ABSTRACT
In humans, an increase in internal core temperature elicits large increases in skin blood flow and sweating. The increase in skin blood flow serves to transfer heat via convection from the body core to the skin surface while sweating results in evaporative cooling of the skin. Cutaneous vasodilation and sudomotor activity are controlled by a sympathetic cholinergic active vasodilator system that is hypothesized to operate through a co-transmission mechanism. To date, mechanisms of cutaneous active vasodilation remain equivocal despite many years of research by several productive laboratory groups. The purpose of this review is to highlight recent advancements in the field of cutaneous active vasodilation framed in the context of some of the historical findings that laid the groundwork for our current understanding of cutaneous active vasodilation.
KEYWORDS: hyperthermia, microdialysis, neurokinin-1 receptors, neuropeptides EDHF, nitric oxide, sympathetic nervous system, thermoregulation, vasoactive intestinal polypeptide
Introduction
It is well accepted that a sympathetic cholinergic active vasodilator system is responsible for the sizeable increase in skin blood flow during hyperthermia; however, the precise mechanisms underlying cutaneous active vasodilation remain unsettled despite decades of research by many notable individuals and laboratories. Over the years, several elegant theories have been proposed and, to date, the most compelling theory underlying cutaneous active vasodilation is the co-transmission theory.1 The original proposition for this theory suggested that acetycholine and an unknown neurotransmitter are released from sympathetic cholinergic nerve terminals, where acetylcholine mediates sudomotor activity (sweating) and the unknown neurotransmitter mediates cutaneous vasodilation. While the general notion of acetylcholine mediating a large portion of sudomotor activity is fairly well established, identification of the unknown co-transmitter responsible for cutaneous vasodilation has proven to be elusive.
Part of the problem in unraveling the details of cutaneous active vasodilation is the redundancy and complexity of the system. As discussed later, very rarely does blockade or inhibition of one pathway eliminate the entirety of cutaneous active vasodilation. Moreover, several lines of data indicate there are several substances, molecules, and peptides involved, with varying magnitudes, in cutaneous active vasodilation, which effectively eliminates the idea of a single co-transmitter mediating cutaneous active vasodilation. With the exception of acetylcholine, resolving the temporal component of the various pathways involved in cutaneous active vasodilation is also largely unknown. This redundancy and complexity is not unlike the vasodilator response to exercising skeletal muscle; despite decades of research, the mechanisms underlying skeletal muscle vasodilation during exercise are not fully clarified. The complexity and redundancy of cutaneous active vasodilation make it difficult to study yet the complexity and the requisite nature of cutaneous active vasodilation for maintaining homeostasis are what make the system equally fascinating.
We will begin this review by discussing the sympathetic innervation of the skin and highlight some of the early, historical studies that provided the foundation for the study of cutaneous active vasodilation. Specifically, these early studies provided the evidence that cutaneous vasodilation during heat stress is of reflex origin and mediated by a sympathetic active vasodilator system. Second, we will review the current state of knowledge of cutaneous active vasodilation. The focus will be on our current understanding of basic mechanisms as elucidated in young, healthy humans. Lastly, we will briefly discuss how cutaneous active vasodilation can be altered by conditions such as exercise, aging, menstrual cycle and oral contraceptives, hypertension, diabetes, multiple sclerosis, and skin grafts. This review will focus predominantly on mechanisms in human skin, specifically nonglabrous (hairy) skin. Glabrous skin, such as the palm, lacks an active vasodilator system and changes in skin blood flow are largely mediated by alterations in sympathetic adrenergic vasoconstriction, such that an increase in palmar blood flow during heating is mainly due to a withdrawal of sympathetic adrenergic vasoconstriction.
Early studies of human temperature regulation and skin blood flow
Sympathetic innervation of human skin
The innervation of human non-glabrous skin is unique in that 2 distinct branches of the sympathetic nervous system innervate it: an adrenergic vasoconstrictor system and a cholinergic active vasodilator system. The adrenergic vasoconstrictor system behaves as in most other vascular beds, such as skeletal muscle: norepinephrine is released from sympathetic nerve terminals, binds to α receptors, and induces vasoconstriction. The adrenergic vasoconstrictor system imparts tonic vasoconstriction to the skin under thermoneutral, resting conditions and becomes more active in response to a reduction in skin and core temperature.
The cholinergic active vasodilator system is activated in response to an increase in core body temperature to elicit large increases in skin blood flow. The initial increase in skin blood flow during heat stress is simply a withdrawal of tonic adrenergic vasoconstriction; however, the substantial increase in skin blood flow with increasing core body temperature is due to the cholinergic active vasodilator system.
Early evidence for reflex cutaneous vasodilation
In 1899, Hough and Ballantyne2 published the earliest data of the effect of external air and water temperature, ranging from 5–45°C, on skin blood flow. Using changes in capillary pressure and observing the color of the skin, these investigators noted that air/water temperature above room temperature turned the skin red and increased skin blood flow.2 Conversely, air/water temperature below room temperature first turned the skin red and then blue, suggesting a decrease in blood flow.2 Although direct measurements of blood flow were not made, these observations established the idea that limb (skin) blood flow is altered by changes in temperature.
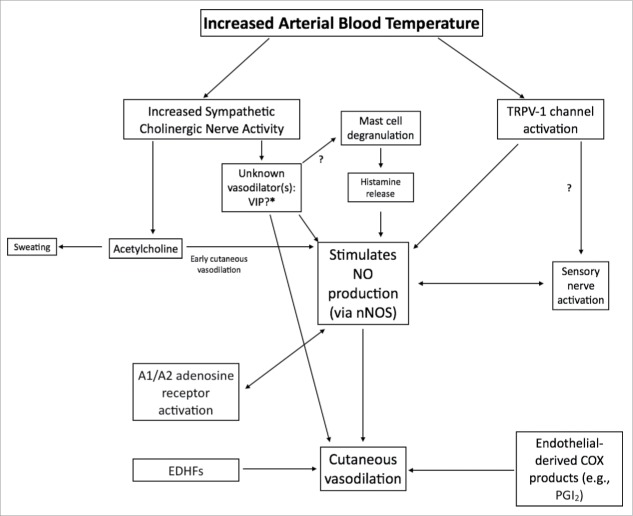
The first evidence the increase in limb blood flow may be reflexively mediated came from the observation that immersion of one limb, or one half of the body (forearms), in warm water (i.e., indirect heating) resulted in an increase in blood flow to the non-heated contralateral limb or non-heated half of the body (legs).3,4 Landmark studies by Lewis & Pickering,3 Grant and Holling,5 and Grant and Pearson6 provided the initial evidence for sympathetically mediated vasodilation. Although different methods were used in the different studies, the strongest evidence was the observation that elimination of the sympathetic nerves abolished the increase in limb blood flow in response to indirect heating,3,5-7 (Fig. 1). Grant and Holling5 also noted that the increase in limb blood flow seemed to coincide with sweating and proposed the hypothesis that sudomotor activity was responsible for the active vasodilation (this hypothesis will be discussed in more detail below). These studies collectively provided the initial evidence that a sympathetic reflex mediated the increase in limb blood flow during passive, indirect body heating.
Figure 1.
Cutaneous nerve block eliminates the increase in forearm blood flow during heat stress. One of the first studies to provide evidence that the increase in forearm blood flow during heat stress was due to a reflex vasodilation rather than withdrawal of vasoconstriction. Forearm blood flow with cutaneous nerve block is shown in the filled symbols and forearm blood flow to the control limb is shown in open symbols. Note the near elimination of forearm vasodilation during heating in the nerve-blocked limb. Adapted, with permission, from Edholm et al.7
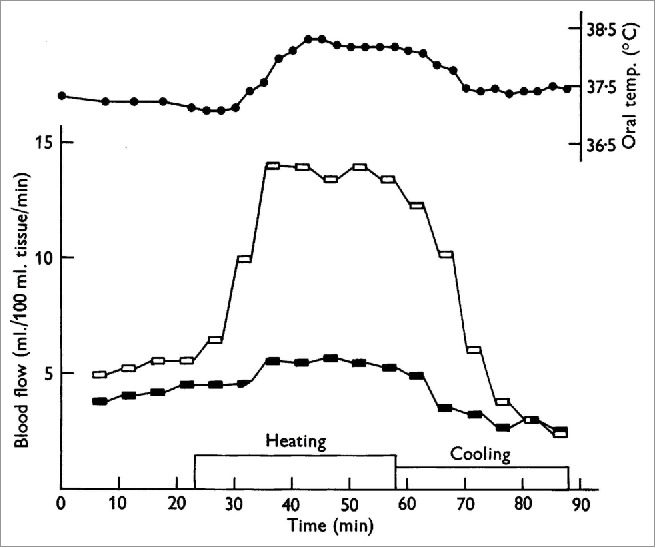
The studies described above made use of venous occlusion plethysmography to measure changes in limb blood flow. This technique takes into account changes in both muscle and skin blood flow and, as such, it remained unclear as to whether the change in limb blood flow during body heating occurred in the skin, muscle, or both. As skeletal muscle represents the majority of the tissue of the limbs, it was thought most of the increase in limb blood flow during body heating was due to an increase in skeletal muscle blood flow.8,9 Arresting the cutaneous circulation with epinephrine subsequently determined that the increase in limb blood flow during body heating was directed primarily to the cutaneous vasculature10-14 (Fig. 2).
Figure 2.
Increase in forearm blood flow is due largely to an increase in skin, rather than skeletal muscle, blood flow. Iontophoresis of adrenaline completely arrests the skin circulation but has little to no effect on skeletal muscle circulation. There was little to no increase in forearm blood flow during heat stress following iontophoresis of adrenaline to the right forearm (filled symbols) compared to the substantial increase in forearm blood flow to the control limb (open symbols). Adapted, with permission, from Edholm et al.13
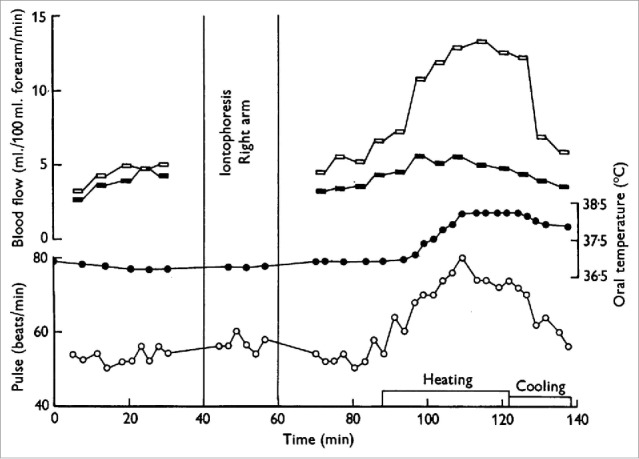
Subsequent studies determined that the initial increases in skin blood flow during body heating were due to withdrawal of sympathetic adrenergic nerve activity.7,15 An elegant study by Roddie et al.15 established 3 key concepts. First, these authors demonstrated that the increase in forearm blood flow during body heating occurs in 2 stages, the first being withdrawal of tonic vasoconstriction and the second being an increase in vasodilator nerve activity. Second, the increase in forearm blood flow due to vasodilator nerve activity was temporally linked to the increase in sweating, thus confirming the initial findings of Grant and Holling.5 Third, atropine delayed and reduced forearm vasodilation and eliminated the sweat response, which provided the first key piece of evidence that the active vasodilator system may be mediated by cholinergic mechanisms.
The studies highlighted in this section collectively indicate: 1) the increase in blood flow during body heating was of reflex origin, 2) the reflex was of sympathetic nature, 2) the increase in blood flow was directed primarily to the skin, 3) cutaneous vasodilation and sweating appear to occur simultaneously, and 3) both cutaneous vasodilation and sweating may be mediated by cholinergic mechanisms. A key question that was left unresolved: what is the nature of the vasodilator(s)?
The sudomotor activity and active vasodilation: the bradykinin hypothesis
Because sweating and cutaneous vasodilation appeared to occur simultaneously led to the early hypothesis that active vasodilation was not only temporally, but also mechanistically, linked to sudomotor activity.5 Based on this close temporal association between sweating and vasodilation, it was proposed that some vasoactive factor released by sweat gland activity caused vasodilation of the skin.
Fox & Hilton16 initially proposed that bradykinin was produced and released from activated sweat glands and was responsible for the cutaneous vasodilation. These authors were able to measure an appreciable increase in kininogenase, the enzyme responsible for bradykinin formation, in the interstitium during body heating. The initial findings were later supported by Fox & Edholm.17 More recent evidence supporting the sudomotor hypothesis were obtained by Brengelmann et al.,18 who observed a lack of cutaneous vasodilation in patients with a congenital absence of sweat glands; however, whether the lack of cutaneous vasodilation is due to lack of bradykinin production is unclear.
Although there are some recent conflicting findings,19,20 most of the recent evidence argues against the bradykinin hypothesis. Previous studies measured bradykinin either in sweat or in the interstitial fluid during heat stress, which leaves open the possibility that bradykinin is formed by sweat glands but is not involved in the cutaneous vasodilation.16,19,20 To directly assess the contribution of bradykinin to cutaneous active vasodilation, Kellogg et al.21 blocked bradykinin B2 receptors via intradermal microdialysis infusion of a specific B2 receptor antagonist. These authors found that inhibition of B2 receptors had no effect on either the threshold or magnitude of cutaneous vasodilation.21 The current thinking is that bradykinin formation from sweat gland activity does not contribute to cutaneous active vasodilation; however, whether some other vasodilator(s) produced from sweat gland activity effects cutaneous active vasodilation is unknown. It also remains equivocal as to whether there are separate sudomotor and active vasodilator nerves.
Establishment of the cholinergic co-transmission theory
Roddie et al.,15,22 using intra-arterial infusion of atropine, first demonstrated that cutaneous vasodilation in response to body heating contained a cholinergic component. These authors consistently observed that atropine delayed the threshold for the onset of cutaneous vasodilation; however, the effect of atropine on the magnitude of cutaneous vasodilation varied as a function of when the atropine was administered. Atropine administration prior to the onset of hyperthermia attenuated the magnitude of cutaneous vasodilation whereas atropine administration during established hyperthermia was without effect on cutaneous vasodilation,15,22 suggesting any cholinergic-induced vasodilation was time-sensitive and could not account for the entirety of the vasodilation. In contrast to the effect on skin blood flow, atropine abolished the sweat response regardless of when atropine was administered, suggesting cholinergic contributions to sweating are not time sensitive and sweating is mediated almost entirely by cholinergic mechanisms.15,22 Subsequent studies mostly,15,16,22-24 but not always,25-27 confirmed the initial findings of Roddie et al.15,22 The current consensus is that sweating is mediated by cholinergic mechanisms but any cholinergic component to cutaneous active vasodilation is responsible for the initial increases in skin blood flow but has minimal, if any, effect during the late stages of heat stress.15,16,22,24,28,29 Readers are referred to a recent review by Smith and Johnson for a more complete discussion of sudomotor mechanisms.30
A study by Kellogg et al.23 not only confirmed the contribution of acetylcholine to sweating and cutaneous vasodilation but also provided an important framework for our current understanding of cutaneous active vasodilation. Kellogg et al. used botulinum toxin A, which inhibits the presynaptic release of all cholinergic transmitters and co-transmitters,31 to determine whether cutaneous active vasodilation works through cholinergic co-transmission. There were 2 hallmark findings from this study. First, as described above, atropine eliminated sweating and delayed, but did not attenuate, cutaneous vasodilation. Second, and most significantly, intradermal injection of botulinum toxin A eliminated both sweating and cutaneous vasodilation. The data from Kellogg et al. suggest that acetylcholine mediates the sweat response and the early component of cutaneous vasodilation while some other unidentified factor that is co-released with acetylcholine mediates the majority of cutaneous vasodilation.23
The findings of Kellogg et al.,23 combined with previous historical findings, established our current working theory of cutaneous active vasodilation: a cholinergic co-transmission theory of cutaneous active vasodilation. The establishment of a cholinergic co-transmission theory proved important in furthering our understanding of cutaneous active vasodilation; however, an important question as to the nature of the unidentified vasodilator that is co-released with acetylcholine remains unanswered. The cholinergic co-transmission theory has provided the framework for recent studies that are aimed at identifying the co-transmitter. Recent work in the field has identified several vasodilators that contribute to cutaneous active vasodilation yet none can fully account for the stout increase in skin blood flow during heat stress; as will become evident in the discussion that follows, it is unlikely that, even if identified, a single co-transmitter will be able to account for the entirety of cutaneous active vasodilation.
Vasodilators implicated in cutaneous active vasodilation: evidence from recent studies
This section will highlight our current state of knowledge with emphasis placed on recent studies that have provided evidence for the contribution of several vasodilators to cutaneous active vasodilation. For a more thorough review of this topic, readers are referred to the recent work by Johnson, Minson, and Kellogg.32
Nitric oxide and nitric oxide synthase
The first evidence of a role for nitric oxide (NO) to active vasodilation was obtained from experiments using the rabbit ear model.33,34 Subsequent studies using the rabbit ear model established that NO acted in a permissive role in active vasodilation.35,36 In this model, only a basal amount of NO was required to permit full expression of active vasodilation.35,36
The first study in humans found that NO did not contribute to cutaneous active vasodilation.37 The initial study by Dietz et al. utilized intra-arterial infusion of the non-specific NOS inhibitor, L-NMMA, and venous occlusion plethysmography to measure forearm blood flow. Later studies using intradermal microdialysis by Shastry et al.38 and Kellogg et al.39 independently observed that non-specific NO synthase (NOS) inhibition (L-NMMA38 or L-NAME39) attenuated cutaneous active vasodilation, but not sweating, suggesting a role for NO in cutaneous active vasodilation.
To determine whether NO was permissive for active vasodilation in human skin, Crandall and MacLean40 attempted to measure NO breakdown products and found no measureable increase, suggesting a permissive role for NO in human skin. Kellogg et al.,41 conversely, using direct measurements of interstitial bioavailable NO, was able to detect significant increases in bioavailable NO, suggesting NO was not acting permissively for active vasodilation. If NO were permissive, then bioavailable concentrations should not have increased. Wilkins and colleagues42 sought to clarify the question as to whether NO was permissive for active vasodilation. In this study, Wilkins et al. simultaneously inhibited endogenous NO production with microdialysis delivery of L-NAME while maintaining a low, yet vasoactive, amount of NO present via delivery of sodium nitroprusside.42 Inhibition of NO attenuated cutaneous active vasodilation but low dose nitroprusside only partially restored active vasodilation, suggesting NO is not permissive and may have a direct effect on cutaneous active vasodilation. The findings from Wilkins et al.,42 in conjunction with those of Kellogg et al.,41 established the current prevailing notion that NO is not permissive for active vasodilation in human skin and NO is a direct effector and directly contributes ∼30–45% to cutaneous active vasodilation. Nitric oxide also appears to work through several of the proposed vasodilators that will be discussed below.
With a direct role for NO established, more recent studies sought to determine which NOS isoform mediated the NO-component of cutaneous active vasodilation. Data from Kellogg et al.43-45 suggest that neuronal NOS (nNOS or NOS I) contribute to the NO component of cutaneous active vasodilation while endothelial NOS (eNOS or NOS III) contributes to the NO component in response to local heating of the skin (Fig. 3). In contrast, recent data from our laboratory indicates eNOS contributes to reflex cutaneous vasodilation during dynamic exercise46 (see below). Whether the NO derived from nNOS is released from sympathetic cholinergic nerves, nitroxidergic nerves, or some other source remains unknown.
Figure 3.
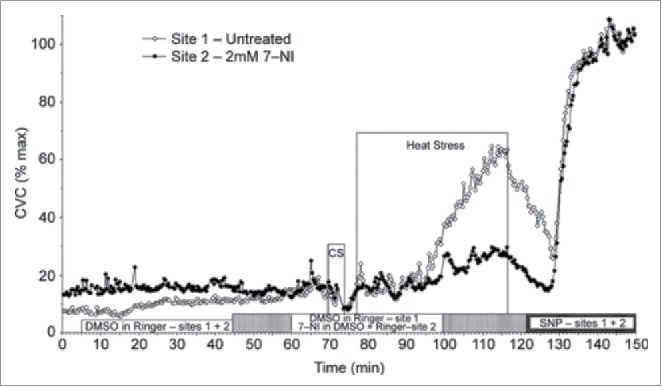
NO component of cutaneous active vasodilation is mediated by nNOS. Inhibition of nNOS with 7-NI (filled symbols) attenuates cutaneous active vasodilation, suggesting nNOS is responsible for the NO component of active vasodilation. NOS inhibition had no effect on skin blood flow during cold stress (CS). Adapted, with permission, from Kellogg et al.45
Lastly, Kellogg et al. recently demonstrated that the NO component of cutaneous active vasodilation works through the soluble guanylyl cyclase-cGMP pathway.47 Although the NO contributes ∼30–45% to cutaneous active vasodilation, antagonism of soluble guanylyl cyclase attenuated active vasodilation by approximately 15%, suggesting that at least one half of the known NO component exerts its effect independent of solubly guanylyl cyclase and cGMP.47
Vasoactive intestinal polypeptide, pituitary adenylate cyclase activating polypeptide, and associated receptors
Vasoactive intestinal polypeptide (VIP) was one of the first peptides suggested to be the putative co-transmitter of cutaneous active vasodilation. Immunohistochemical studies in human skin indicate that VIP is co-localized with acetylcholine in nerves associated with blood vessels and sweat glands thus making VIP a strong candidate for the unknown co-transmitter.48-50 Unfortunately, experimental evidence of a role for VIP in cutaneous active vasodilation is not as strong as the background information suggests.
The earliest test of a role for VIP came from Savage et al. who assessed cutaneous active vasodilation during heat stress in patients with cystic fibrosis, who have reduced levels of VIP in nerves of sweat glands.51,52 Savage et al. observed a normal cutaneous vasodilation to heat stress in cystic fibrosis patients, suggesting VIP may not be involved in cutaneous active vasodilation. Kellogg et al.53 and Wilkins et al.54 found that neither an increased contribution of acetylcholine nor NO, respectively, could account for the well-preserved cutaneous active vasodilation in patients with cystic fibrosis. These collective data suggest VIP may not be involved in cutaneous active vasodilation; however, whether some other vasodilator pathway is upregulated in patients with cystic fibrosis is unknown.
In healthy humans, independent studies by Bennett et al.55 and Wilkins et al.56 delivered the VIP fragment, VIP10–28, to the skin via microdialysis to inhibit the VIP receptors, VPAC1 and VPAC2, and obtained divergent results. Bennett et al. found that VIP10–28 attenuated cutaneous active vasodilation while Wilkins et al. found that VIP10–28 augmented both cutaneous active vasodilation as well as the vasodilation to exogenous VIP.55–57 Wilkins et al. further observed that NOS inhibition combined with VIP10–28 had no effect on the enhanced cutaneous vasodilation during heat stress.56
Further adding to the ambiguity of the involvement of VIP to cutaneous active vasodilation are the findings from a series of studies by Kellogg et al.58,59 In these studies Kellogg et al. found that cutaneous active vasodilation was attenuated when VPAC2 and PAC1 receptors, both of which bind VIP as well as pituitary adenylate cyclase activating polypeptide (PACAP), were inhibited and that functional NO was required for VPAC2/PAC receptors to elicit vasodilation.59
In summary, the role of VIP in cutaneous active vasodilation remains ambiguous. There are compelling data to suggest VIP and its receptors are involved in cutaneous active vasodilation.55,58,59 yet equally compelling data against a role for VIP.51,53,54,56 VIP, PACAP, and PHM can all bind, with varying affinity, to VPAC and PAC receptors and it is unclear which ligand(s) are responsible for the aforementioned receptor blockade studies.55,56,58,59
Histamine receptors
Data from our laboratory suggest that H1 histamine receptors contribute to cutaneous active vasodilation; however, the H2 isoform of the histamine receptor does not appear to be involved.60 Using intradermal microdialysis, we observed an attenuated cutaneous vasodilation during hyperthermia in the presence of an H1 antagonist (pyrilamine) while an H2 antagonist (cimetidine) was without effect.60 We also observed that a portion of the known NO-component of cutaneous active vasodilation could be explained by H1 receptor activation.60 An interesting finding from this study was the observation that acute systemic oral administration of an H1 receptor antagonist (360 mg fexofenadine) did not attenuate cutaneous vasodilation to local infusion of histamine, suggesting oral antihistamines do not reach skin in sufficient quantities to alter cutaneous vasodilation.60 Whether chronic antihistamine administration attenuates cutaneous vasodilation is unknown. Further support for H1 histamine receptors comes from the aforementioned study by Wilkins et al. who found that VIP-induced cutaneous vasodilation was mediated, in part, by H1 histamine receptor activation.57 Taken together, it is possible that, during hyperthermia, VIP is released from sympathetic cholinergic nerves and induces mast cell degranulation and release of histamine. Histamine would then bind to, and activate, H1 receptors, which would then induce production of NO via nNOS. As discussed above for VIP, receptor antagonist studies only allow for determination of the involvement of specific receptors to cutaneous active vasodilation; conclusions as to which ligand(s) activate the receptors cannot be made. Whether histamine is released from mast cells and accumulates in the interstitial space during hyperthermia and is responsible for the observed H1 component of cutaneous active vasodilation is unknown.
Prostaglandins
McCord et al. found that inhibition of vasoactive prostaglandins with ketorolac attenuated cutaneous active vasodilation, suggesting a role for the cyclooxygenase (COX) pathway to cutaneous active vasodilation.61 The combined effect of COX inhibition and NOS inhibition attenuated cutaneous active vasodilation to a greater extent than the independent effects of COX and NOS inhibition, suggesting that the COX pathway is independent of NOS and NO.61 The findings of COX contribution to cutaneous active vasodilation in young, healthy participants was subsequently confirmed by Holowatz et al. using low-dose (81 mg) systemic aspirin (discussed in more detail below).62
Neurokinin-1 receptors
Data from Wong and Minson suggest neurokinin-1 (NK1) receptors contribute to cutaneous active vasodilation.63 To circumvent problems associated with specific NK1 antagonists, the authors used repeated infusions of substance P to desensitize the NK1 receptors and found that desensitization of NK1 receptors attenuated cutaneous active vasodilation by approximately 30%.63,64 Combined NK1 receptor desensitization and NOS inhibition attenuated cutaneous active vasodilation by approximately 80% such that the magnitude of the vasodilation during hyperthermia was only slightly above baseline levels of skin blood flow. These data collectively suggest NK1 receptor activation and NO make largely independent contributions to cutaneous active vasodilation.
Although NK1 receptor activation appears to make an independent contribution to cutaneous active vasodilation, it remains unknown as to whether substance P is what activates the NK1 receptors. Other peptides of the kinin family that are structurally similar to substance P could, in theory, also bind to NK1 receptors but the affinity of the receptor for substance P makes substance P the most likely candidate. If substance P is involved in cutaneous active vasodilation, it raises the question as to the source of the substance P. The majority of substance P in human skin is usually co-localized with CGRP in sensory nerve terminals, which would raise the additional question as to whether sensory nerves are somehow involved in cutaneous active vasodilation; however, there is evidence for an endothelial source of substance P, which could be liberated from vascular endothelial cells during hyperthermia.65,66 The potential role of cutaneous sensory nerves is discussed next.
Sensory nerves and transient receptor potential vanilloid type 1 (TRPV-1) channels
Charkoudian et al. sought to determine whether cutaneous sensory nerves contribute to cutaneous active vasodilation by using topical capsaicin cream in 2 separate protocols67: an acute application to stimulate an increase in sensory nerve activity and a chronic (7 day) application to desensitize cutaneous sensory nerves. Charkoudian et al. found that neither acute nor chronic capsaicin attenuated cutaneous active vasodilation, suggesting that cutaneous sensory nerves do not contribute to cutaneous active vasodilation.67
Support, albeit indirect, for sensory nerves comes from recent data from our laboratory suggesting TRPV-1 channels contribute to cutaneous active vasodilation. We found that microdialysis infusion of the TRPV-1 channel specific antagonist, capsazepine, attenuated cutaneous active vasodilation by approximately 25%68 while combined inhibition of TRPV-1 channels and NOS attenuated cutaneous active vasodilation more than the independent effects of TRPV-1 and NOS inhibition.68 These data collectively suggest TRPV-1 channels make a direct contribution to cutaneous active vasodilation and a portion of the NO-component may be explained by TRPV-1 channel activation. Inasmuch as TRPV-1 channels are located predominantly on sensory nerve terminals, these data provide indirect evidence for cutaneous sensory nerves to cutaneous vasodilation; however, TRPV-1 channels are also found in endothelial cells, which could explain how TPRV-1 channel activation increases NO production. Whether the observed TRPV-1 channel component is reflective of TRPV-1 channel activation on sensory nerves or endothelial cells is unknown.
We recently took a more direct approach to investigate a potential contribution of cutaneous sensory nerves to cutaneous active vasodilation. Use of topical anesthetic to inhibit sensory nerves (EMLA; 2.5% lidocaine and 2.5% prilocaine) did not affect the magnitude of cutaneous active vasodilation; however, anesthetic did shift the onset of cutaneous active vasodilation to a higher oral temperature (ΔTor = 0.2°C for control sites vs. ΔTor = 0.5°C for EMLA sites), suggesting that sensory nerves are required for initiating the increase in skin blood flow during hyperthermia.69 Combined inhibition of sensory nerves and NOS attenuated cutaneous active vasodilation by approximately 80%, which suggests intact sensory nerves and NO are required for full expression of cutaneous active vasodilation.69 An intriguing area for future research is to reconcile the differences between our data and those of Charkoudian et al.63,67-69
Adenosine receptors
A recent study by Fieger and Wong70 provided evidence suggesting that A1/A2 adenosine receptor activation does not directly contribute to cutaneous active vasodilation as inhibition of A1/A2 receptors with theophylline did not attenuate cutaneous vasodilation during heat stress; however, a role for A1/A2 receptors was unmasked when A1/A2 receptors and NOS were simultaneously inhibited.70 That is, active vasodilation was attenuated to a greater extent when both A1/A2 receptors and NOS were inhibited compared to when only NOS was inhibited, suggesting any role for A1/A2 receptors may normally be masked by the actions of NO and/or other vasodilators.
Calcium-activated potassium (kca) channels and endothelial-derived hyperpolarizing factors (edhfs)
Brunt & Minson71 found that KCa channels and EDHFs make a substantial contribution to cutaneous thermal hyperemia induced by local heating of the skin. A logical next step was to address the question as to whether KCa channels and EDHFs also contribute to cutaneous active vasodilation. Interestingly, these authors found that KCa channels and EDHFs do not directly contribute to cutaneous active vasodilation while concomitant inhibition of NOS and KCa channels augments cutaneous active vasodilation.72 Following up on these observations, these authors provided evidence to suggest that the augmented vasodilation with combined NOS and KCa channel inhibition is due to cross-talk and activation of KIR and/or KATP channels.72 Thus, while EDHFs do not appear to directly contribute to cutaneous active vasodilation it does appear that EDHFs are indirectly involved via cross-talk with other pathways.72
Modulators of cutaneous active vasodilation
The final section of this review will focus on modulators of cutaneous active vasodilation. Although several conditions are known to effect cutaneous vasodilation, we will only briefly focus on a select few. For a more detailed and thorough review of modulators of cutaneous active vasodilation, readers are referred to the recent review by Johnson et al.32
Dynamic exercise
The initial cutaneous vascular response to dynamic exercise is a reduction in skin blood flow brought about by an increase in sympathetic adrenergic activity but this vasoconstrictor response may be attenuated by prior hyperthermia.73-77 The threshold for the onset of cutaneous active vasodilation is also shifted to a higher internal temperature during dynamic exercise.76,78-80 The shift in threshold for cutaneous active vasodilation during exercise does not appear to be due to enhanced adrenergic vasoconstriction but rather a delay in the active vasodilator system and the delay may be due to changes in plasma osmolality.79-82
As exercise progresses and the core temperature threshold for active vasodilation is surpassed, skin blood flow increases nearly linearly with increasing exercise duration and core temperature73,76 until core temperature reaches ∼38–39°C at which point skin blood flow begins to plateau.73,76,83 The plateau in skin blood flow at high core temperatures during exercise appears to be due to a limitation in the active vasodilator system and not due to increased adrenergic vasoconstriction.84-86
Despite the known effects described above, it is unclear as to whether the mechanisms of cutaneous vasodilation during dynamic exercise are the same as during passive heat stress. Blair et al. were the first to report that cutaneous vasodilation during exercise is of reflex origin87; however, studies investigating mechanisms of cutaneous vasodilation during dynamic exercise are sparse. A recent study from our laboratory suggests cutaneous vasodilation during dynamic exercise contains a significant NO component that is similar in magnitude as that observed with passive heat stress46 (Fig. 4). Unlike passive heat stress, the NO component during dynamic exercise is due to increased NO production from eNOS rather than nNOS, which suggests that the stimulus for NO production differs between dynamic exercise and passive heat stress.43-46,88 Whether vasodilators suggested contributing to cutaneous active vasodilation (e.g., VIP, H1 histamine receptors) are also involved during dynamic exercise remains to be determined.
Figure 4.
Contribution of NO to cutaneous vasodilation during dynamic exercise. Similar to passive heat stress, NO contributes ∼35% to cutaneous vasodilation during dynamic exercise sufficient to increase in core temperature ∼0.8°C above baseline. Whereas NO appears to be derived via nNOS during passive heat stress, the NO component during dynamic exercise appears to be derived from eNOS. Adapted, with permission, from McNamara et al.46
Female sex hormones
Female reproductive hormones, estrogen and progesterone, are known to alter thermoregulation. Concentrations of these hormones fluctuate throughout phases of the menstrual cycle and with administration of oral contraceptives. Estrogen is highest during the late follicular phase, just before ovulation, and second highest during the mid-luteal phase.89 Progesterone is low throughout the menstrual cycle until the mid-luteal phase, where it is higher than all other female reproductive hormones.89 Estrogen and progesterone are both elevated with normal (estrogen + progesterone) oral contraceptive use.90 Cyclic alterations of these hormone levels are known to change core temperature as well as the temperature threshold for the onset of active cutaneous vasodilation.
Core temperature and the core temperature threshold for the onset of cutaneous active vasodilation vary with different levels of estrogen and progesterone during the normal menstrual cycle and with oral contraceptive use. During the late follicular phase, core temperature is reduced both at rest and during exercise and there is a leftward shift in the core temperature threshold for the onset of active vasodilation.91-93 Conversely, during the mid-luteal phase, core temperature is higher both at rest and during dynamic exercise and there is a rightward shift of the core temperature threshold for the onset of active vasodilation onset.94-99
The most common forms of oral contraceptives are typically comprised of a combination of estrogen and progesterone. With these forms of oral contraceptives, the same rightward shift observed during the mid-luteal phase of the normal menstrual cycle occurs with oral contraceptive use both during passive heating90 and dynamic exercise.96,99,100 Initially, the elicited response from oral contraceptive use led to the hypotheses that progesterone predominates over core temperature and temperature threshold for the onset of active vasodilation more so than estrogen.96 Subsequent studies investigating the effects of estrogen and progesterone on thermoregulation suggest estrogen plays a larger modifying role than previously suspected.101,102 For example, Stachenfeld et al. demonstrated that progestin-only oral contraceptive use results in a higher core temperature and threshold for the onset of active vasodilation. The use of oral contraceptives with combined estrogen and progestin resulted in a complete reversal of the effects of progestin-only.102 Therefore, estrogen must produce some modulating effect when co-administered with progestin. A later study by Houghton et al. found that core temperature does not change with varied levels of progestin with oral contraceptive administration, suggesting that progestin is not as powerful on these alterations as previously thought.101 Data from Houghton et al. further demonstrated that there is a greater NO-dependent contribution to active cutaneous vasodilation in women using low-dose progestin contraceptives versus women using high-dose progestin contraceptives.101 This finding suggests estrogen is necessary for endothelial-dependent NO-mediated cutaneous vasodilation.101 Therefore, estrogen may upregulate NO production.
Healthy aging
The effect of healthy aging is one of the most well studied effectors of cutaneous active vasodilation and is well known to alter both the central cardiovascular responses to heat stress and cutaneous active vasodilation.103-107 The reduction in cutaneous active vasodilation may be due to decreased sensitivity of the active vasodilator system or decreased vascular responsiveness in the skin.
As discussed above, NO contributes ∼30–45% to cutaneous active vasodilation in young, healthy humans. An interesting finding is that at high levels of heat stress, aged individuals rely more on a diminished NO component than do younger individuals.108 That is, despite an attenuated NO component, aged individuals rely more on NO to increase skin blood flow during hyperthermia than on other mechanisms. Thus, it appears that not only does aging attenuate the NO component but aging appears to also severely compromise the unknown vasodilator(s) component.108
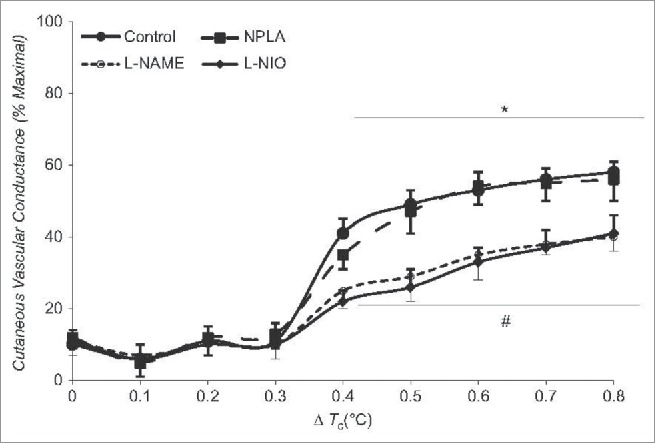
A series of studies by Holowatz et al. have provided a wealth of information as to how aging alters cutaneous active vasodilation, in particular with regards to the NO component. Acute arginase inhibition, acute L-arginine supplementation, and a combination of the 2, all result in augmented cutaneous active vasodilation within the skin of aged humans.109 These data suggest arginase activity is increased in aged skin and may result in reduced bioavailability of L-arginine, and therefore, NO109 (Fig. 5). Holowatz et al. further found that acute ascorbate supplementation independently increases cutaneous active vasodilation while acute ascorbate supplementation plus arignase inhibition produces an additive effect of increasing AVD, suggesting that oxidative stress contributes to the age-related decrease in NO-dependent vasodilation.110 Data from Stanhewicz et al. further suggest that reduced bioavailable BH4 contributes to the reduced NO-dependent vasodilation as both local and systemic BH4 supplementation can improve cutaneous active vasodilation via NO-dependent mechanisms.111,112 Folic acid supplementation also improves cutaneous active vasodilation in aged skin via NO-dependent mechanisms.113
Figure 5.
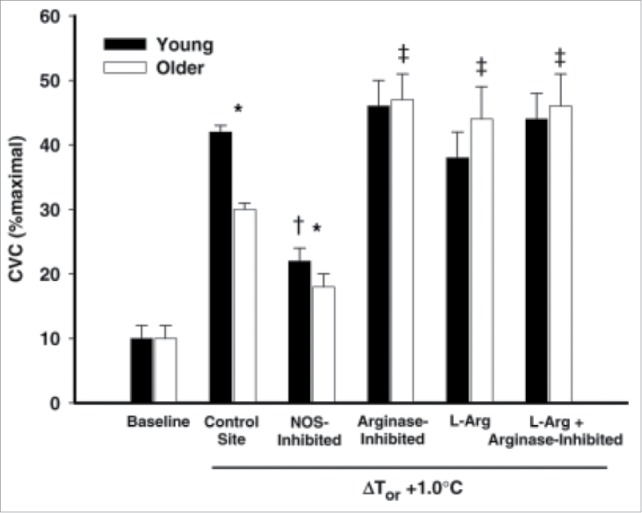
Effect of arginase inhibition and L-Arginine supplementation on cutaneous active vasodilation in young and older humans. Cutaneous active vasodilation is attenuated in older (open bars) compared to younger (black bars) subjects. Inhibition of arginase, L-Arginine supplementation, or a combination of the 2 restored cutaneous active vasodilation in older subjects but had no effect in younger subjects. These data suggest that healthy aging results in an increase in arginase activity that reduces bioavailability of L-Arginine, an important substrate for NOS. Adapted, with permission, from Holowatz et al.109
In addition to the NO pathway, Holowatz and colleagues also examined the COX pathway and platelets. Localized COX-inhibition (via microdialysis infusion of ketorolac) increased baseline, thermoneutral skin blood flow but there was no difference between COX-inhibited sites and control sites, suggesting that local COX-derived vasodilators do not functionally contribute to the increase in skin blood flow in response to passive heating in the skin of middle-aged human.114 Interestingly, chronic low-dose aspirin therapy attenuates cutaneous active vasodilation in middle-aged humans.115 Similarly, the specific platelet ADP receptor inhibitor, clopidogrel, was shown to attenuate cutaneous active vasodilation and increase the core temperature threshold for active vasodilation.115,116 These data suggest platelets may be activated during hyperthermia and release vasodilator factors that result in cutaneous vasodilation.115,116 The aforementioned low-dose aspirin and clopidogrel studies were performed in middle-aged participants; whether similar responses occur in younger and/or older individuals is unclear.
Type 1 and Type 2 diabetes
There is some evidence that type 1 diabetes negatively affects the cutaneous microvasculature but this finding is not consistent117,118; some of the discrepancy may be explained by differences in blood glucose levels during the time the experiments were performed. For a more comprehensive review of temperature regulation in diabetics, the reader is referred to a recent review by Kenny et al.119 To date, the most comprehensive information regarding thermoregulation in type 1 diabetes comes from a series of studies from the Kenny lab. These studies suggest type 1 diabetes does not affect heat loss during exercise in the heat except at high levels of heat stress.120-122 To our knowledge there are no studies aimed at systematically investigating mechanisms of cutaneous active vasodilation in type 1 diabetes and thus remains an important area for future research.
A series of studies by Charkoudian and colleagues suggests type 2 diabetics have impaired cutaneous active vasodilation that cannot be explained by attenuated NO-dependent vasodilation.123 That is, type 2 diabetics have an attenuated cutaneous vasodilation during hyperthermia but a well-preserved NO component.123 This group also found that, in addition to a reduced magnitude of cutaneous vasodilation, type 2 diabetes also shifted the threshold for cutaneous active vasodilation to higher core temperatures.123,124 These data collectively suggest type 2 diabetes impairs thermoregulation and negatively affects cutaneous vasodilation during hyperthermia. It should be noted that in the studies described that the type 2 diabetic participants were all relatively healthy, were free of neuropathy, and their blood glucose was relatively well controlled123,124; the data from these studies, therefore, may not translate to more severe cases to type 2 diabetes. It is also unknown how type 2 diabetes affects other pathways of cutaneous active vasodilation
Hypertension
Essential hypertension reduces the magnitude of cutaneous active vasodilation during both passive heat stress and during exercise in the heat; however, this observation is not consistently reported.125-129 The attenuation of cutaneous active vasodilation appears to be due to impairment of both NO- dependent (Fig. 6) and independent components of the active vasodilator system rather than to enhanced adrenergic vasoconstriction. Hypertension also alters the relative contribution of NOS and neurogenic control mechanisms to skin vasomotion as assessed by spectral analysis.130 This same group also found that upregulation of iNOS is at least partly responsible for the decrement in cutaneous vasodilation in response to local heating in patients with hypertension; whether iNOS also contributes to the attenuated cutaneous active vasodilation is unknown.131 An interesting series of studies from Holowatz et al. suggests that at least part of the deficit in cutaneous active vasodilation associated with hypertension can be reversed by local microdialysis infusion of antioxidants as well as with arginase inhibition.128,129 Inasmuch as chronic, systemic antioxidant therapy is inconsistent in reversing oxidative stress and in lowering blood pressure in hypertensive humans, it remains unknown whether chronic, systemic antioxidant therapy can correct deficits in cutaneous active vasodilation in hypertensive humans.
Figure 6.
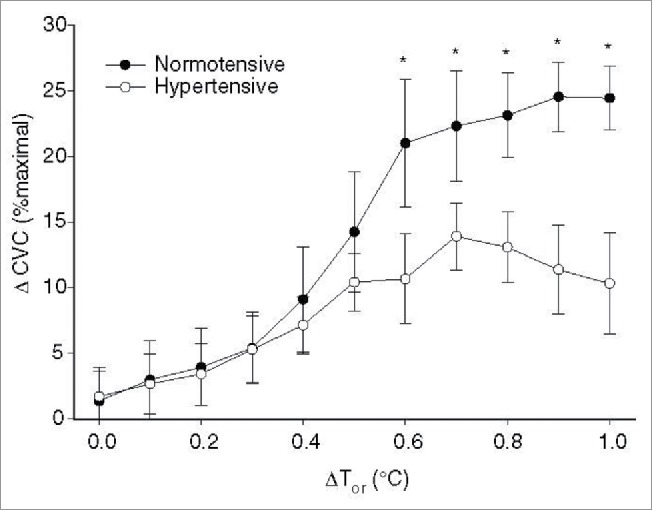
Attenuated NO-dependent cutaneous active vasodilation in patients with essential hypertension. Patients with essential hypertension have a reduced NO component to cutaneous active vasodilation during heat stress at core temperatures >0.5°C. ΔCVC was calculated as the difference between control and NOS inhibited sites to obtain an index of the NO-contribution for each 0.1°C increase in core temperature. Adapted, with permission, from Holowatz et al.129
Multiple sclerosis
Multiple sclerosis (MS) results in demyelination of axons in the central nervous system.132 Heat sensitivity, in which symptoms get worse during exposure to heat or during exercise, is one of the many symptoms of MS.133 Although information pertaining to cutaneous active vasodilation in MS patients is sparse, interesting work from Davis et al. suggests that MS patients have reduced sweating responses during passive heat stress but augmented skin blood flow responses compared with healthy controls, suggesting skin blood flow compensates for reduced sweating.133-135
Burns and skin grafts
Burns requiring skin grafts can alter sweating and cutaneous vasodilation during heat stress. A series of studies by Davis and colleagues have provided the most detailed information pertaining to thermoregulation in skin grafts.136-138 Data from this group suggests that skin grafts result in severely attenuated sweating responses as well as attenuated cutaneous vasodilation during passive heat stress when compared to adjacent intact skin. The impaired cutaneous vasodilation appears to be due to impairment in endothelium-dependent control of the cutaneous vasculature. The degree of denervation in the burn region also seems to be important in determining the cutaneous vasodilator response to heat stress. A striking feature of the skin graft is that the attenuated cutaneous vasodilation persists even after long-term recovery (up to 4 y post-skin graft).136-138
Perspectives
Based on the information presented in this review, it is clear that there are several important questions that still need to be addressed in the field of cutaneous active vasodilation, both in health and disease. In this section, we describe 3 areas that we deem important for furthering our understanding of active vasodilation. This list is not meant to be all inclusive but it is hoped that this list will, at least, foster new research in this field. First, most of the present data do not allow us to draw conclusions about specific vasodilator substances, only about vasodilator receptors and/or pathways. In vivo mechanistic studies utilizing intradermal microdialysis have largely used pharmacological agents to block/inhibit various receptors and data from these studies can only be used to describe the receptor being inhibited. For example, it is possible that amine compounds other than just histamine activate H1 histamine receptors; inhibiting H1 histamine receptors does not inform as to what is specifically activating the receptor during hyperthermia. Thus, future studies aimed at determining specific vasodilator substances will be important. Second, as discussed above, it has been difficult to resolve the temporal component of the identified vasodilator pathways; however, understanding the temporal component of these pathways is important, particularly as changes in the temporal component may underlie some of the decrements associated with healthy aging and various disease states. Lastly, race/ethnicity can affect vascular and endothelial function that may partly be explained by heightened levels of inflammatory mediators. For example, emerging evidence indicates that African Americans have a reduced forearm blood flow response to vascular occlusion that is associated with polymorphisms for nuclear factor E2-related factor 2 (Nrf2), which is important for innate antioxidant defense.139 Whether similar polymorphisms affect cutaneous active vasodilation remains to be determined.
Conclusion
Our goal in this review was to concisely report nearly 100 years' worth of research on the effect of heat on skin blood flow and the mechanisms of cutaneous active vasodilation. It is now well established that cutaneous vasodilation during hyperthermia is controlled by a sympathetic cholinergic active vasodilator system where acetylcholine largely mediates an increase in sweating but also contributes to an early increase in cutaneous vasodilation and an unknown neurotransmitter is responsible for the vast majority of cutaneous vasodilation. Data from several laboratories have identified several neurotransmitters, neuropeptides, and vasodilator molecules that contribute to cutaneous active vasodilation; however no single ‘unknown’ transmitter has been identified that can fully account for the substantial increase in skin blood flow during hyperthermia. Nitric oxide appears to be an important contributor to cutaneous active vasodilation. Although NO only accounts for ∼30–45% of cutaneous active vasodilation, several vasodilator pathways converge on the NO system (Fig. 7). Although great progress has been made, there are clearly several unresolved questions and areas for future research in the field of cutaneous active vasodilation.
Figure 7.
Schematic flowchart of vasodilators and pathways involved in cutaneous active vasodilation. Although most of the identified vasodilators are depicted, for clarity, not all receptors and downstream second messenger pathways are shown. Arrows marked with a “?” indicate pathways that are speculative and lack empirical data.
Abbreviations
- BH4
tetrahydrobiopterin
- CGRP
calcitonin gene-related peptide
- COX
cyclooxygenase
- EDHF
endothelial-derived hyperpolarizing factor
- KATP
ATP-sensitive potassium channels
- KCa
calcium-activated potassium channels
- KIR
inwardly rectifying potassium channels
- L-NAME
Nω-nitro-L-arginine methyl ester;
- L-NMMA
NG-monomethyl-L-arginine
- MS
multiple sclerosis
- NK1
neurokinin-1
- NO
nitric oxide
- NOS
nitric oxide synthase
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- PAC
pituitary adenylate cyclase receptor
- PACAP
pituitary adenylate cyclase activating polypeptide
- PHM
peptide histidine methionine
- TRPV-1
transient receptor potential vanilloid type 1 channel
- VIP
vasoactive intestinal polypeptide
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This review is the product of the teaching, encouragement, and mentorship, both direct and indirect, of several people including: Drs. Christopher T. Minson, John M. Johnson, Craig G. Crandall, W. Larry Kenney, and Don D. Sheriff. We also acknowledge several contemporary colleagues who have provided insightful discussions and motivation to continue to investigate mechanisms of cutaneous active vasodilation: Drs. Lacy M. Alexander, Brad W. Wilkins, Grant H. Simmons, Gary J. Hodges, and Scott L. Davis. Lastly, we thank former students in the lab who have helped us continue to contribute to the body of knowledge: Ms. Sarah M. Fieger, Mr. Tanner C. McNamara, Ms. Erica L. Levitt, Mr. Jeremy T. Keen, and Ms. Jennifer C. Harvey.
References
- [1].Kellogg DL Jr, Pérgola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res 1995; 77:1222-8; PMID:7586235; http://dx.doi.org/ 10.1161/01.RES.77.6.1222 [DOI] [PubMed] [Google Scholar]
- [2].Hough T, Ballantyne BL. Preliminary note on the effects of changes in external temperature on the circulation of blood in the skin. J Boston Society Medical Sci 1899; 3:330-4. [PMC free article] [PubMed] [Google Scholar]
- [3].Lewis T, Pickering GW. Vasodilatation in the limbs in response to warming the body, with evidence for sympathetic vasodilator nerves in man. Heart 1931; 16:33-51. [Google Scholar]
- [4].Gibbon JHH, Landis EM. Vasodilatation in the lower extremities in response to immersing the forearms in water. J Clin Invest 1932; 11:1019-36.; http://dx.doi.org/ 10.1172/JCI100456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Grant RT, Holling HE. Further observations on the vascular responses of the human limb to body warming: evidence for sympathetic vasodilator nerves in the normal subject. Clin Sci (Lond) 1938; 3:273-85. [Google Scholar]
- [6].Grant RT, Pearson RSB. The blood circulation in the human limb: observations on the differences between the proximal and distal parts and remarks on the regulation of core body temperature. Clin Sci (Lond) 1938; 3:119–39. [Google Scholar]
- [7].Edholm OG, Fox RH, Macpherson RK. Vasomotor control of the cutaneous blood vessels in the human forearm. J Physiol 1957; 139:455-65; PMID:13492236; http://dx.doi.org/ 10.1113/jphysiol.1957.sp005904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Barcroft H, Bonnar WM, Edholm OG. Reflex vasodilatation in human skeletal muscle in response to heating the body. J Physiol 1947; 106:271-8; PMID:16991759; http://dx.doi.org/ 10.1113/jphysiol.1947.sp004210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barcroft H, Edholm OG. The effect of temperature on blood flow and deep temperature in the human forearm. J Physiol 1943; 102:5-20; PMID:16991588; http://dx.doi.org/ 10.1113/jphysiol.1943.sp004009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cooper KE, Edholm OG, Fletcher JG, Fox RH, Macpherson RK. Vasodilatation in the forearm during indirect heating. J Physiol 1954; 125:347-58. [PubMed] [Google Scholar]
- [11].Cooper KE, Edholm OG, Mottram RF. The partition of the blood flow between skin and muscle in the human forearm. J Physiol 1954; 123:33-4P; PMID:13143532 [PubMed] [Google Scholar]
- [12].Cooper KE, Edholm OG, Mottram RF. The blood flow in skin and muscle of the human forearm. J Physiol 1955; 128:258-67; PMID:14392606; http://dx.doi.org/ 10.1113/jphysiol.1955.sp005304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Edholm OG, Fox RH, Macpherson RK. The effect of body heating on the circulation in skin and muscle. J Physiol 1956; 134:612-9; PMID:13398947; http://dx.doi.org/ 10.1113/jphysiol.1956.sp005669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roddie IC, Shepherd JT, Whelan RF. Evidence from venous oxygen saturation measurements that the increase in forearm blood flow during body heating is confined to the skin. J Physiol 1956; 134:444-50; PMID:13398924; http://dx.doi.org/ 10.1113/jphysiol.1956.sp005656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Roddie IC, Shepherd JT, Whelan RF. The vasomotor nerve supply to the skin and muscle of the human forearm. Clin Sci (Lond) 1957; 16:67-74; PMID:13414140 [PubMed] [Google Scholar]
- [16].Fox RH, Hilton SM. Bradykinin formation in human skin as a factor in heat vasodilatation. J Physiol 1958; 142:219-32; PMID:13564431; http://dx.doi.org/ 10.1113/jphysiol.1958.sp006011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fox RH, Edholm OG. Nervous control of the cutaneous circulation. Br Med Bull 1963; 19:110-4; PMID:13959044 [DOI] [PubMed] [Google Scholar]
- [18].Brengelmann GL, Freund PR, Rowell LB, Olerud JE, Kraning KK. Absence of active cutaneous vasodilation associated with congenital absence of sweat glands in humans. Am J Physiol (Heart Circ Physiol) 1981; 240:H571-5. [DOI] [PubMed] [Google Scholar]
- [19].Frewin DB, McConnell DJ, Downey JA. Is a kininogenase necessary for human sweating? Lancet 1973; 2:744.; PMID:4125839; http://dx.doi.org/ 10.1016/S0140-6736(73)92587-7 [DOI] [PubMed] [Google Scholar]
- [20].Hibino T, Takemura T, Sato K. Human eccrine sweat contains tissue killikrein and kininase II. J Invest Dermatol 1994; 102:214-20; PMID:7508964; http://dx.doi.org/ 10.1111/1523-1747.ep12371765 [DOI] [PubMed] [Google Scholar]
- [21].Kellogg DL, Liu Y, McAllister K, Friel C, Pergola PE. Bradykinin does not mediate cutaneous active vasodilation during heat stress in humans. J Appl Physiol 2002; 93:125-1221; http://dx.doi.org/ 10.1152/japplphysiol.01142.2001 [DOI] [PubMed] [Google Scholar]
- [22].Roddie IC, Shepherd JT, Whelan RF. The contribution of constrictor and dilator nerves to the skin vasodilation during body heating. J Physiol 1957; 136:489-97; PMID:13429515; http://dx.doi.org/ 10.1113/jphysiol.1957.sp005775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Grossmann M, Jamieson MJ, Kellogg DL Jr, Kosiba WA, Pergola PE, Crandall CG, Shepherd AM. The effect of iontophoresis on the cutaneous vasculature: evidence for current-induced hyperemia. Microvasc Res 1995; 50:444-52; PMID:8583956; http://dx.doi.org/ 10.1006/mvre.1995.1070 [DOI] [PubMed] [Google Scholar]
- [24].Shastry S, Minson CT, Wilson SA, Dietz NM, Joyner MJ. Effects of atropine and L-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol 2000; 88:467-72; PMID:10658012 [DOI] [PubMed] [Google Scholar]
- [25].Kolka MA, Stephenson LA. Cutaneous blood flow and local sweating after systemic atropine administration. Pflügers Archiv 1987; 410:524-9; PMID:3432054; http://dx.doi.org/ 10.1007/BF00586536 [DOI] [PubMed] [Google Scholar]
- [26].Love AHG, Shanks RG. The relationship between the onset of sweating and vasodilation in the forearm during body heating. J Physiol 1962; 162:121-8; PMID:14466872; http://dx.doi.org/ 10.1113/jphysiol.1962.sp006918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kolka MA, Stephenson LA. Atropine-induced vasodilation decreases esophageal temperature during exercise. Am J Physiol Reg Integ Comp Physiol 1989; 257:R1089-R95. [DOI] [PubMed] [Google Scholar]
- [28].Shibasaki M, Wilson TE, Cui J, Crandall CG. Acetylcholine released from cholinergic nerves contributes to cutaneous vasodilation during heat stress. J Appl Physiol 2002; 93:1947-51; PMID:12391110; http://dx.doi.org/ 10.1152/japplphysiol.00036.2002 [DOI] [PubMed] [Google Scholar]
- [29].Kamijo YI, Lee K, Mack GW. Active cutaneous vasodilation in resting humans during mild heat stress. J Appl Physiol 2005; 98:829-37; PMID:15489258; http://dx.doi.org/ 10.1152/japplphysiol.00235.2004 [DOI] [PubMed] [Google Scholar]
- [30].Smith CJ, Johnson JM. Responses to hyperthermia. Optimizing heat dissipation by convection and evaporation: Neural control of skin blood flow and sweating in humans. Auton Neurosci 2016; 196:25-36; http://dx.doi.org/ 10.1016/j.autneu.2016.01.002 [DOI] [PubMed] [Google Scholar]
- [31].Simpson LL. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev 1981; 33:155-88; PMID:6119708 [PubMed] [Google Scholar]
- [32].Johnson JM, Minson CT, Kellogg DL. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Comp Physiol ; 2014; 4:33-89; http://dx.doi.org/ 10.1002/cphy.c130015 [DOI] [PubMed] [Google Scholar]
- [33].Taylor WF, Bishop VS. A role for nitric oxide in active thermoregulatory vasodilation. Am J Physiol Heart Circ Physiol 1993; 264:H1355-H9. [DOI] [PubMed] [Google Scholar]
- [34].Taylor WF, DiCarlo SE, Bishop VS. Neurogenic vasodilator control of rabbit ear blood flow. Am J Physiol Reg Integ Comp Physiol 1992; 262:R766-R70. [DOI] [PubMed] [Google Scholar]
- [35].Farrell DM, Bishop VS. Permissive role for nitric oxide in active thermoregulatory vasodilation in rabbit ear. Am J Physiol Heart Circ Physiol 1995; 269:H1613-H8. [DOI] [PubMed] [Google Scholar]
- [36].Farrell DM, Bishop VS. The roles of cGMP and cAMP in active thermoregulatory vasodilation in rabbit ear. Am J Physiol Reg Integ Comp Physiol 1997; 272:R975-R81. [DOI] [PubMed] [Google Scholar]
- [37].Dietz NM, Rivera JM, Warner DO, Joyner MJ. Is nitric oxide involved in cutaneous vasodilation during body heating in humans? J Appl Physiol 1994; 76:2047-53; PMID:7520431 [DOI] [PubMed] [Google Scholar]
- [38].Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol 1998; 85:830-4; PMID:9729554 [DOI] [PubMed] [Google Scholar]
- [39].Kellogg DL Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol 1998; 85:824-9; PMID:9729553 [DOI] [PubMed] [Google Scholar]
- [40].Shibasaki M, Crandall CG. Effect of local acetylcholinesterase inhibition on sweat rate in humans. J Appl Physiol 2001; 90:757-62; PMID:11181580 [DOI] [PubMed] [Google Scholar]
- [41].Kellogg DL, Zhao JL, Friel C, Roman LJ. Nitric oxide concentration increases in the cutaneous interstitial space during heat stress in humans. J Appl Physiol 2003; 94:1971-7; PMID:12679350; http://dx.doi.org/ 10.1152/japplphysiol.00826.2002 [DOI] [PubMed] [Google Scholar]
- [42].Wilkins BW, Holowatz LA, Wong BJ, Minson CT. Nitric oxide is not permissive for cutaneous active vasodilatation in humans. J Physiol 2003; 548:963-9; PMID:12651918; http://dx.doi.org/ 10.1113/jphysiol.2002.035931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kellogg D, Zhao J, Wu Y. Roles of nitric oxide synthase isoforms in cutaneous vasodilation induced by local warming of the skin and whole body heat stress in humans. J Appl Physiol 2009; 107:1438-44; PMID:19745188; http://dx.doi.org/ 10.1152/japplphysiol.00690.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kellogg DL Jr, Zhao JL, Wu Y. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. Am J Physiol Heart Circ Physiol 2008; 295:H123-9; PMID:18469149; http://dx.doi.org/ 10.1152/ajpheart.00082.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kellogg DL, Zhao JL, Wu Y. Neuronal nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. J Physiol 2008; 586:847-57; PMID:18048451; http://dx.doi.org/ 10.1113/jphysiol.2007.144642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].McNamara TC, Keen JT, Simmons GH, Alexander LM, Wong BJ. Endothelial nitric oxide synthase mediates the nitric oxide component of reflex cutaneous vasodilatation during dynamic exercise in humans. J Physiol 2014; 592:5317-26; PMID:25260636; http://dx.doi.org/ 10.1113/jphysiol.2014.272898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kellogg DL, Zhao JL, Wu Y, Johnson JM. Antagonism of soluble guanylyl cyclase attenuates cutaneous vasodilation during whole body heat stress and local warming in humans. J Appl Physiol 2011; 110:1406-13; PMID:21292837; http://dx.doi.org/ 10.1152/japplphysiol.00702.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hartschuh W, Reinecke M, Weihe E, Yanaihara N. VIP-immunoreactivity in the skin of various mammals: immunohistochemical, radioimmunological and experimental evidence for a dual localization in cutaneous nerves and merkel cells. Peptides 1984; 5:239-45; PMID:6382194; http://dx.doi.org/ 10.1016/0196-9781(84)90213-4 [DOI] [PubMed] [Google Scholar]
- [49].Hökfelt T, Johansson O, Ljungdahl A, Lundberg JM, Schultzberg M. Peptidergic neurones. Nature 1980; 284:515-21; PMID:6154244; http://dx.doi.org/ 10.1038/284515a0 [DOI] [PubMed] [Google Scholar]
- [50].Vaalasti A, Tainio H, Rechardt L. Vasoactive intestinal polypeptide (VIP)-like immunoreactivity in the nerves of human axillary sweat glands. J Invest Dermatol 1985; 85:246-8; PMID:4031541; http://dx.doi.org/ 10.1111/1523-1747.ep12276717 [DOI] [PubMed] [Google Scholar]
- [51].Savage MV, Brengelmann GL, Buchan AM, Freund PR. Cystic fibrosis, vasoactive intestinal polypeptide, and active cutaneous vasodilation. J Appl Physiol 1990; 69:2149-54; PMID:1706332 [DOI] [PubMed] [Google Scholar]
- [52].Heinz-Erian P, Dey RD, Flux M, Said SI. Deficient vasoactive intestinal peptide innervation in the sweat glands of cystic fibrosis patients. Science 1985; 229:1407-8; PMID:4035357; http://dx.doi.org/ 10.1126/science.4035357 [DOI] [PubMed] [Google Scholar]
- [53].Kellogg DL Jr, Hodges GJ, Orozco CR, Phillips TM, Zhao JL, Johnson JM. Cholinergic mechanisms of cutaneous active vasodilation during heat stress in cystic fibrosis. J Appl Physiol 2007; 103:963-8; PMID:17600158; http://dx.doi.org/ 10.1152/japplphysiol.00278.2007 [DOI] [PubMed] [Google Scholar]
- [54].Wilkins BW, Martin E, Roberts SK, Joyner MJ. Preserved reflex cutaneous vasodilation in cystic fibrosis does not include an enhanced nitric oxide-dependent mechanism. J Appl Physiol 2007; 102:2301-6; PMID:17412796; http://dx.doi.org/ 10.1152/japplphysiol.00013.2007 [DOI] [PubMed] [Google Scholar]
- [55].Bennett LAT, Johnson JM, Stephens DP, Saad AR, Kellogg DL. Evidence for a Role for Vasoactive Intestinal Peptide in Active Vasodilatation in the Cutaneous Vasculature of Humans. J Physiol 2003; 552:223-32; PMID:12847205; http://dx.doi.org/ 10.1113/jphysiol.2003.042135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wilkins BW, Wong BJ, Tublitz NJ, McCord GR, Minson CT. Vasoactive intestinal peptide fragment VIP10-28 and active vasodilation in human skin. J Appl Physiol 2005; 99:2294-301; PMID:16109832; http://dx.doi.org/ 10.1152/japplphysiol.00500.2005 [DOI] [PubMed] [Google Scholar]
- [57].Wilkins BW, Chung LH, Tublitz NJ, Wong BJ, Minson CT. Mechanisms of vasoactive intestinal peptide-mediated vasodilation in human skin. J Appl Physiol 2004; 97:1291-8; PMID:15155712; http://dx.doi.org/ 10.1152/japplphysiol.00366.2004 [DOI] [PubMed] [Google Scholar]
- [58].Johnson JM, Kellogg DL. Local thermal control of the human cutaneous circulation. J Appl Physiol 2010; 109:1229-38; PMID:20522732; http://dx.doi.org/ 10.1152/japplphysiol.00407.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kellogg DL, Zhao JL, Wu Y, Johnson JM. Nitric oxide and receptors for VIP and PACAP in cutaneous active vasodilation during heat stress in humans. J Appl Physiol 2012; 113:1512-8; PMID:22961270; http://dx.doi.org/ 10.1152/japplphysiol.00859.2012 [DOI] [PubMed] [Google Scholar]
- [60].Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol 2004; 560:941-8; PMID:15375193; http://dx.doi.org/ 10.1113/jphysiol.2004.071779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].McCord GR, Cracowski J-L, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 2006; 291:R596-R602; PMID:16484440; http://dx.doi.org/ 10.1152/ajpregu.00710.2005 [DOI] [PubMed] [Google Scholar]
- [62].Holowatz LA, Kenney WL. Chronic low-dose aspirin therapy attenuates reflex cutaneous vasodilation in middle-aged humans. J Appl Physiol 2009; 106:500-5; PMID:19036898; http://dx.doi.org/ 10.1152/japplphysiol.91215.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wong BJ, Minson CT. Neurokinin-1 receptor desensitization attenuates cutaneous active vasodilatation in humans. J Physiol 2006; 577:1043-51; PMID:17023511; http://dx.doi.org/ 10.1113/jphysiol.2006.112508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wong BJ, Tublitz NJ, Minson CT. Neurokinin-1 receptor desensitization to consecutive microdialysis infusions of substance P in human skin. J Physiol 2005; 568:1047-56; PMID:16123103; http://dx.doi.org/ 10.1113/jphysiol.2005.095372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Milner P, Kirkpatrick KA, Ralevic V, Toothill V, Pearson J, Burnstock G. Endothelial cells cultured from human umbilical vein release ATP, substance P and acetylcholine in response to increased flow. Proc R Soc Lond B Biol Sci 1990; 241:245-8; http://dx.doi.org/ 10.1098/rspb.1990.0092 [DOI] [PubMed] [Google Scholar]
- [66].Milner P, Ralevic V, Hopwood AM, Fehér E, Lincoln J, Kirkpatrick KA, Burnstock G. Ultrastructural localisation of substance P and choline acetyltransferase in endothelial cells of rat coronary artery and release of substance P and acetylcholine during hypoxia. Experientia 1989; 45:121-5; PMID:2465912; http://dx.doi.org/ 10.1007/BF01954843 [DOI] [PubMed] [Google Scholar]
- [67].Charkoudian N, Fromy Brr, Saumet JL. Reflex control of the cutaneous circulation after acute and chronic local capsaicin. J Appl Physiol 2001; 90:1860-4; PMID:11299278; http://dx.doi.org/ 10.1063/1.1384853 [DOI] [PubMed] [Google Scholar]
- [68].Wong BJ, Fieger SM. Transient receptor potential vanilloid type 1 channels contribute to reflex cutaneous vasodilation in humans. J Appl Physiol 2012; 112:2037-42; PMID:22518827; http://dx.doi.org/ 10.1152/japplphysiol.00209.2012 [DOI] [PubMed] [Google Scholar]
- [69].Wong BJ. Sensory nerves and nitric oxide contribute to reflex cutaneous vasodilation in humans. Am J Physiol Reg Integ Comp Physiol 2013; 304:R651-R6; http://dx.doi.org/ 10.1152/ajpregu.00464.2012 [DOI] [PubMed] [Google Scholar]
- [70].Fieger SM, Wong BJ. No direct role for A1/A2 adenosine receptor activation to reflex cutaneous vasodilatation during whole-body heat stress in humans. Acta Physiol 2012; 205:403-10; http://dx.doi.org/ 10.1111/j.1748-1716.2012.02426.x [DOI] [PubMed] [Google Scholar]
- [71].Brunt VE, Minson CT. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol 2012; 590:3523-34; PMID:22674719; http://dx.doi.org/ 10.1113/jphysiol.2012.236398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Brunt VE, Fujii N, Minson CT. No independent, but an interactive, role of calcium-activated potassium channels in human cutaneous active vasodilation. J Appl Physiol 2013; 115:1290-6; PMID:23970531; http://dx.doi.org/ 10.1152/japplphysiol.00358.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Johnson JM. Exercise and the cutaneous circulation. Exerc Sport Sci Rev 1992; 20:59-97; PMID:1623893; http://dx.doi.org/ 10.1249/00003677-199200200-00003 [DOI] [PubMed] [Google Scholar]
- [74].Johnson JM, Park MK. Effect of heat stress on cutaneous vascular responses to the initiation of exercise. J Appl Physiol 1982; 53:744-9; PMID:7129999; http://dx.doi.org/ 10.1063/1.329940 [DOI] [PubMed] [Google Scholar]
- [75].Kellogg DL, Johnson JM, Kosiba WA. Competition between cutaneous active vasoconstriction and active vasodilation during exercise in humans. Am J Physiol 1991; 261:H1184-H9; PMID:1928401 [DOI] [PubMed] [Google Scholar]
- [76].Barlett HL, Kenney WL, Buskirk ER. Body composition and the expiratory reserve volume of pre-pubertal lean and obese boys and girls. Int J Obes Relat Metab Disord 1992; 16:653-6; PMID:1328089 [PubMed] [Google Scholar]
- [77].Taylor WF, Johnson JM, O'Leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol 1984; 57:191-6; PMID:6469780 [DOI] [PubMed] [Google Scholar]
- [78].Johnson JM, Park MK. Effect of upright exercise on threshold for cutaneous vasodilation and sweating. J Appl Physiol 1981; 50:814-8; PMID:7263365 [DOI] [PubMed] [Google Scholar]
- [79].Kellogg DL, Johnson JM, Kosiba WA. Control of internal temperature threshold for active cutaneous vasodilation by dynamic exercise. J Appl Physiol 1991; 71:2476-82; PMID:1778949 [DOI] [PubMed] [Google Scholar]
- [80].Takamata A, Nagashima K, Nose H, Morimoto T. Role of plasma osmolality in the delayed onset of thermal cutaneous vasodilation during exercise in humans. Am J Physiol Reg Integ Comp Physiol 1998; 275:R286-R90. [DOI] [PubMed] [Google Scholar]
- [81].Mitono H, Endoh H, Okazaki K, Ichinose T, Masuki S, Takamata A, Nose H. Acute hypoosmolality attenuates the suppression of cutaneous vasodilation with increased exercise intensity. J Appl Physiol 2005; 99:902-8; PMID:15845777; http://dx.doi.org/ 10.1152/japplphysiol.00156.2005 [DOI] [PubMed] [Google Scholar]
- [82].Shibasaki M, Aoki K, Morimoto K, Johnson JM, Takamata A. Plasma hyperosmolality elevates the internal temperature threshold for active thermoregulatory vasodilation during heat stress in humans. Am J Physiol Reg Integ Comp Physiol 2009; 297:R1706-12.; http://dx.doi.org/ 10.1152/ajpregu.00242.2009 [DOI] [PubMed] [Google Scholar]
- [83].Brengelmann GL, Johnson JM, Hermansen L, Rowell LB. Altered control of skin blood flow during exercise at high internal temperatures. J Appl Physiol 1977; 43:790-4; PMID:591471 [DOI] [PubMed] [Google Scholar]
- [84].Kellogg DL Jr, Johnson JM, Kenney WL, Pérgola PE, Kosiba WA. Mechanisms of control of skin blood flow during prolonged exercise in humans. Am J Physiol Heart Circ Physiol 1993; 265:H562-H8. [DOI] [PubMed] [Google Scholar]
- [85].Tankersley CG, Smolander J, Kenney WL, Fortney SM. Sweating and skin blood flow during exercise: effects of age and maximal oxygen uptake. J Appl Physiol 1991; 71:236-42; PMID:1917747 [DOI] [PubMed] [Google Scholar]
- [86].Bernard TE, Kenney WL. Rationale for a personal monitor for heat strain. Am Ind Hyg Assoc J 1994; 55:505-14; PMID:8017291; http://dx.doi.org/ 10.1080/15428119491018772 [DOI] [PubMed] [Google Scholar]
- [87].Blair DA, Glover WE, Roddie IC. Vasomotor responses in the human arm during leg exercise. Circ Res 1961; 9:264-74; http://dx.doi.org/ 10.1161/01.RES.9.2.264 [DOI] [Google Scholar]
- [88].Fujii N, Meade RD, Alexander LM, Akbari P, Foudil-bey I, Louie JC, Boulay P, Kenny GP. iNOS-dependent sweating and eNOS-dependent cutaneous vasodilation are evident in younger adults, but are diminished in older adults exercising in the heat. J Appl Physiol 2016; 120:318-27; PMID:26586908; http://dx.doi.org/ 10.1152/japplphysiol.00714.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Stachenfeld NS, Taylor HS. Sex hormone effect on body fluid and sodium regulation in women with and without exercise-associated hyponatremia. J Appl Physiol (1985) 2009; 107:864-72; PMID:19556454; http://dx.doi.org/ 10.1152/japplphysiol.91211.2008 [DOI] [PubMed] [Google Scholar]
- [90].Charkoudian N, Johnson JM. Modification of active cutaneous vasodilation by oral contraceptive hormones. J Appl Physiol 1997; 83:2012-8; PMID:9390975 [DOI] [PubMed] [Google Scholar]
- [91].Tankersley CG, Nicholas WC, Deaver DR, Mikita D, Kenney WL. Estrogen replacement in middle-aged women: thermoregulatory responses to exercise in the heat. J Appl Physiol 1992; 73:1238-45; PMID:1447065 [DOI] [PubMed] [Google Scholar]
- [92].Stephenson LA, Kolka MA. Endogenous 17B-estradiol decreases core temperature threshold for sweating in women. Faseb Journal 1995; 9:A357. [DOI] [PubMed] [Google Scholar]
- [93].Stephenson LA, Kolka MA. Esophogeal temperature threshold for sweating decreases before ovulation in premenopausal women. J Appl Physiol (1985) 1999; 86:22-8; PMID:9887109 [DOI] [PubMed] [Google Scholar]
- [94].Israel SL, Schneller O. The thermogenic property of progesterone. Fertil Steril 1950; 1:53-65; http://dx.doi.org/ 10.1016/S0015-0282(16)30066-8 [DOI] [Google Scholar]
- [95].Prior JC, McKay DW, Vigna YM, Barr SI. Medroxyprogesterone increases basal temperature: a placebo-controlled crossover trial in post-menopausal women. Fertil Steril 1995; 63:1222-6; PMID:7750591; http://dx.doi.org/ 10.1016/S0015-0282(16)57601-8 [DOI] [PubMed] [Google Scholar]
- [96].Stephenson LA, Kolka MA. Menstrual cycle phase and time of day alter reference signal controlling arm blood flow and sweating. Am J Physiol Reg Integ Comp Physiol 1985; 18:R186-R91. [DOI] [PubMed] [Google Scholar]
- [97].Hessemer V, Bruck K. Influence of menstrualy cycle on shivering, skin blood flow and sweating responses at night. J Appl Physiol (1985) 1985; 59:1902-10; PMID:4077797 [DOI] [PubMed] [Google Scholar]
- [98].Kolka MA, Stephenson LA. Control of sweating during the human menstrual cycle. Eur J Appl Physiol 1989; 58:890-5; http://dx.doi.org/ 10.1007/BF02332224 [DOI] [PubMed] [Google Scholar]
- [99].Hirata K, Nagasaka T, Hirashita M, Takahata T, Nuriomura T. Effects of human menstrual cycle on thermoregulatory vasodilation during exercise. Eur J Appl Physiol 1986; 54:559-65; http://dx.doi.org/ 10.1007/BF00943341 [DOI] [PubMed] [Google Scholar]
- [100].Rogers SM, Baker MA. Thermoregulation during exercise in women who are taking oral contraceptives. Eur J Appl Physiol 1997; 75:34-8; http://dx.doi.org/ 10.1007/s004210050123 [DOI] [PubMed] [Google Scholar]
- [101].Houghton B, Holowatz L, Minson C. Influence of progestin bioactivity on cutaneous vascular responses to passive heating. Med Sci Sports Exerc 2005; 37:45-51; PMID:15632666; http://dx.doi.org/ 10.1249/01.MSS.0000150075.81511.FE [DOI] [PubMed] [Google Scholar]
- [102].Stachenfeld NS, Silva C, Keefe DL. Estrogen modifies the temperature effects of progesterone. J Appl Physiol (1985) 2000; 88:1634-49. [DOI] [PubMed] [Google Scholar]
- [103].Kenney WL. Control of heat-induced cutaneous vasodilatation in relation to age. European J Applied Physiol Occupational Physiol 1988; 57:120-5; http://dx.doi.org/ 10.1007/BF00691250 [DOI] [PubMed] [Google Scholar]
- [104].Kenney WL, Morgan AL, Farquhar WB, Brooks EM, Pierzga JM, Derr JA. Decreased active vasodilator sensitivity in aged skin. Am J Physiol 1997; 272(4) Pt 2:H1609-14; PMID:9139942 [DOI] [PubMed] [Google Scholar]
- [105].Martin HL, Loomis JL, Kenney WL. Maximal skin vascular conductance in subjects aged 5-85 yr. J Appl Physiol 1995; 79:297-301; PMID:7559234 [DOI] [PubMed] [Google Scholar]
- [106].Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol 1998; 84:1323-32; PMID:9516200 [DOI] [PubMed] [Google Scholar]
- [107].Sagawa S, Shiraki K, Yousef MK, Miki K. Sweating and cardiovascular responses of aged men to heat exposure. J Gerontol 1988; 43:M1-M8; PMID:3335747; http://dx.doi.org/ 10.1093/geronj/43.1.M1 [DOI] [PubMed] [Google Scholar]
- [108].Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol 2003; 284:H1662-7; PMID:12505876; http://dx.doi.org/ 10.1152/ajpheart.00871.2002 [DOI] [PubMed] [Google Scholar]
- [109].Holowatz LA, Thompson CS, Kenney WL. L-Arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. J Physiol 2006; 574:573-81; PMID:16675494; http://dx.doi.org/ 10.1113/jphysiol.2006.108993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Holowatz LA, Thompson CS, Kenney WL. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. Am J Physiol Heart Circ Physiol 2006; 291:H2965-70; PMID:16905599; http://dx.doi.org/ 10.1152/ajpheart.00648.2006 [DOI] [PubMed] [Google Scholar]
- [111].Stanhewicz AE, Alexander LM, Kenney WL. Oral sapropterin acutely augments reflex vasodilation in aged human skin through nitric oxide-dependent mechanisms. J Appl Physiol (1985) 2013; 115:972-8; PMID:23743404; http://dx.doi.org/ 10.1152/japplphysiol.00481.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Stanhewicz AE, Bruning RS, Smith CJ, Kenney WL, Holowatz LA. Local tetrahydrobiopterin administration augments reflex cutaneous vasodilation through nitric oxide-dependent mechanisms in aged human skin. J Appl Physiol (1985) 2012; 112:791-7; PMID:22162527; http://dx.doi.org/ 10.1152/japplphysiol.01257.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Stanhewicz AE, Alexander LM, Kenney WL. Folic acid supplementation improves microvascular function in older adults through nitric oxide-dependent mechanisms. Clin Sci (Lond) 2015; 129:159-67; PMID:25748442; http://dx.doi.org/ 10.1042/CS20140821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Holowatz LA, Jennings JD, Lang JA, Kenney WL. Ketorolac alters blood flow during normothermia but not during hyperthermia in middle-aged human skin. J Appl Physiol 2009; 107:1121-7; PMID:19661446; http://dx.doi.org/ 10.1152/japplphysiol.00750.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Holowatz LA, Jennings JD, Lang JA, Kenney WL. Systemic low-dose aspirin and clopidogrel independently attenuate reflex cutaneous vasodilation in middle-aged humans. J Appl Physiol 2010; 108:1575-81; PMID:20360429; http://dx.doi.org/ 10.1152/japplphysiol.01362.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Bruning RS, Dahmus JD, Kenney WL, Alexander LM. Aspirin and clopidogrel alter core temperature and skin blood flow during heat stress. Med Sci Sports Exerc 2013; 45:674-82; PMID:23135368; http://dx.doi.org/ 10.1249/MSS.0b013e31827981dc [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Heimhalt-El Hamriti M, Schreiver C, Noerenberg A, Scheffler J, Jacoby U, Haffner D, Fischer DC. Impaired skin microcirculation in paediatric patients with type 1 diabetes mellitus. Cardiovasc Diabetol 2013; 12:115-; PMID:23937662; http://dx.doi.org/ 10.1186/1475-2840-12-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Naylor LH, Yusof NM, Paramalingam N, Jones TW, Davis EA, Green DJ. Acute hyperglycaemia does not alter nitric oxide-mediated microvascular function in the skin of adolescents with type 1 diabetes. Eur J Appl Physiol 2014; 114:435-41; PMID:24337670; http://dx.doi.org/ 10.1007/s00421-013-2785-6 [DOI] [PubMed] [Google Scholar]
- [119].Kenny GP, Sigal RJ, McGinn R. Body temperature regulation in diabetes. Temperature 2016; 3:119-45; http://dx.doi.org/ 10.1080/23328940.2015.1131506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Carter M, McGinn R, Barrera Ramirez J, Sigal R, Kenny G. Impairments in local heat loss in type 1 diabetes during exercise in the heat. Med Sci Sports Exerc 2014; 46:2224-33; PMID:24784146; http://dx.doi.org/ 10.1249/MSS.0000000000000350 [DOI] [PubMed] [Google Scholar]
- [121].McGinn R, Carter MR, Barrera-Ramirez J, Sigal RJ, Flouris AD, Kenny GP. Does type 1 diabetes alter post-exercise thermoregulatory and cardiovascular function in young adults? Scandinavian J Med Sci Sports 2015; 25:e504-e14; http://dx.doi.org/ 10.1111/sms.12344 [DOI] [PubMed] [Google Scholar]
- [122].Stapleton J, Yardley J, Boulay P, Sigal R, Kenny G. Whole-body heat loss during exercise in the heat is not impaired in type 1 diabetes. Med Sci Sports Exerc 2013; 45:1656-64; PMID:23475170; http://dx.doi.org/ 10.1249/MSS.0b013e31829002f3 [DOI] [PubMed] [Google Scholar]
- [123].Sokolnicki LA, Strom NA, Roberts SK, Kingsley-Berg SA, Basu A, Charkoudian N. Skin blood flow and nitric oxide during body heating in type 2 diabetes mellitus. J Appl Physiol 2009; 106:566-70; PMID:19056994; http://dx.doi.org/ 10.1152/japplphysiol.91289.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Wick DE, Roberts SK, Basu A, Sandroni P, Fealey RD, Sletten D, Charkoudian N. Delayed threshold for active cutaneous vasodilation in patients with Type 2 diabetes mellitus. J Appl Physiol 2006; 100:637-41; PMID:16210432; http://dx.doi.org/ 10.1152/japplphysiol.00943.2005 [DOI] [PubMed] [Google Scholar]
- [125].Kenney WL. Decreased core-to-skin heat transfer in mild essential hypertensives exercising in the heat. Clin Exp Hypertens A 1985; 7:1165-72; PMID:4042389 [DOI] [PubMed] [Google Scholar]
- [126].Kenney WL, Kamon E. Comparative physiological responses of normotensive and essentially hypertensive men to exercise in the heat. Eur J Applied Physiol Occupational Physiol 1984; 52:196-201; PMID:6538835; http://dx.doi.org/ 10.1007/BF00433392 [DOI] [PubMed] [Google Scholar]
- [127].Kenney WL, Kamon E, Buskirk ER. Effect of mild essential hypertension on control of forearm blood flow during exercise in the heat. J Applied Physiol 1984; 56:930-5; PMID:6725071 [DOI] [PubMed] [Google Scholar]
- [128].Holowatz LA, Kenney WL. Local ascorbate administration augments NO- and non-NO-dependent reflex cutaneous vasodilation in hypertensive humans. Am J Physiol Heart Circ Physiol 2007; 293:H1090-H6; PMID:17483240; http://dx.doi.org/ 10.1152/ajpheart.00295.2007 [DOI] [PubMed] [Google Scholar]
- [129].Holowatz LA, Kenney WL. Up-regulation of arginase activity contributes to attenuated reflex cutaneous vasodilatation in hypertensive humans. J Physiol 2007; 581:863-72; PMID:17347269; http://dx.doi.org/ 10.1113/jphysiol.2007.128959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Bruning RS, Kenney WL, Alexander LM. Altered skin flowmotion in hypertensive humans. Microvasc Res 2015; 97:81-7; PMID:24418051; http://dx.doi.org/ 10.1016/j.mvr.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Smith C, Santhanam L, Bruning R, Stanhewicz A, Berkowitz D, Holowatz L. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension 2011; 58:935-42; PMID:21931069; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.111.178129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Frohman EM, Racke MK, Raine CS. Multiple sclerosis - the plaque and its pathogenesis. N Engl J Med 2006; 354:942-55; PMID:16510748; http://dx.doi.org/ 10.1056/NEJMra052130 [DOI] [PubMed] [Google Scholar]
- [133].Davis SL, Wilson TE, White AT, Frohman EM. Thermoregulation in multiple sclerosis. J Appl Physiol 2010; 109:1531-7; PMID:20671034; http://dx.doi.org/ 10.1152/japplphysiol.00460.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Davis SL, Korkmas MA, Crandall CG, Frohman EM. Impaired sweating in multiple sclerosis leads to increased reliance on skin blood flow for heat dissipation (abstract). Faseb Journal 2010; 24:991.25. [Google Scholar]
- [135].Davis SL, Wilson TE, Vener JM, Crandall CG, Petajan JH, White AT. Pilocarpine-induced sweat gland function in individuals with multiple sclerosis. J Appl Physiol 2005; 98:1740-4; PMID:15640392; http://dx.doi.org/ 10.1152/japplphysiol.00860.2004 [DOI] [PubMed] [Google Scholar]
- [136].Crandall CG, Davis SL. Cutaneous vascular and sudomotor responses in human skin grafts. J Appl Physiol 2010; 109:1524-30; PMID:20558761; http://dx.doi.org/ 10.1152/japplphysiol.00466.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Davis S, Shibasaki M, Low D, Cui J, Keller D, Purdue G, Hunt J, Arnoldo TBD, Kowalske K, Crandall C. Impaired cutaneous vasodilation and sweating in grafted skin during whole-body heating. J Burn Care Res 2007; 28:427-34; PMID:17438492; http://dx.doi.org/ 10.1097/BCR.0B013E318053D312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Davis SL, Shibasaki M, Low DA, Cui J, Keller DM, Wingo JE, Purdue GF, Hunt JL, Arnoldo BD, Kowalske KJ, et al.. Sustained impairments in cutaneous vasodilation and sweating in grafted skin following long-term recovery. J Burn Care Res 2009; 30:675-85; PMID:19506504; http://dx.doi.org/ 10.1097/BCR.0b013e3181abfd43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Marczak ED, Marzec J, Zeldin DC, Kleeberger SR, Brown NJ, Pretorius M, Lee CR. Polymorphisms in the transcription factor NRF2 and forearm vasodilator responses in humans. Pharmacogenet Genomics 2012; 22:620-8; PMID:22668754; http://dx.doi.org/ 10.1097/FPC.0b013e32835516e5 [DOI] [PMC free article] [PubMed] [Google Scholar]