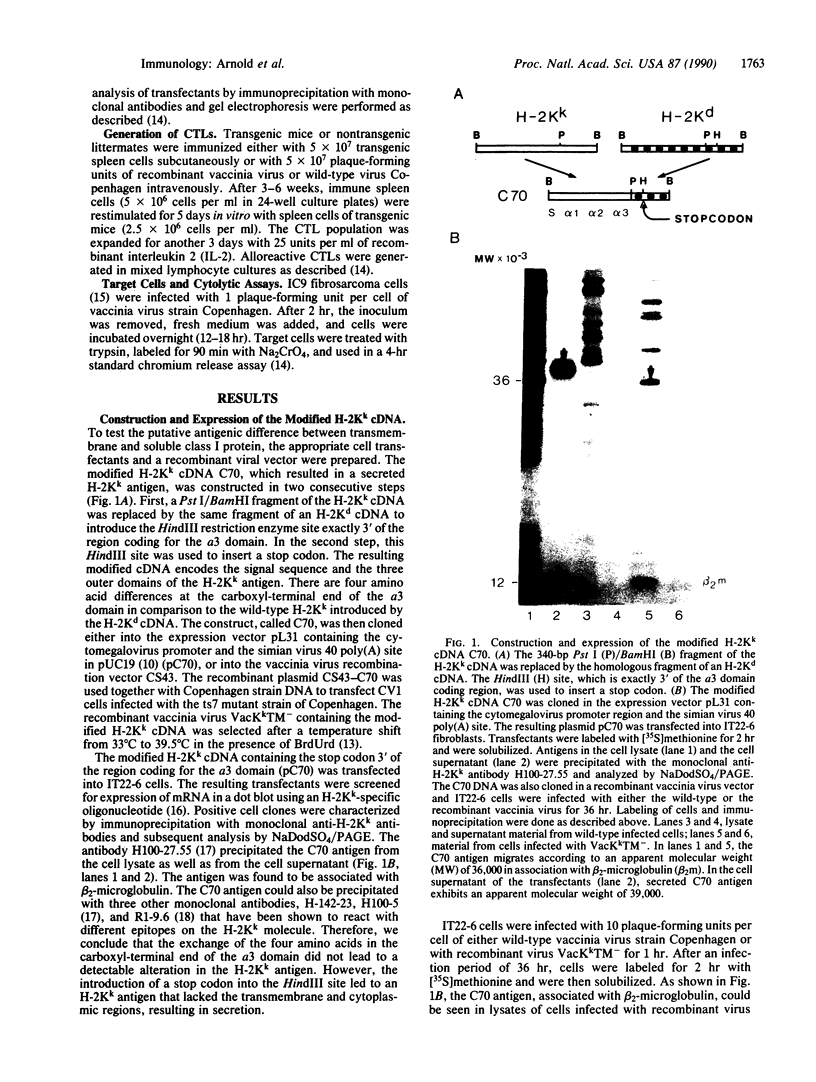
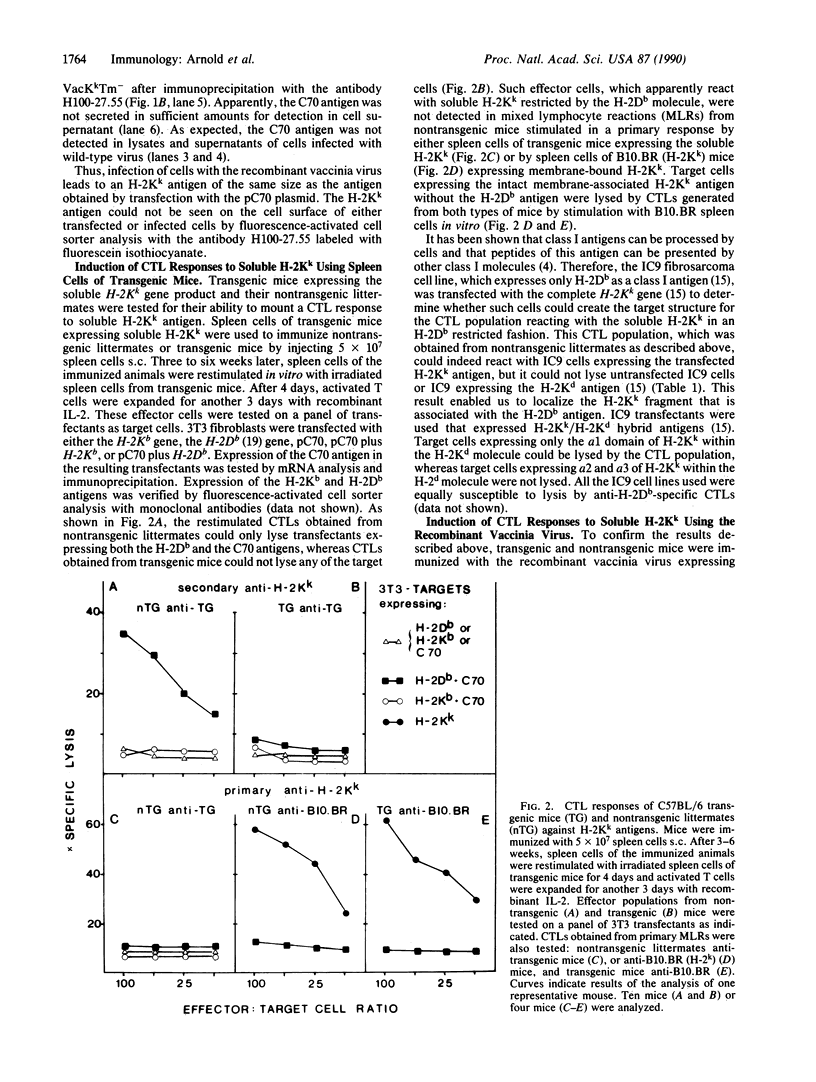
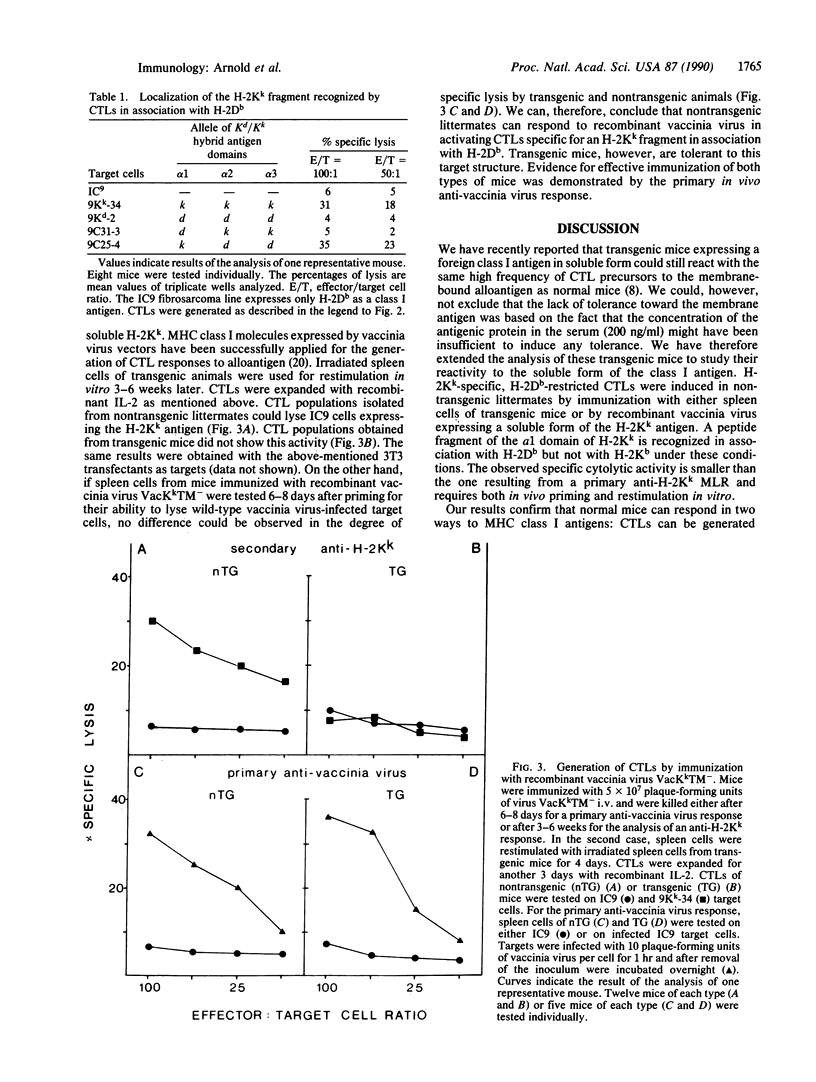
Abstract
The properties of transmembrane and soluble transplantation antigens were compared with respect to the induction of tolerance and the selection of the T-cell repertoire. For this purpose, transgenic (H-2b x H-2d)F1 mice were constructed that carry integrated copies of a modified H-2Kk gene resulting in the secretion from various cell types including thymocytes of soluble H-2Kk molecules. Despite the presence of H-2Kk antigen, these mice were still able to generate an H-2Kk-specific T-cell response. This response was comparable to that produced by normal littermates when stimulated with cells expressing membrane H-2Kk in a mixed lymphocyte reaction. In contrast, only transgenic mice failed to generate a cytolytic T-cell response to soluble H-2Kk antigen expressed by recombinant vaccinia virus and presented by the H-2Db molecule. These data imply the presence of two populations of alloreactive cytolytic T cells. A small fraction of T cells recognizes alloantigen as antigenic peptide(s) presented by other major histocompatibility complex class I molecules and tolerance can be induced in this population by soluble alloantigen. The majority of T cells, however, require the whole cell membrane-expressed class I molecule for recognition. This population is not affected by tolerance induction to the soluble major histocompatibility complex class I molecule.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen H., Wraith D., Pala P., Askonas B., Flavell R. A. Domain interactions of H-2 class I antigens alter cytotoxic T-cell recognition sites. Nature. 1984 May 17;309(5965):279–281. doi: 10.1038/309279a0. [DOI] [PubMed] [Google Scholar]
- Allison J., Campbell I. L., Morahan G., Mandel T. E., Harrison L. C., Miller J. F. Diabetes in transgenic mice resulting from over-expression of class I histocompatibility molecules in pancreatic beta cells. Nature. 1988 Jun 9;333(6173):529–533. doi: 10.1038/333529a0. [DOI] [PubMed] [Google Scholar]
- Arnold B., Burgert H. G., Hamann U., Hämmerling G., Kees U., Kvist S. Cytolytic T cells recognize the two amino-terminal domains of H-2 K antigens in tandem in influenza A infected cells. Cell. 1984 Aug;38(1):79–87. doi: 10.1016/0092-8674(84)90528-2. [DOI] [PubMed] [Google Scholar]
- Arnold B., Dill O., Küblbeck G., Jatsch L., Simon M. M., Tucker J., Hämmerling G. J. Alloreactive immune responses of transgenic mice expressing a foreign transplantation antigen in a soluble form. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2269–2273. doi: 10.1073/pnas.85.7.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold B., Horstmann U., Kuon W., Burgert H. G., Hämmerling G. J., Kvist S. Alloreactive cytolytic T-cell clones preferentially recognize conformational determinants on histocompatibility antigens: analysis with genetically engineered hybrid antigens. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7030–7034. doi: 10.1073/pnas.82.20.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Coupar B. E., Andrew M. E., Boyle D. B., Blanden R. V. Immune responses to H-2Kd antigen expressed by recombinant vaccinia virus. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7879–7882. doi: 10.1073/pnas.83.20.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranage M. P., Kouzarides T., Bankier A. T., Satchwell S., Weston K., Tomlinson P., Barrell B., Hart H., Bell S. E., Minson A. C. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 1986 Nov;5(11):3057–3063. doi: 10.1002/j.1460-2075.1986.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drillien R., Spehner D. Physical mapping of vaccinia virus temperature-sensitive mutations. Virology. 1983 Dec;131(2):385–393. doi: 10.1016/0042-6822(83)90506-8. [DOI] [PubMed] [Google Scholar]
- Heath W. R., Hurd M. E., Carbone F. R., Sherman L. A. Peptide-dependent recognition of H-2Kb by alloreactive cytotoxic T lymphocytes. Nature. 1989 Oct 26;341(6244):749–752. doi: 10.1038/341749a0. [DOI] [PubMed] [Google Scholar]
- Koch S., Koch N., Robinson P., Hämmerling G. Comparison of allogeneic and xenogeneic determinants on the H-2Kk molecule. Transplantation. 1983 Aug;36(2):177–180. doi: 10.1097/00007890-198308000-00013. [DOI] [PubMed] [Google Scholar]
- Machy P., Arnold B., Aliño S., Leserman L. D. Interferon sensitive and insensitive MHC variants of a murine thymoma differentially resistant to methotrexate-containing antibody-directed liposomes and immunotoxin. J Immunol. 1986 Apr 15;136(8):3110–3115. [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanski J. L., Pala P., Corradin G., Jordan B. R., Cerottini J. C. H-2-restricted cytolytic T cells specific for HLA can recognize a synthetic HLA peptide. Nature. 1986 Dec 11;324(6097):578–579. doi: 10.1038/324578a0. [DOI] [PubMed] [Google Scholar]
- Morahan G., Brennan F. E., Bhathal P. S., Allison J., Cox K. O., Miller J. F. Expression in transgenic mice of class I histocompatibility antigens controlled by the metallothionein promoter. Proc Natl Acad Sci U S A. 1989 May;86(10):3782–3786. doi: 10.1073/pnas.86.10.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson C. A., Williams L. C., McLaughlin-Taylor E., McMillan M. Creation of H-2 class I epitopes using synthetic peptides: recognition by alloreactive cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1031–1035. doi: 10.1073/pnas.86.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P., Clayberger C., Zorn S. L., Ludwig D. S., Schoolnik G. K., Krensky A. M. Inhibition of alloreactive cytotoxic T lymphocytes by peptides from the alpha 2 domain of HLA-A2. Nature. 1987 Feb 12;325(6105):625–628. doi: 10.1038/325625a0. [DOI] [PubMed] [Google Scholar]
- Schneck J., Maloy W. L., Coligan J. E., Margulies D. H. Inhibition of an allospecific T cell hybridoma by soluble class I proteins and peptides: estimation of the affinity of a T cell receptor for MHC. Cell. 1989 Jan 13;56(1):47–55. doi: 10.1016/0092-8674(89)90982-3. [DOI] [PubMed] [Google Scholar]
- Song E. S., Linsk R., Olson C. A., McMillan M., Goodenow R. S. Allospecific cytotoxic T lymphocytes recognize an H-2 peptide in the context of a murine major histocompatibility complex class I molecule. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1927–1931. doi: 10.1073/pnas.85.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]