatty acid
- N-terminus
N-terminus
- OA
Oleic cid
- PC12
Pheochromocytoma
- PIP
Phosphoinositide
- PIP2
Phosphatidylinositol 4,5-biphosphate
- PI(3)P
phosphatidylinositol 3-phosphate
- PtdIns(4)P
phosphaditylinositol 4-phosphate
- PIP5K1C
Phosphatidylinositol 4-phosphate 5-kinase type-1 gamma
- PLA2
Phospholipase A2
- PLC
Phospholipase C
- PS
Pregnenolone sulfate
- POA
Palmitoleic acid
- PUFA
Polyunsatured fatty acid
- RA
Ricinoleic acid
- TG
Trigeminal Ganglia
- TRP
Transient Receptor Potential
- TRPA
Transient Receptor Potential-Ankyrin
- TRPC
Transient Receptor Potential-Canonical
- TRPM
Transient Receptor Potential-Melastatin
- TRPML
Transient Receptor Potential-Mucolipin
- TRPN
Transient Receptor Potential-No mechanoreceptor potential C
- TRPP
Transient Receptor Potential-Polycistin
- TRPV
Transient Receptor Potential-Vanilloid
- VBP
Vanilloid binding pocket
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Itzel Alejandra Llorente Gil, Tonatiuh Figueroa Vanegas, Ana Escalante and Francisco Pérez at Instituto de Fisiología Celular, UNAM for expert technical support. We thank León Islas at Facultad de Medicina, UNAM for valuable comments.
Funding
This work was supported by grants from Dirección General de Asuntos del Personal Académico (DGAPA)- Programa de Apoyo a Proyectos de Investigación e Inovación Tecnológica (PAPIIT) IN200314, Consejo Nacional de Ciencia y Tecnología (CONACyT) CB-2014-01-238399 and a grant from Fronteras en la Ciencia No. 77 from CONACyT to T.R; DGAPA-PAPIIT IA202815 to S.L.M.L; CONACyT 256767 and DGAPA-PAPIIT IA201513 to L.L.
References
- [1].Pak WL, Grossfield J, Arnold KS. Mutants of the visual pathway of drosophila melanogaster. Nature 1970; 227:518-20; PMID:5428475; http://dx.doi.org/ 10.1038/227518b0 [DOI] [PubMed] [Google Scholar]
- [2].Montell C, Rubin GM. Molecular characterization of the drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron 1989; 2:1313-23; PMID:2516726; http://dx.doi.org/ 10.1016/0896-6273(89)90069-X [DOI] [PubMed] [Google Scholar]
- [3].Venkatachalam K, Montell C. Trp channels. Annu Rev Biochem 2007; 76:387-417; PMID:17579562; http://dx.doi.org/ 10.1146/annurev.biochem.75.103004.142819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang H, Siemens J. Trp ion channels in thermosensation, thermoregulation and metabolism. Temperature 2015; 2:178-87; PMID:27227022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Smani T, Shapovalov G, Skryma R, Prevarskaya N, Rosado JA. Functional and physiopathological implications of trp channels. Biochim Biophys Acta 2015; 1853:1772-82; PMID:25937071; http://dx.doi.org/ 10.1016/j.bbamcr.2015.04.016 [DOI] [PubMed] [Google Scholar]
- [6].Taberner FJ, Fernandez-Ballester G, Fernandez-Carvajal A, Ferrer-Montiel A. Trp channels interaction with lipids and its implications in disease. Biochim Biophys Acta 2015; 1848:1818-27; PMID:25838124; http://dx.doi.org/ 10.1016/j.bbamem.2015.03.022 [DOI] [PubMed] [Google Scholar]
- [7].Suh BC, Hille B. Pip2 is a necessary cofactor for ion channel function: How and why? Annu Rev Biophys 2008; 37:175-95; PMID:18573078; http://dx.doi.org/ 10.1146/annurev.biophys.37.032807.125859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997; 389:816-24; PMID:9349813; http://dx.doi.org/ 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- [9].Salazar H, Llorente I, Jara-Oseguera A, Garcia-Villegas R, Munari M, Gordon SE, Islas LD, Rosenbaum T. A single n-terminal cysteine in trpv1 determines activation by pungent compounds from onion and garlic. Nat Neurosci 2008; 11:255-61; PMID:18297068; http://dx.doi.org/ 10.1038/nn2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Prescott ED, Julius D. A modular pip2 binding site as a determinant of capsaicin receptor sensitivity. Science 2003; 300:1284-8; PMID:12764195; http://dx.doi.org/ 10.1126/science.1083646 [DOI] [PubMed] [Google Scholar]
- [11].Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to trpv1 and mediates ngf-stimulated trpv1 trafficking to the plasma membrane. J Gen Physiol 2006; 128:509-22; PMID:17074976; http://dx.doi.org/ 10.1085/jgp.200609576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor trpv1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci 2005; 25:4835-43; PMID:15888659; http://dx.doi.org/ 10.1523/JNEUROSCI.1296-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of trpv1 by phosphoinositides. J Neurosci 2007; 27:7070-80; PMID:17596456; http://dx.doi.org/ 10.1523/JNEUROSCI.1866-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rohacs T. Phosphoinositide signaling in somatosensory neurons. Adv Biol Regul 2016; 61:2-16; PMID:26724974; http://dx.doi.org/ 10.1016/j.jbior.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Collins MD, Gordon SE. Short-chain phosphoinositide partitioning into plasma membrane models. Biophys J 2013; 105:2485-94; PMID:24314079; http://dx.doi.org/ 10.1016/j.bpj.2013.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Senning EN, Collins MD, Stratiievska A, Ufret-Vincenty CA, Gordon SE. Regulation of trpv1 ion channel by phosphoinositide (4,5)-bisphosphate: The role of membrane asymmetry. J Biol Chem 2014; 289:10999-1006; PMID:24599956; http://dx.doi.org/ 10.1074/jbc.M114.553180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Klein RM, Ufret-Vincenty CA, Hua L, Gordon SE. Determinants of molecular specificity in phosphoinositide regulation. Phosphatidylinositol (4,5)-bisphosphate (pi(4,5)p2) is the endogenous lipid regulating trpv1. J Biol Chem 2008; 283:26208-16; PMID:18574245; http://dx.doi.org/ 10.1074/jbc.M801912200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. Trpv1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron 2013; 77:667-79; PMID:23439120; http://dx.doi.org/ 10.1016/j.neuron.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brauchi S, Orta G, Mascayano C, Salazar M, Raddatz N, Urbina H, Rosenmann E, Gonzalez-Nilo F, Latorre R. Dissection of the components for pip2 activation and thermosensation in trp channels. Proc Natl Acad Sci U S A 2007; 104:10246-51; PMID:17548815; http://dx.doi.org/ 10.1073/pnas.0703420104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ufret-Vincenty CA, Klein RM, Hua L, Angueyra J, Gordon SE. Localization of the pip2 sensor of trpv1 ion channels. J Biol Chem 2011; 286:9688-98; PMID:21224382; http://dx.doi.org/ 10.1074/jbc.M110.192526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ufret-Vincenty CA, Klein RM, Collins MD, Rosasco MG, Martinez GQ, Gordon SE. Mechanism for phosphoinositide selectivity and activation of trpv1 ion channels. J Gen Physiol 2015; 145:431-42; PMID:25918361; http://dx.doi.org/ 10.1085/jgp.201511354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Poblete H, Oyarzun I, Olivero P, Comer J, Zuniga M, Sepulveda RV, Baez-Nieto D, Gonzalez Leon C, Gonzalez-Nilo F, Latorre R. Molecular determinants of phosphatidylinositol 4,5-bisphosphate (pi(4,5)p2) binding to transient receptor potential v1 (trpv1) channels. J Biol Chem 2015; 290:2086-98; PMID:25425643; PMID:24305160; http://dx.doi.org/ 10.1074/jbc.M114.613620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liao M, Cao E, Julius D, Cheng Y. Structure of the trpv1 ion channel determined by electron cryo-microscopy. Nature 2013; 504:107-12; http://dx.doi.org/ 10.1038/nature12822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wright BD, Loo L, Street SE, Ma A, Taylor-Blake B, Stashko MA, Jin J, Janzen WP, Frye SV, Zylka MJ. The lipid kinase pip5k1c regulates pain signaling and sensitization. Neuron 2014; 82:836-47; PMID:24853942; http://dx.doi.org/ 10.1016/j.neuron.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. Trpv1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron 2013; 77:667-79; PMID:23439120; http://dx.doi.org/ 10.1016/j.neuron.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Berman DE, Dall'Armi C, Voronov SV, McIntire LB, Zhang H, Moore AZ, Staniszewski A, Arancio O, Kim TW, Di Paolo G. Oligomeric amyloid-beta peptide disrupts phosphatidylinositol-4,5-bisphosphate metabolism. Nat Neurosci 2008; 11:547–54; PMID:18391946; http://dx.doi.org/10.1038/nn.2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, et al. Ltrpc2 ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 2002; 9:163–73; PMID:11804595; http://dx.doi.org/10.1016/S1097-2765(01)00438-5 [DOI] [PubMed] [Google Scholar]
- [28]. Roberts JC, Davis JB, Benham CD. [3h]resiniferatoxin autoradiography in the cns of wild-type and trpv1 null mice defines trpv1 (vr-1) protein distribution. Brain Res 2004; 995:176–83; PMID:14672807; http://dx.doi.org/10.1016/j.brainres.2003.10.001 [DOI] [PubMed] [Google Scholar]
- [29]. Mori F, Ribolsi M, Kusayanagi H, Monteleone F, Mantovani V, Buttari F, Marasco E, Bernardi G, Maccarrone M, Centonze D. Trpv1 channels regulate cortical excitability in humans. J Neurosci 2012; 32:873–79; PMID:22262885; http://dx.doi.org/10.1523/JNEUROSCI.2531-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Eslamizade MJ, Saffarzadeh F, Mousavi SM, Meftahi GH, Hosseinmardi N, Mehdizadeh M, Janahmadi M. Alterations in ca1 pyramidal neuronal intrinsic excitability mediated by ih channel currents in a rat model of amyloid beta pathology. Neuroscience 2015; 305:279–92; PMID:26254243; http://dx.doi.org/10.1016/j.neuroscience.2015.07.087 [DOI] [PubMed] [Google Scholar]
- [31]. Toth B, Csanady L. Pore collapse underlies irreversible inactivation of trpm2 cation channel currents. Proc Natl Acad Sci U S A 2012; 109:13440–13445; PMID:22847436; http://dx.doi.org/10.1073/pnas.1204702109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Xu C, Macciardi F, Li PP, Yoon IS, Cooke RG, Hughes B, Parikh SV, McIntyre RS, Kennedy JL, Warsh JJ. Association of the putative susceptibility gene, transient receptor potential protein melastatin type 2, with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet 2006; 141B:36–43; PMID:16252251; http://dx.doi.org/10.1002/ajmg.b.30239 [DOI] [PubMed] [Google Scholar]
- [33]. Benham CD, Gunthorpe MJ, Davis JB. Trpv channels as temperature sensors. Cell Calcium 2003; 33:479–87; PMID:12765693; http://dx.doi.org/10.1016/S0143-4160(03)00063-0 [DOI] [PubMed] [Google Scholar]
- [34]. Bender FL, Mederos YSM, Li Y, Ji A, Weihe E, Gudermann T, Schafer MK. The temperature-sensitive ion channel trpv2 is endogenously expressed and functional in the primary sensory cell line f-11. Cell Physiol Biochem 2005; 15(1-4):183–94; PMID:15665528; http://dx.doi.org/10.1159/000083651 [DOI] [PubMed] [Google Scholar]
- [35]. Mercado J, Gordon-Shaag A, Zagotta WN, Gordon SE. Ca2+-dependent desensitization of trpv2 channels is mediated by hydrolysis of phosphatidylinositol 4,5-bisphosphate. J Neurosci 2010; 30:13338–47; PMID:20926660; http://dx.doi.org/10.1523/JNEUROSCI.2108-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Nilius B, Biro T, Owsianik G. Trpv3: Time to decipher a poorly understood family member! J Physiol 2014; 592:295–304; PMID:23836684; http://dx.doi.org/10.1113/jphysiol.2013.255968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. Farnesyl pyrophosphate is a novel pain-producing molecule via specific activation of trpv3. J Biol Chem 2010; 285:19362–71; PMID:20395302; http://dx.doi.org/10.1074/jbc.M109.087742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Doerner JF, Hatt H, Ramsey IS. Voltage- and temperature-dependent activation of trpv3 channels is potentiated by receptor-mediated pi(4,5)p2 hydrolysis. J Gen Physiol 2011; 137:271–88; PMID:21321070; http://dx.doi.org/10.1085/jgp.200910388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. White JP, Cibelli M, Urban L, Nilius B, McGeown JG, Nagy I. Trpv4: Molecular conductor of a diverse orchestra. Physiol Rev 2016; 96:911–73; PMID:27252279; http://dx.doi.org/10.1152/physrev.00016.2015 [DOI] [PubMed] [Google Scholar]
- [40]. Garcia-Elias A, Mrkonjic S, Pardo-Pastor C, Inada H, Hellmich UA, Rubio-Moscardo F, Plata C, Gaudet R, Vicente R, Valverde MA. Phosphatidylinositol-4,5-biphosphate-dependent rearrangement of trpv4 cytosolic tails enables channel activation by physiological stimuli. Proc Natl Acad Sci U S A 2013; 110:9553–8; PMID:23690576; http://dx.doi.org/10.1073/pnas.1220231110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Steinberg X, Lespay-Rebolledo C, Brauchi S. A structural view of ligand-dependent activation in thermotrp channels. Front Physiol 2014; 5:171; PMID:24847275; http://dx.doi.org/10.3389/fphys.2014.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Takahashi N, Hamada-Nakahara S, Itoh Y, Takemura K, Shimada A, Ueda Y, Kitamata M, Matsuoka R, Hanawa-Suetsugu K, Senju Y, et al. . Trpv4 channel activity is modulated by direct interaction of the ankyrin domain to pi(4,5)p(2). Nat Commun 2014; 5:4994; PMID:25256292; http://dx.doi.org/10.1038/ncomms5994 [DOI] [PubMed] [Google Scholar]
- [43]. Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, Amalfitano A, Cheung EL, Derfler BH, Duggan A, et al. Trpa1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 2004; 432:723–730. [DOI] [PubMed] [Google Scholar]
- [44]. Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al. . Anktm1, a trp-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003; 112:819–29; PMID:12654248; http://dx.doi.org/10.1016/S0092-8674(03)00158-2 [DOI] [PubMed] [Google Scholar]
- [45]. Kamakura T, Ishida Y, Nakamura Y, Yamada T, Kitahara T, Takimoto Y, Horii A, Uno A, Imai T, Okazaki S, et al. . Functional expression of trpv1 and trpa1 in rat vestibular ganglia. Neurosci Lett 2013; 552:92–7; PMID:23916509; http://dx.doi.org/10.1016/j.neulet.2013.07.019 [DOI] [PubMed] [Google Scholar]
- [46]. Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, et al. A trp channel that senses cold stimuli and menthol. Cell 2002; 108:705–715. [DOI] [PubMed] [Google Scholar]
- [47]. Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel trpa1 is activated by pungent compounds and bradykinin. Neuron 2004; 41:849–57; PMID:15046718; http://dx.doi.org/10.1016/S0896-6273(04)00150-3 [DOI] [PubMed] [Google Scholar]
- [48]. Kim D, Cavanaugh EJ, Simkin D. Inhibition of transient receptor potential a1 channel by phosphatidylinositol-4,5-bisphosphate. Am J Physiol Cell Physiol 2008; 295:C92–9; PMID:18495815; http://dx.doi.org/10.1152/ajpcell.00023.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Karashima Y, Prenen J, Meseguer V, Owsianik G, Voets T, Nilius B. Modulation of the transient receptor potential channel trpa1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflugers Arch 2008; 457:77–89; PMID:18461353; http://dx.doi.org/10.1007/s00424-008-0493-6 [DOI] [PubMed] [Google Scholar]
- [50]. Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential trpa1 channel desensitization in sensory neurons is agonist dependent and regulated by trpv1-directed internalization. J Physiol 2007; 583:175–93; PMID:17584831; http://dx.doi.org/10.1113/jphysiol.2007.133231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, et al. . Adp-ribose gating of the calcium-permeable ltrpc2 channel revealed by nudix motif homology. Nature 2001; 411:595–9; PMID:11385575; http://dx.doi.org/10.1038/35079100 [DOI] [PubMed] [Google Scholar]
- [52]. Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, Tominaga M. Trpm2 activation by cyclic adp-ribose at body temperature is involved in insulin secretion. EMBO J 2006; 25:1804–15; PMID:16601673; http://dx.doi.org/10.1038/sj.emboj.7601083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Toth B, Iordanov I, Csanady L . Ruling out pyridine dinucleotides as true trpm2 channel activators reveals novel direct agonist adp-ribose-2'-phosphate. J Gen Physiol 2015; 145:419–30; PMID:25918360; http://dx.doi.org/10.1085/jgp.201511377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Held K, Voets T, Vriens J . Trpm3 in temperature sensing and beyond. Temperature 2015; 2:201–13; PMID:27227024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, Benoit M, Xue F, Janssens A, Kerselaers S, et al. . Trpm3 is a nociceptor channel involved in the detection of noxious heat. Neuron 2011; 70:482–94; PMID:21555074; http://dx.doi.org/10.1016/j.neuron.2011.02.051 [DOI] [PubMed] [Google Scholar]
- [56]. Holendova B, Grycova L, Jirku M, Teisinger J. Ptdins(4,5)p2 interacts with cam binding domains on trpm3 n-terminus. Channels 2012; 6:479–82; PMID:22989896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Toth BI, Konrad M, Ghosh D, Mohr F, Halaszovich CR, Leitner MG, Vriens J, Oberwinkler J, Voets T. Regulation of the transient receptor potential channel trpm3 by phosphoinositides. J Gen Physiol 2015; 146:51–63; PMID:26123194; http://dx.doi.org/10.1085/jgp.201411339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Uchida K, Demirkhanyan L, Asuthkar S, Cohen A, Tominaga M, Zakharian E. Stimulation-dependent gating of trpm3 channel in planar lipid bilayers. FASEB J 2016; 30:1306–16; PMID:26655382; http://dx.doi.org/10.1096/fj.15-281576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Guinamard R, Salle L, Simard C. The non-selective monovalent cationic channels trpm4 and trpm5. Adv Exp Med Biol 2011; 704:147–71; PMID:21290294; http://dx.doi.org/10.1007/978-94-007-0265-3_8 [DOI] [PubMed] [Google Scholar]
- [60]. Nilius B, Prenen J, Janssens A, Voets T, Droogmans G. Decavanadate modulates gating of trpm4 cation channels. J Physiol 2004; 560:753–65; PMID:15331675; http://dx.doi.org/10.1113/jphysiol.2004.070839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Julius D, Nathans J. Signaling by sensory receptors. Cold Spring Harb Perspect Biol 2012; 4:a005991; PMID:22110046; http://dx.doi.org/10.1101/cshperspect.a005991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Zhang Z, Okawa H, Wang Y, Liman ER. Phosphatidylinositol 4,5-bisphosphate rescues trpm4 channels from desensitization. J Biol Chem 2005; 280:39185–92; PMID:16186107; http://dx.doi.org/10.1074/jbc.M506965200 [DOI] [PubMed] [Google Scholar]
- [63]. Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. The ca2+-activated cation channel trpm4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J 2006; 25:467–78; PMID:16424899; http://dx.doi.org/10.1038/sj.emboj.7600963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Yamaguchi S, Tanimoto A, Otsuguro K, Hibino H, Ito S. Negatively charged amino acids near and in transient receptor potential (trp) domain of trpm4 channel are one determinant of its ca2+ sensitivity. J Biol Chem 2014; 289:35265–82; PMID:25378404; http://dx.doi.org/10.1074/jbc.M114.606087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65]. Liu D, Liman ER. Intracellular ca2+ and the phospholipid pip2 regulate the taste transduction ion channel trpm5. Proc Natl Acad Sci U S A 2003; 100:15160–5; PMID:14657398; http://dx.doi.org/10.1073/pnas.2334159100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Yudin Y, Rohacs T. Regulation of trpm8 channel activity. Mol Cell Endocrinol 2012; 353:68–74; PMID:22061619; http://dx.doi.org/10.1016/j.mce.2011.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Liu B, Qin F. Functional control of cold- and menthol-sensitive trpm8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci 2005; 25:1674–81; PMID:15716403; http://dx.doi.org/10.1523/JNEUROSCI.3632-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Rohacs T, Lopes CM, Michailidis I, Logothetis DE. Pi(4,5)p2 regulates the activation and desensitization of trpm8 channels through the trp domain. Nat Neurosci 2005; 8:626–34; PMID:15852009; http://dx.doi.org/10.1038/nn1451 [DOI] [PubMed] [Google Scholar]
- [69]. Daniels RL, Takashima Y, McKemy DD. Activity of the neuronal cold sensor trpm8 is regulated by phospholipase c via the phospholipid phosphoinositol 4,5-bisphosphate. J Biol Chem 2009; 284:1570–82; PMID:19019830; http://dx.doi.org/10.1074/jbc.M807270200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Rivera R, Chun J. Biological effects of lysophospholipids. Rev Physiol Biochem Pharmacol 2008; 160:25–46; PMID:18481029 [DOI] [PubMed] [Google Scholar]
- [71]. D'Arrigo P, Servi S. Synthesis of lysophospholipids. Molecules 2010; 15:1354–77; PMID:20335986; http://dx.doi.org/10.3390/molecules15031354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72]. Lundbaek JA, Andersen OS. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J Gen Physiol 1994; 104:645–73; PMID:7530766; http://dx.doi.org/10.1085/jgp.104.4.645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Vanden Abeele F, Zholos A, Bidaux G, Shuba Y, Thebault S, Beck B, Flourakis M, Panchin Y, Skryma R, Prevarskaya N. Ca2+-independent phospholipase a2-dependent gating of trpm8 by lysophospholipids. J Biol Chem 2006; 281:40174–82; PMID:17082190; http://dx.doi.org/10.1074/jbc.M605779200 [DOI] [PubMed] [Google Scholar]
- [74]. Andersson DA, Nash M, Bevan S. Modulation of the cold-activated channel trpm8 by lysophospholipids and polyunsaturated fatty acids. J Neurosci 2007; 27:3347–55; PMID:17376995; http://dx.doi.org/10.1523/JNEUROSCI.4846-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Gentry C, Stoakley N, Andersson DA, Bevan S. The roles of ipla2, trpm8 and trpa1 in chemically induced cold hypersensitivity. Mol Pain 2010; 6:4; PMID:20092626; http://dx.doi.org/10.1186/1744-8069-6-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76]. Schilling T, Eder C. Non-selective cation channel activity is required for lysophosphatidylcholine-induced monocyte migration. J Cell Physiol 2009; 221:325–34; PMID:19562685; http://dx.doi.org/10.1002/jcp.21857 [DOI] [PubMed] [Google Scholar]
- [77]. Monet M, Gkika D, Lehen'kyi V, Pourtier A, Vanden Abeele F, Bidaux G, Juvin V, Rassendren F, Humez S, Prevarsakaya N. Lysophospholipids stimulate prostate cancer cell migration via trpv2 channel activation. Biochim Biophys Acta 2009; 1793:528–39; PMID:19321128; http://dx.doi.org/10.1016/j.bbamcr.2009.01.003 [DOI] [PubMed] [Google Scholar]
- [78]. Fallah A, Pierre R, Abed E, Moreau R. Lysophosphatidylcholine-induced cytotoxicity in osteoblast-like mg-63 cells: Involvement of transient receptor potential vanilloid 2 (trpv2) channels. Mol Membr Biol 2013; 30:315–26; PMID:23964684; http://dx.doi.org/10.3109/09687688.2013.828855 [DOI] [PubMed] [Google Scholar]
- [79]. Shibasaki K, Ishizaki Y, Mandadi S. Astrocytes express functional trpv2 ion channels. Biochem Biophys Res Commun 2013; 441:327–32; PMID:24161738; http://dx.doi.org/10.1016/j.bbrc.2013.10.046 [DOI] [PubMed] [Google Scholar]
- [80]. Sheikh AM, Michikawa M, Kim SU, Nagai A. Lysophosphatidylcholine increases the neurotoxicity of alzheimer's amyloid beta1-42 peptide: Role of oligomer formation. Neuroscience 2015; 292:159–69; PMID:25727637; http://dx.doi.org/10.1016/j.neuroscience.2015.02.034 [DOI] [PubMed] [Google Scholar]
- [81]. Yung YC, Stoddard NC, Chun J. Lpa receptor signaling: Pharmacology, physiology, and pathophysiology. J Lipid Res 2014; 55:1192–214; PMID:24643338; http://dx.doi.org/10.1194/jlr.R046458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82]. Jang Y, Lee MH, Lee J, Jung J, Lee SH, Yang DJ, Kim BW, Son H, Lee B, Chang S, et al. . Trpm2 mediates the lysophosphatidic acid-induced neurite retraction in the developing brain. Pflugers Arch 2014; 466:1987–98; PMID:24413888; http://dx.doi.org/10.1007/s00424-013-1436-4 [DOI] [PubMed] [Google Scholar]
- [83]. Pan HL, Zhang YQ, Zhao ZQ. Involvement of lysophosphatidic acid in bone cancer pain by potentiation of trpv1 via pkcepsilon pathway in dorsal root ganglion neurons. Mol Pain 2010; 6:85; PMID:21118579; http://dx.doi.org/10.1186/1744-8069-6-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84]. Nieto-Posadas A, Picazo-Juarez G, Llorente I, Jara-Oseguera A, Morales-Lazaro S, Escalante-Alcalde D, Islas LD, Rosenbaum T. Lysophosphatidic acid directly activates trpv1 through a c-terminal binding site. Nat Chem Biol 2012; 8:78–85; http://dx.doi.org/10.1038/nchembio.712 [DOI] [PubMed] [Google Scholar]
- [85]. Vigna SR, Shahid RA, Liddle RA. Ethanol contributes to neurogenic pancreatitis by activation of trpv1. FASEB J 2014; 28:891–6; PMID:24221085; http://dx.doi.org/10.1096/fj.13-236208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86]. Vieira C, Fetzer S, Sauer SK, Evangelista S, Averbeck B, Kress M, Reeh PW, Cirillo R, Lippi A, Maggi CA, et al. . Pro- and anti-inflammatory actions of ricinoleic acid: Similarities and differences with capsaicin. Naunyn Schmiedebergs Arch Pharmacol 2001; 364:87–95; PMID:11534859; http://dx.doi.org/10.1007/s002100100427 [DOI] [PubMed] [Google Scholar]
- [87]. Morales-Lazaro SL, Serrano-Flores B, Llorente I, Hernandez-Garcia E, Gonzalez-Ramirez R, Banerjee S, Miller D, Gududuru V, Fells J, Norman D, et al. . Structural determinants of the transient receptor potential 1 (trpv1) channel activation by phospholipid analogs. J Biol Chem 2014; 289:24079–90; PMID:25035428; http://dx.doi.org/10.1074/jbc.M114.572503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88]. Kozlowska M, Gruczynska E, Scibisz I, Rudzinska M. Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seeds. Food Chem 2016; 213:450–6; PMID:27451203; http://dx.doi.org/10.1016/j.foodchem.2016.06.102 [DOI] [PubMed] [Google Scholar]
- [89]. Matta JA, Miyares RL, Ahern GP. Trpv1 is a novel target for omega-3 polyunsaturated fatty acids. J Physiol 2007; 578:397–411; PMID:17038422; http://dx.doi.org/10.1113/jphysiol.2006.121988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. Morales-Lázaro SLI, Sierra-Ramírez F, López-Romero AE, Ortíz-Rentería M, Serrano-Flores B, Simon SA, Islas LD, Rosenbaum T. Inhibition of trpv1 channels by a naturally occurring omega-9 fatty acid reduces pain and itch. Nat Commun 2016; in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91]. Zhang X, Koronowski KB, Li L, Freeman BA, Woodcock S, de Groat WC. Nitro-oleic acid desensitizes trpa1 and trpv1 agonist responses in adult rat drg neurons. Exp Neurol 2014; 251:12–21; PMID:24212047; PMID:865625; http://dx.doi.org/10.1016/j.expneurol.2013.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92]. Shieh HS, Hoard LG, Nordman CE. Crystal structure of anhydrous cholesterol. Nature 1977; 267:287–9; http://dx.doi.org/10.1038/267287a0 [DOI] [PubMed] [Google Scholar]
- [93]. Naylor J, Li J, Milligan CJ, Zeng F, Sukumar P, Hou B, Sedo A, Yuldasheva N, Majeed Y, Beri D, et al. . Pregnenolone sulphate- and cholesterol-regulated trpm3 channels coupled to vascular smooth muscle secretion and contraction. Circ Res 2010; 106:1507–15; PMID:20360246; http://dx.doi.org/10.1161/CIRCRESAHA.110.219329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94]. Morenilla-Palao C, Pertusa M, Meseguer V, Cabedo H, Viana F. Lipid raft segregation modulates trpm8 channel activity. J Biol Chem 2009; 284:9215–24; PMID:19176480; http://dx.doi.org/10.1074/jbc.M807228200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95]. Veliz LA, Toro CA, Vivar JP, Arias LA, Villegas J, Castro MA, Brauchi S. Near-membrane dynamics and capture of trpm8 channels within transient confinement domains. PLoS One 2010; 5:e13290; PMID:20948964; http://dx.doi.org/10.1371/journal.pone.0013290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96]. Liu M, Huang W, Wu D, Priestley JV. Trpv1, but not p2x, requires cholesterol for its function and membrane expression in rat nociceptors. Eur J Neurosci 2006; 24:1–6; PMID:16800863; http://dx.doi.org/10.1111/j.1460-9568.2006.04889.x [DOI] [PubMed] [Google Scholar]
- [97]. Picazo-Juarez G, Romero-Suarez S, Nieto-Posadas A, Llorente I, Jara-Oseguera A, Briggs M, McIntosh TJ, Simon SA, Ladron-de-Guevara E, Islas LD, et al. . Identification of a binding motif in the s5 helix that confers cholesterol sensitivity to the trpv1 ion channel. J Biol Chem 2011; 286:24966–76; PMID:21555515; http://dx.doi.org/10.1074/jbc.M111.237537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98]. Jansson ET, Trkulja CL, Ahemaiti A, Millingen M, Jeffries GD, Jardemark K, Orwar O. Effect of cholesterol depletion on the pore dilation of trpv1. Mol Pain 2013; 9:1; PMID:23279936; http://dx.doi.org/10.1186/1744-8069-9-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99]. Klein AS, Tannert A, Schaefer M. Cholesterol sensitises the transient receptor potential channel trpv3 to lower temperatures and activator concentrations. Cell Calcium 2014; 55:59–68; PMID:24406294; http://dx.doi.org/10.1016/j.ceca.2013.12.001 [DOI] [PubMed] [Google Scholar]
- [100]. Kumari S, Kumar A, Sardar P, Yadav M, Majhi RK, Kumar A, Goswami C. Influence of membrane cholesterol in the molecular evolution and functional regulation of trpv4. Biochem Biophys Res Commun 2015; 456:312–9; PMID:25434996; http://dx.doi.org/10.1016/j.bbrc.2014.11.077 [DOI] [PubMed] [Google Scholar]


