Summary
Background
Disease-related symptoms impair the quality of life of countless patients with chronic lymphocytic leukemia (CLL) who do not require systemic therapy. Currently available therapies are not specifically aimed at symptom control. Because stimulation of the B-cell receptor activates Janus kinase (JAK)-2 in CLL cells and the JAK2 inhibitor ruxolitinib improves symptoms of patients with myelofibrosis, we hypothesized that ruxolitinib would improve disease-related symptoms in CLL patients.
Methods
Ruxolitinib (10 mg twice daily) was administered to symptomatic CLL patients who did not require systemic therapy for CLL. Scores on the brief fatigue inventory (BFI), CLL module of the MD Anderson symptom inventory (MDASI) and symptom-associated interference in daily activities (interference score; IS), were assessed prior to treatment and after 3 months of treatment. Plasma cytokine/chemokine levels were measured at baseline and at 3 months.
Findings
Forty-one CLL patients (25 untreated and 16 previously treated) were enrolled. Thirty-two (78%) of the participants experienced ≥20% reduction in the average BFI score or in the average MDASI score. 59% of the participants had ≥2 units reduction in worst fatigue score in 24 hours as assessed by the BFI. The mean percentage reductions in BFI, MDASI, and IS scores were >42% (p<0.0001). Improvements in the three symptom scores correlated with reductions in levels of IL-6, C-reactive protein, CXCL10, osteopontin, TNF-α, ICAM-1/CD54, VCAM-1/CD106, and beta-2 microglobulin. Furthermore, treatment with ruxolitinib increased and then decreased lymphocyte counts to baseline levels or lower. Grade 3/4 cytopenias were recorded in three patients.
Interpretation
In CLL patients, ruxolitinib significantly improved disease-related symptoms, reduced cytokine and chemokine levels, and increased and then decreased lymphocyte counts, likely through mobilization followed by apoptosis of CLL cells. Further studies aimed at testing the therapeutic efficacy of ruxolitinib in CLL are warranted.
Funding
Supported by the Incyte Corp., MD Anderson Cancer Center Support Grant CA016672 and Award Number P01 CA049639 from the National Cancer Institute.
Keywords: Ruxolitinib, symptoms, fatigue, chronic lymphocytic leukemia, inflammatory cytokines/chemokines
INTRODUCTION
A significant number of patients with chronic lymphocytic leukemia (CLL) who do not meet the international working group on CLL (iwCLL) 1 criteria for treatment of CLL experience fatigue and/or other disease-related debilitating symptoms that significantly impair their quality of life (QoL) and bring about severe distress and depression.2–5 Disease-associated symptoms such as fatigue, low-grade fever, night sweats, and weight loss are associated with elevated circulating levels of inflammatory cytokines and/or chemokines. Most symptomatic patients are treated with supportive care measures that provide little clinical benefit. As a consequence, a small fraction of patients with severe QoL impairment are offered systemic therapy for CLL.1. Our group has previously assessed the symptoms of 126 consecutive patients with CLL. The most severe symptoms were fatigue, disturbed sleep, drowsiness, distress, and difficulty remembering. About 70% of patients presented with a significantly abnormal symptom score. 6
Inflammatory cytokines induce disease-related symptoms7 in a variety of neoplastic diseases, 8 including hematologic malignancies such as primary myelofibrosis9,10 and CLL. 11 Elevated levels of TNF- α, IL-1β, IL-6, IL-1RA, and IFN-α were previously reported to significantly correlate with cancer associated fatigue, depression, night sweats. 12 The role of cytokines and chemokines in the initiation, maintenance, and progression of CLL has been the subject of intense research over the past two decades, 13 as elevated plasma cytokine levels10 correlate with unfavorable clinical outcome in patients with CLL. 13
We recently found that stimulation of the B-cell receptor (BCR) of CLL cells activates the Janus kinase (JAK)-2/signal transducer and activator of transcription (STAT)-3 pathway and that the JAK1/2 inhibitor ruxolitinib inhibits the phosphorylation of JAK2 and STAT3 on tyrosine residues of these cells, inducing their apoptosis. 14 Ruxolitinib is known to reduce the levels and inhibit the effects of a number of cytokines, chemokines, and hematopoietic growth factors, providing significant clinical benefits. In patients with myelofibrosis, ruxolitinib alleviates symptoms, improves QoL, and reduces spleen size and tumor burden. 15 Similarly, ruxolitinib was found to improve symptoms and decrease inflammation biomarkers such as the acute phase reactant C-reactive protein (CRP) in patients with steroid-refractory graft-versushost disease16 or pancreatic cancer. 17 Therefore, we sought to investigate the effect of ruxolitinib on disease-related symptoms in patients with CLL.
PATIENTS AND METHODS
Patient enrollment and treatment
This prospective, single arm, single institution, phase II clinical trial designed to test the effect of ruxolitinib on disease-related symptoms of patients with CLL was approved by The University of Texas MD Anderson Cancer Center institutional review board and supported by Incyte Corporation (clinicaltrials.gov identifier number NCT02131584). All patients in this study were enrolled between 15th September 2014 to 20th September 2015. Informed consent (verbal and written) was obtained in accordance with institutional guidelines and the Declaration of Helsinki. Patients with previously untreated or previously treated CLL that did not require therapeutic intervention according to the iwCLL guidelines1 were eligible. CLL patients with performance status ≤ 2 were eligible. According to the 2008 iwCLL guidelines, active disease should be considered if there is significant fatigue, defined as ECOG performance status 2 or worse and inability to work or perform usual activities). All participants were assessed for disease-related symptoms18 by using their medical history and the symptom questionnaires including the brief fatigue inventory (BFI), the CLL module of the MD Anderson symptom inventory (MDASI), and the symptom interference scale (IS) of the MDASI. The MDASI is widely used in evaluating the most prevalent cancer-related symptoms in both liquid and solid tumors and has been validated in CLL6. The BFI has been used to assess fatigue in myelofibrosis19, whereas both the BFI and MDASI scoring systems have been used in leukemia and lymphomas studies20 and BFI was used to assess the effect of ruxolitinib on symptom scores of patients with myeloproliferative neoplasms treated with ruxolitinib. 21 The symptom assessment tools are presented in Supplement file, page 1–2. 22 Symptoms were rated on a 0 to 10 scale (10 most severe), symptom interference was also rated on a 0 to 10 scale (10 interferes completely), and symptomatic patients with a baseline average symptom severity scale score of 2 points or greater on BFI scale were enrolled in the study. Patients were taken off study if they experienced no improvement in their symptoms. Patients who responded, continued treatment for up to 2 years based on the clinical judgment of the treating physician.
Ruxolitinib was administered orally at 10 mg twice daily, and dose adjustment (escalation or deescalation) was allowed. Symptom scores were assessed at baseline and after 2 weeks and 3 months of treatment. The primary endpoints of the trial was improvement in the average fatigue score (BFI) from baseline at time of enrollment to 3 months of treatment and/or a 2 point reduction in patients’ rating of their “worst fatigue” in the last 24 hours, assessed according to the BFI score. Improvement in MDSAI score and disease response assessment were the secondary endpoints of the study. Participants with ≥20% reduction in the average BFI, MDASI, or IS scores or ≥2 units reduction in the patient’s rating of their “worst fatigue” in the last 24 hours, were considered responders. For continuous calculations, we used all available observations and responder-based analyses. Adverse events were reported in accordance with the leukemiaspecific adverse event recording and reporting guidelines.
Correlative studies
Plasma cytokine and chemokine levels
The effect of ruxolitinib treatment on plasma levels of cytokines and chemokines, known to be reduced by ruxolitinib in patients with myelofibrosis treated with ruxolitinib, 23 was assessed by analysis with a multiplex cytokine array (customized biochip array; Randox Biosciences, County Antrim, U.K.) at baseline and at 3 months of treatment. Correlative studies were conducted in accordance with the research protocol. The array included 24 cytokine/chemokines and we have analyzed only those cytokines/chemokines which were possibly relevant to CLL microenvironment.
Fractionation of peripheral blood low-density cells
Participants’ peripheral blood cells were fractionated by using Ficoll Hypaque 1077 (Sigma-Aldrich, St. Louis, MO). Fractionated cells were used immediately or frozen for additional studies. More than 90% of peripheral blood CLL cells were CD5+/CD19+ lymphocytes.
IL-6 levels in CLL cells
Changes in interleukin (IL)-6 levels were determined by western immunoblotting in peripheral blood cells obtained from CLL patients prior to and 3 months into treatment. Mouse anti-human IL-6 antibodies (Sigma-Aldrich) were used as previously described. 24
Chemokine and cytokine mRNA expression in CLL cells
To determine whether treatment with ruxolitinib affected expression of cytokine/chemokines in CLL cells, peripheral blood cells obtained from four randomly selected patients who showed a response to ruxolitinib were subjected to analysis for various cytokine and chemokine mRNAs. Total RNA was isolated from these cells by using Trizol (Ambion/Life Technologies, Waltham, MA). Quality and RNA concentration were assessed by an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). cDNA was synthesized from 5 µg samples of total RNA with the SuperScript strand synthesis system for polymerase chain reaction (PCR; Invitrogen/Life Technologies, Waltham, MA). An RT2 Profiler PCR array (Qiagen, Valencia, CA) analysis was performed in accordance with the manufacturer’s instructions by using the 7900HT fast realtime (RT) PCR system (Applied Biosystems, Foster City, CA). The array’s results were validated by quantitative RT-PCR using the TaqMan gene expression assay for CCL2, CCL5, CXCR5, IL-8, and GAPDH according to the manufacturer’s instructions. Samples were analyzed in triplicate, and relative quantification was determined by the comparative CT method.
Because plasma level of CRP, in addition to cytokines and chemokines, has been found to correlate with disease burden, 25 and beta-2 microglobulin (β2M) is an established prognostic indicator in CLL, we also measured changes in the levels of CRP and β2M during treatment with ruxolitinib.
Statistical analysis
The average BFI, IS, and MDASI scores i.e. the means of the fatigue severity scores and the fatigue interference scores from the BFI and the total symptom severity scores from the MDASI from baseline to 2 weeks and 3 months of treatment were calculated and waterfall plots were generated showing subject-level changes across all subjects. Changes in plasma levels of cytokines and chemokines over the same period were analyzed for all participants, and a linear regression analysis was performed to estimate the association between changes in cytokine/chemokine plasma levels and percentage change in symptom scores at 3 months. Log2 of fold change was calculated as change from baseline values for the levels of cytokines and chemokines. The subset (composite) of cytokines produced largest adjusted R-square for the percent change of BFI, Interference, or MDASI using a linear regression model. The composite was calculated at subject level, which was a linear combination of the best subset of 10 cytokines using the slopes from the linear regression model as the coefficients. All statistical analyses were carried out by using SAS version 9.2 statistical software (Stata Corp. LP, College Station, TX).
Role of the funding source
The study sponsor for this study was Incyte Pharma. The clinical trials was designed by ZE. The supporters reviewed provided drug and partial financial support for the conduct of the study. The supporters had no role in the final inference of the analysis. All authors had access to the raw data. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
RESULTS
Patient characteristics
Overall, 41 CLL patients (25 previously untreated and 16 previously treated) who did not require systemic therapy according to the iwCLL criteria were enrolled on the study. The disease characteristics of the participants at the time of enrollment are presented in Table 1. As expected, the majority of these patients had favorable disease characteristics, such as early Rai stage, low expression of Zap-70/CD38, low β2M level, and absence of deletion 17p and mutated IgVH. Fatigue, lack of energy, and night sweats were the most common presenting symptoms. The median follow-up interval from enrollment was 11.3 months (IQR 5.3 months), and the median duration of ruxolitinib intake was 7.7 months (IQR 6.0 months). Twenty-four participants took ruxolitinib for ≥6 months and continued to demonstrate consistent improvement in symptoms. The participants’ comorbidities and clinical status prior to starting ruxolitinib therapy are depicted in supplemental file page 3–4. Since none of the patients met the criteria for treatment and none had a significant disease burden, we could not systematically assess disease response.
Table 1.
Participant characteristics
| Characteristic | Measure/Category | Overall |
|---|---|---|
|
| ||
| Age, years | Median (IQR) | 57 (11) |
|
| ||
| WBC ×109/L | Median (IQR) | 13.9 (13.0) |
|
| ||
| ALC ×109/L* | Median (IQR) | 7.5 (18.0) |
|
| ||
| Rai stage^ | 1–2 (%) | 100% |
| 3–4 (%) | 0% | |
|
| ||
| CD38 | ≤30 % | 67% |
| >30% | 33% | |
|
| ||
| Zap-70 | Negative | 73% |
| Positive | 27% | |
|
| ||
| β2M (mg/L) | <4 | 93% |
| ≥4 | 7% | |
|
| ||
| IGHV mutation# | Mutated | 64% |
| Unmutated | 36% | |
|
| ||
| FISH result, n | del17p | 0 |
| del11q | 3 | |
| Trisomy12 | 10 | |
| del13q | 14 | |
| Negative | 12 | |
| not done | 2 | |
|
| ||
| Karyotype, n | Diploid | 24 |
| Others | 2 | |
| Complex | 0 | |
| not done | 15 | |
|
| ||
| Treatment status, n | Previously untreated | 25 |
| prior treated | 16 | |
WBC, white blood cell count; ALC, absolute lymphocyte count; β2M, beta-2 microglobulin; M, mutated; UM, unmutated; FISH, fluorescence in situ hybridization
There were 16 patients with ALC < 5000 (4 were previously untreated, and 12 were previously treated)
Six patients had Rai stage 0, 7 had Rai stage 1 and 2 had Rai stage 2.
Overall, mutation status of IGVH was available in 31 patients. 18 were previously untreated (5 were UM and 13 M) while 13 patients were previously treated (6 were UM and 7 were M).
Effect of ruxolitinib on symptom scores
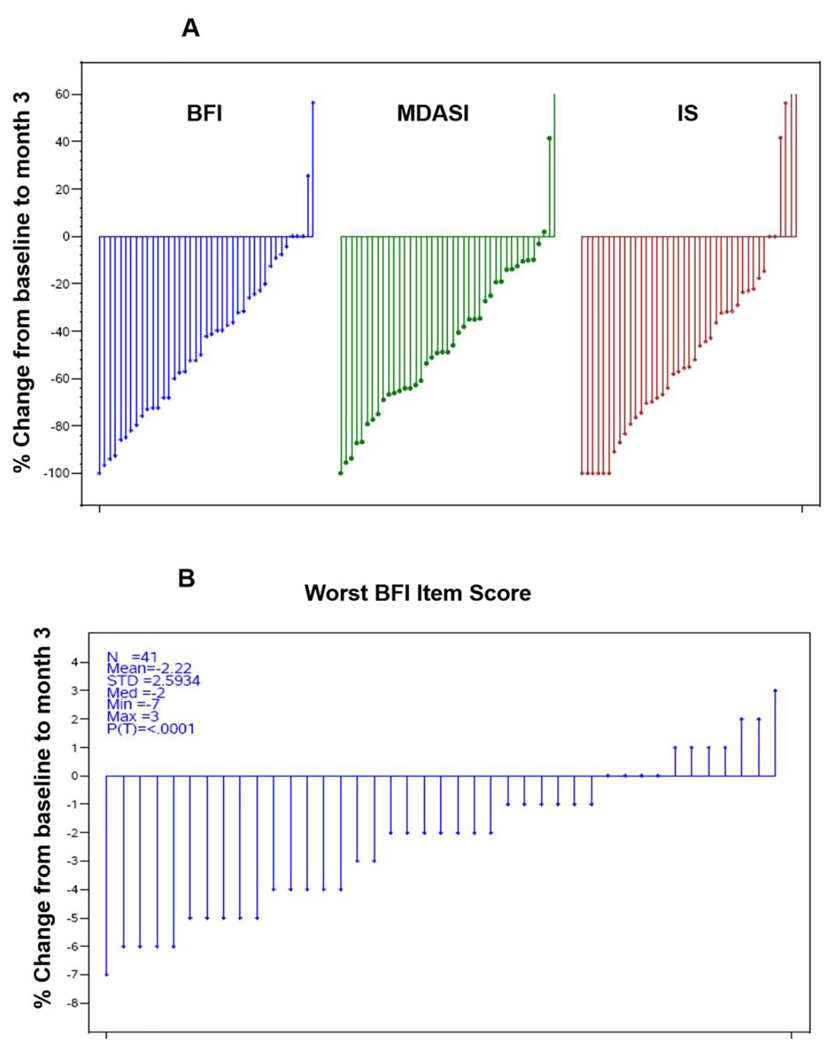
Mean (SD) of absolute scores at baseline were 5.5 (1.7), 4.3 (2.5), 3.0 (1.7) and 7.3 (1.4) for BFI, IS and MDASI and worst fatigue in 24 hours respectively. The average symptom scores of all 41 participants were calculated as mean percentage change from baseline at 2 weeks and 3 months. Overall, 78% of participants (n=32) had ≥20% reduction in the average BFI and 59% (24 out of 41) had ≥2 unit reduction in the worst fatigue in last 24 hours (Figure-1A-B). 40% of the participants had ≥20% reduction in the average IS, or MDASI scores. At 3 months, the mean percentage change in the BFI score was 44.3% (SD=35%, p<0.0001). The mean percentage change in the IS score was 43.4% (SD=51.5%, p<0.0001). Similarly, the mean percentage change in the MDASI score was 42.1% (SD=37.4%, p<0.0001; Figure 1B). At 2 weeks, the mean percentage changes in the BFI, MDASI, and IS scores were 29.3% (SD=34.7%, p<0.0001), 11.2% (SD=67.8%, p=0.012), and 26.2% (SD=50.5%, p=0.0009), respectively. Overall, at 3 months, there was a significant improvement in the severity of fatigue and symptoms interfering with daily activities and a decrease in the severity of CLL-related symptoms compared to baseline. Remarkably, ≥50% improvement was noted in specific symptom scores including fatigue that interfered with enjoyment of life or mood and/or walking ability in the last 24 hours, improved mood and/or relations with other people improvement in the feeling of being sad or not feeling well at its worst (Supplemental file page 5–6).
Figure 1. (A-B). Ruxolitinib treatment reduces symptom scores of patients with CLL.
Waterfall plot showing the mean percentage change from baseline to 3-month scores on BFI (brief fatigue inventory), MDASI (CLL module of the MD Anderson symptom inventory), IS (symptom-associated interference in daily activities) and worst fatigue in 24 hours on BFI scale. Each vertical line represents an individual participant. Significant reductions in symptom scores were observed after ruxolitinib treatment (P<0.0001 in all three scoring systems). The proportion of participants with ≥20% reduction in BFI and/or MDASI score was 78% (32 of 41).
To explore further, we did a subset analysis to compare the changes in symptom scores at 3 months with respect to the participants’ treatment status. The BFI and IS scores showed more pronounced improvement at 3 months in previously treated participants than in untreated participants (mean percentage change in BFI, 51% (± 28) vs 40% (± 39); mean percentage change in IS, 50% (± 41 vs 39% ± 57.3; p=0.23), whereas the mean percentage changes in the MDASI score were similar [43% (± 35) vs 41% (± 39) ; p=0.93)].
Effect of ruxolitinib on plasma cytokine and chemokine levels
To determine whether ruxolitinib treatment affected the participants’ plasma cytokine and/or chemokine levels, we assessed those levels prior to and at 3 months of ruxolitinib treatment (Supplemental file page 12) Plasma cytokine and chemokine levels were high prior to treatment, as previously reported. 13 Three months into therapy, the levels of the acute phase reactant CRP, and IL-6, IL-1RA, ICAM1, IP- 10/CXCL10, MIP-1β/CCL4, osteopontin, TNF-α, and CXCL13/BCAC1 were significantly reduced (Supplemental file pages 7–8). Notably, levels of IL-6, CRP, and CCL4 were reduced by >0.50 fold (p<0.05). Remarkably, participants with previously untreated disease had more pronounced reductions in cytokine/chemokine levels, particularly CRP, IL-6, and CCL4.
To determine whether CLL cell IL-6 levels were affected by ruxolitinib treatment, frozen peripheral blood low-density cells obtained prior to treatment and 3 months into treatment from four participants who showed a response to the treatment were analyzed for IL-6 by western immunoblotting. As shown in Supplemental file page 13, ruxolitinib treatment significantly reduced IL-6 levels. Furthermore, as shown in Supplemental Figure 2 and Supplemental file page 14, the expression of chemokine and cytokine mRNAs in CLL cells from the same four participants was reduced after 3 months of ruxolitinib treatment compared to baseline, although the levels varied among these patients.
Correlation between symptom scores and plasma cytokine/chemokine levels
Because cancer-related symptoms are associated with increased cytokine levels8 and because ruxolitinib treatment improved CLL-related symptoms and reduced cytokine and chemokine plasma levels, we analyzed whether there was a correlation between the mean percent reduction in symptom score (mean score calculated from all items within the individual questionnaires) and cytokine/chemokine levels. Using a multivariable regression analysis, we found that a composite of plasma levels of CRP, IL-6, IP- 10/CXCL10, osteopontin, TNF-α, ICAM1, and VCAM1, that produced the highest adjusted R2 value of 0.4246 in the regression model (Table 2), linearly correlated with the reduction in the participants’ symptom scores Supplemental file page 15 (Figure 3A-D).
Table 2.
Composite of cytokine and chemokine plasma levels that best correlate with the MDASI score using a multivariable regression model
| Dependent Variable |
Independent Variables ( Log2 fold-change from baseline to month 3) |
Coefficient | Type 3 P-value |
R2 |
|---|---|---|---|---|
|
Percentage reduction from baseline to Month 3 in the MDASI score |
Intercept* | 41.71141 | 0.13 | 0.4246 |
| C-reactive protein | −10.56035 | 0.32 | ||
| Intercellular adhesion molecule 1 (CD54) | 17.59391 | 0.072 | ||
| IFN-γ -induced protein 10 (IP-10; CXCL10) | 16.31205 | 0.0034 | ||
| Interleukin-6 (IL-6) | 9.98691 | 0.079 | ||
| Osteopontin | −8.13491 | 0.018 | ||
| Tumor necrosis factor-α | −18.67772 | 0.057 | ||
| Vascular adhesion molecule-1 (VCAM-1) | −21.90617 | 0.13 |
Intercept refers to the point at which reductions in symptom scores and the selected cytokine levels had zero fold change.
Effect of ruxolitinib on blood cell counts and β2M levels
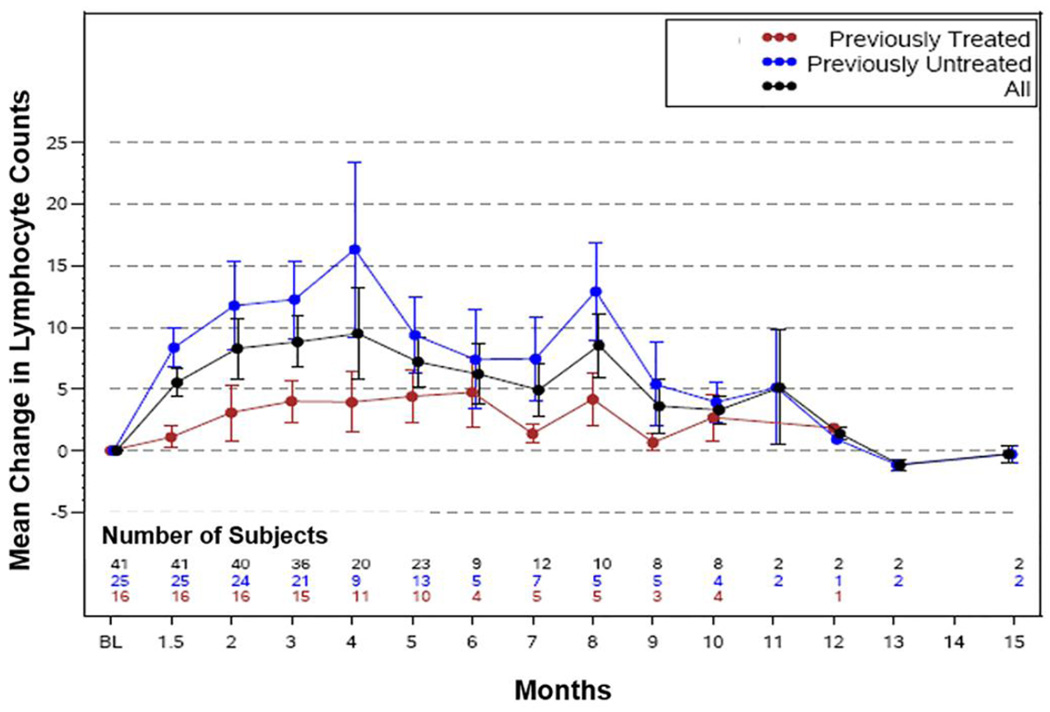
Because cytokine and chemokine levels are known to correlate with disease burden and prognosis, 13 we also assessed the effect of ruxolitinib treatment on absolute lymphocyte count (ALC), WBC count, platelet count, and hemoglobin level, as well as β2M level. As shown in Figure 4, treatment with ruxolitinib induced a rise in ALC which over time returned to baseline levels or lower. The rise and fall in ALC was more pronounced in previously untreated participants. A bimodal distribution of ALC was noted however the number of patients whose counts were available at this time point is relatively small. A similar pattern of change was noted in WBC count (Supplemental file page 17). Computerized tomography performed in three participants showed a reduction in lymph node size by 5%, 6% and more than 50% at 3 and 8 months of treatment, respectively, suggesting that lymphocyte redistribution increased ALC and WBC counts. Only 3 patients underwent CT scans by physician’s choice for unrelated reasons. Because pre-enrolment workup was not aimed at assessing disease burden, we could not assess the effect of ruxolitinib on lymphocyte compartmental distribution. We also noted that plasma β2M levels fell below baseline levels and remained low thereafter (Supplemental file page 17) and that the reduction in β2M level was less pronounced in previously treated participants. Platelet counts also increased and then stabilized. However, hemoglobin levels were reduced below baseline levels in a majority of participants (10% decrease compared to the baseline). Nevertheless, these participants did not require red blood cell transfusions. Conversely, the absolute neutrophil count declined below baseline levels and recovered and stabilized over time (not shown).
Causes of discontinuation and adverse effects of ruxolitinib therapy
Overall, 21 participants discontinued ruxolitinib (Supplemental Table 5). The most common causes of ruxolitinib discontinuation were disease progression (n=5) and lack of response (n=5); other causes were miscellaneous, including worsening myasthenia gravis and HHV-6 infection, dizziness and tinnitus, low platelet count, and weight gain and joint pain (n=4); participant choice (n=3); and loss of response (n=4). One participant died of new-onset progressive myasthenia gravis associated with preexisting HHV-6 infection. Two participants were taken off study because of progressive lymphocytosis, worsening anemia, and lymphadenopathy, that according to the attending physician, required systemic therapy. Three participants were taken off study because of lymphocytosis and fatigue who concerned the patients and their physicians. They went on to receive systemic therapy. Only 2 participants discontinued ruxolitinib before the 3 month assessment. One participant discontinued treatment because of lack of response and the other due to worsening fatigue.
Generally, ruxolitinib was well tolerated. All ruxolitinib-related adverse effects are depicted in Table-3. Only three participants discontinued ruxolitinib because of side effects that were probably related to ruxolitinib. Those included dizziness and tinnitus, low platelet count, weight gain, and joint pain. One participant experienced grade 3 insomnia, two had grade 3 hypertension, one had grade 3 febrile neutropenia, one had a lung infection, and one had grade 4 neutropenia. The death of one participant from progressive myasthenia-induced neuromuscular failure was associated with pre-existing HHV-6 infection and was most likely unrelated to ruxolitinib, although ruxolitinib can be associated with atypical infections or reactivate latent viruses.
Table 3.
Adverse effects likely related to Ruxolitinib
| Specific Adverse Events | Grade 1–2 | Grade 3–4 | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Neutropenia | 1 | 2 | 2 | 5 |
| Hypertension | 3 | - | 2 | 5 |
| Insomnia | 5 | - | 1 | 2 |
| Tinnitus and Dizziness | 6 | - | 1 | −2 |
| Thrombocytopenia | 1 | 2 | 1 | 2 |
| Weight gain | 3 | 7 | - | - |
| Joint pain | 3 | 7 | - | - |
| Diarrhea | 7 | 17 | - | - |
| Headache | 6 | - | - | |
| Nausea | 6 | - | - | |
| Sinus congestion | 4 | - | - | |
| Palpitations | 1 | 2 | - | - |
| Cognitive disturbance (reversible) | 1 | 2 | - | - |
| Anxiety | 2 | 5 | - | - |
| Cough | 3 | 7 | - | - |
DISCUSSION
In this study, we have shown that treatment with the JAK1/2 inhibitor ruxolitinib significantly alleviated disease-related symptoms, as assessed by three different symptoms scores, in both previously treated and untreated CLL patients. Disease related symptoms such as fatigue, night sweats, weight loss, joint pain, depression, and/or other constitutional symptoms compromise the QoL of patients with CLL. 3,5 Inflammatory cytokines can induce disease related symptoms13 and their high levels correlate with unfavorable prognosis. 11 We recently found14 that stimulation of the BCR induces phosphorylation of JAK2, and that the JAK2 inhibitor ruxolitinib induces apoptosis of BCR-stimulated CLL cells. Activation of the JAK/STAT pathway results in overproduction of cytokines and chemokines in a variety of neoplastic or inflammatory diseases. 27 Fatigue in patients with cancer could be caused by anemia or elevated levels of pro-inflammatory cytokines. Although ruxolitinib induced a 10% drop in hemoglobin levels, patient’s symptoms improved, suggesting that elevated cytokine levels induced fatigue in CLL patients. Nevertheless, because this is a single arm study and the variability in symptom scores was not assessed, a placebo effect in improving symptoms could not be completely ruled out. We did not check any patients for deviations on their symptom scores, and could not determine the fluctuation in the symptom scores. However the large magnitude of symptom score changes and the fact that significant differences were observed at three months and not at two weeks, mitigate the likelihood of a placebo effect. Furthermore, although the R2 of BFI score was only 0.076, statistically significant improvements in other symptom scores including MDASI, and worst fatigue suggest that ruxolitinib does improve symptoms in patients with CLL.
Treatment with ruxolitinib decreased plasma levels of cytokines and chemokines in most of the participants, and this reduction correlated with improvement in symptom scores. The improvement in symptom scores correlated with reductions in the levels of IL-6, CRP, MIP-1β (CCL4), and CXCL10, all of which play a role in the pathogenesis of CLL. 13 Osteopontin levels were elevated prior to ruxolitinib treatment and suppressed during treatment, suggesting that, like leukemic lymphoblasts, CLL cells’ adherence to their microenvironment28 is enhanced by osteopontin; this finding also suggests that ruxolitinib-induced reduction in osteopontin levels contributed to the mobilization of CLL cells. Like CRP, β2M levels were reduced by ruxolitinib treatment. β2M is an established prognostic factor in CLL, and reduction in β2M levels is associated with prolonged progression-free survival. 29 Recent studies demonstrated that activation of JAK and its downstream signal transducer and activator of transcription (STAT) pathway affects the levels of CXCL12/CXCR430, 31 and CCL2 chemokines32. Treatment with ruxolitinib suppressed the expression of several chemokine and cytokines, suggesting that gene expression in CLL cells mobilized from their microenvironment33 is different from that of steady state circulating CLL cells. Unlike steady state peripheral blood CLL cells, CLL cells mobilized from their microenvironment by ruxolitinib expressed lower levels of chemokines and cytokines.
Like ibrutinib34 and other BCR signaling inhibitors, 35 ruxolitinib decreased lymph node size and increased peripheral blood lymphocyte counts, likely by reducing chemokine levels and mobilizing CLL cells from bone marrow and lymph nodes into the peripheral blood. After several months of treatment, lymphocyte counts returned to, or dropped below, baseline level. This redistribution of CLL cells34, 35 followed by reduction in peripheral blood lymphocyte counts implies that ruxolitinib might exert a therapeutic effect, possibly by inducing apoptosis of mobilized CLL cells. 14 However, because our trial was designed to assess the effect of ruxolitinib on patients’ symptom and patients with a significant disease burden were not included, these preliminary observations should be taken with caution. Nevertheless, our data suggest that studies aimed at assessing the therapeutic effects of ruxolitinib in CLL are warranted.
Like in our study, ibrutinib was found to affect the levels of STAT3 target genes in ovarian cancer cells36 however the mechanism of this observation is not fully understood. Cytokines whose levels are increased in CLL are produced as a result of activation of the JAK/STAT pathway by the transcription factor nuclear factor (NF)-κB. NF-κB is constitutively activated in CLL37 and is further activated following stimulation of the BCR. 38 NF-κB activates several cytokines such as IL-6 that binds to its corresponding receptor and activate JAK1 and/or JAK2, 39 both of which are inhibited by ruxolitinib. Indeed, we found that treatment with ruxolitinib reduced IL-6 protein and chemokine mRNA levels in CLL cells from a subset of the participants, with variation among them. Because mRNA levels were analyzed only in 4 patients, we could not assess whether cytokine mRNA levels correlated with their protein levels. The reduction in chemokine and cytokine levels correlated with symptom improvement, suggesting that cytokines that induced symptoms were produced by CLL cells or were released as a result of the interaction between CLL cells and accessory cells11, and contributed to CLL-related symptoms as observed in other neoplasms, during disease progression. 10
The symptom assessment scores we used in our study have been utilized in evaluating symptoms of patients with hematologic malignancies and other neoplasms. 22 The significant improvement in symptoms assessed concomitantly by these three scoring systems suggests that excessive activation of JAK1/2 might induce fatigue and a variety of symptoms, including reduced energy level, impaired mood, and depression, consistent with a recent study showing that the JAK/STAT pathway is involved in activating synaptic plasticity in the hippocampus. 40
Among the 21 participants who came off study in only nine patients treatment was discontinued because of lack of symptomatic improvement or toxicity. Five patients were taken of study mainly because of an increase in peripheral blood lymphocyte count. Remarkably, following an initial increase, ALC was decreased in the majority of the 24 participants who continued ruxolitinib treatment beyond 6 months, suggesting that, similar to ibrutinib, ruxolitinib induces redistribution and then apoptosis of CLL cells. 34 A similar observation was made by Spaner et al., 41 who administered ruxolitinib to 13 CLL patients deemed unfit to receive chemoimmunotherapy and found that the increase in ALC was associated with a reduction in lymphadenopathy. In Spaner’s trial ruxolitinib was administered to patients with significant disease burden whereas our trial was designed to assess symptom control in patients not requiring systemic therapy for CLL. Patients’ characteristics were therefore very different and the clinical course was different in the two trials.
In summary, our study demonstrated that ruxolitinib significantly reduced the levels of fatigue and other disease-related symptoms in patients with CLL and that improvement in symptoms correlated with reductions in levels of cytokines/chemokines, CRP, and β2M. Like BTK inhibitors, ruxolitinib increased and then decreased peripheral blood lymphocyte counts, probably because of redistribution followed by apoptosis of CLL cells. An optimal strategy to treat fatigue and disease related symptoms in patients with CLL who do not need systemic therapy should be addressed in larger studies. Although ruxolitinib is effective and likely reduces disease burden, adverse effects of prolonged treatment with kinase inhibitors and their high cost are concerning. Another possible difference between ibrutinib based study and our study could be in the dropout rate of patients which is lower in the treatment protocols than in symptom control protocols in which convenience and adherence to protocol requirements are factored in. Further studies to investigate our findings and explore the effects of ruxolitinib on CLL tumor burden are warranted.
Supplementary Material
Figure 2. Ruxolitinib induced mobilization of CLL cells from lymph nodes to the peripheral blood.
Ruxolitinib treatment increased and then decreased absolute lymphocyte counts (ALC) of CLL patients. Mean changes in peripheral blood ALC are depicted. Overall, ALC increased initially and then decreased. Previously treated participants showed a dampened response compared to previously untreated participants.
Systematic Review.
-
a)
Did you do a systematic review as part of the planning for this trial? - Yes
-
b)
If yes, please provide details of databases searched, search terms used, any restrictions on search, etc. How did you select and combine the evidence? - We did a thorough systematic review of symptom control in cancer especially in leukemia patients before we finalized the manuscript. We searched Medline and PubMed between Jan 1, 1990, and June 1, 2016, with the search terms “CLL”, “chronic lymphocytic leukemia”, “ruxolitinib”, “symptom control”, “cytokine in cancer” for publications in English. We identified relevant research articles (mentioned in reference section) checking for symptom control in cancer and ruxolitinib in myelofibrosis, which formed the basis for this study.
-
d)
What is the existing evidence in this area of research, and how did you identify it? - We identified relevant research articles (mentioned in reference section) checking for strategies to control symptoms in cancer patients, effect of JAK-2 inhibitors in symptom control, CLL, myelofibrosis, which formed the basis for this manuscript.
Interpretation.
-
a)
How does the present trial fit into that evidence? – We could not find any previously published data on ruxolitinib in CLL patients for symptom control in patients not requiring systemic therapy.
-
b)
What do the findings of all the trials and the present trial mean? – Since there was no comparative study for symptom control in CLL, we believe that our findings are novel and provide an effective strategy to control symptoms in patients with CLL.
-
c)
What are the clinical implications of your findings? What should clinicians do now? – Our study demonstrated that ruxolitinib significantly reduced the levels of fatigue and other disease-related symptoms in patients with CLL and that improvement in symptoms correlated with reductions in levels of cytokines/chemokines, CRP, and β2M. Like BTK inhibitors, ruxolitinib increased and then decreased peripheral blood lymphocyte counts, probably because of redistribution followed by apoptosis of CLL cells. An optimal strategy to treat fatigue and disease related symptoms in patients with CLL who do not need systemic therapy should be addressed in larger studies.
Research in context.
Evidence before this study
We searched Medline and PubMed specifically for original research articles published on CLL and symptom control and symptom control in cancer. We also looked into JAK-2 inhibition and cytokine/chemokine levels in patients with different cancers. We identified relevant research articles (mentioned in reference section) which have demonstrated the efficacy of ruxolitinib in clinical trials in myelofibrosis. We identified relevant research articles which have demonstrated the efficacy of ruxolitinib in improving clinical symptoms of patients with myelofibrosis.
Added value of this study
This study provides insight into symptom control in patients with CLL, who do not require systemic therapy. We have previously reported that ruxolitinib can induce the apoptosis in CLL cells and that activation of B cell receptor on CLL cells can activate Jak-2. We have shown that ruxolitinib significantly improved fatigue and other disease related symptoms in patients with CLL not requiring systemic therapy and ruxolitinib induced decrease in symptom burden significantly correlated with reduction in levels of inflammatory cytokines and chemokines. Furthermore, we have shown that ruxolitinib may have an effect as a therapy in CLL since patients can have a lymph node shrinkage and a corresponding increase in lymphocyte counts similar to what is observed with other kinase inhibitors in CLL.
Implications of all the available evidence
We found that ruxolitinib has a potential to develop further as an agent for symptom control in patient with CLL. Our data suggest that Ruxolitinib also has a potential to be used to treat patients with CLL.
Acknowledgments
This study was supported in part by the NIH/NCI under award number P30CA016672 and by the NCI under award number P01CA049639. We thank William Sun from Incyte Corporation for providing support for the statistical analysis and Kathryn Hale for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contributions
Z.E. designed the study. C.C. performed preliminary symptom score analysis and provided the symptom scores. S.R. was the study’s research nurse. P.J., U.R., C.C., S.R., and Z.E. contributed to data collection and analysis. H.X., N.G. designed the study’s statistical method and monitoring. H.X., N.G., I.V., and U.R. analyzed the data. D.H., L.P., and Z.L., performed the laboratory experiments. Z.E., M.K., N.J., P.T., P.B., C.D., A.F., S.V., S.O.B., J.B., W.W., and H.K. treated and monitored the patients. P.J. and Z.E. wrote the paper.
Conflicts-of-Interest Disclosure: Z.E. receives research support from and serves as a consultant to Incyte Corporation. None of the other authors have any conflicts of interest to disclose.
References
- 1.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Efficace F, Kemmler G, Vignetti M, Mandelli F, Molica S, Holzner B. Health-related quality of life assessment and reported outcomes in leukaemia randomised controlled trials - a systematic review to evaluate the added value in supporting clinical decision making. Eur J Cancer. 2008;44(11):1497–1506. doi: 10.1016/j.ejca.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Pashos CL, Flowers CR, Kay NE, et al. Association of health-related quality of life with gender in patients with B-cell chronic lymphocytic leukemia. Support Care Cancer. 2013;21(10):2853–2860. doi: 10.1007/s00520-013-1854-z. [DOI] [PubMed] [Google Scholar]
- 4.Stephens JM, Gramegna P, Laskin B, Botteman MF, Pashos CL. Chronic lymphocytic leukemia: economic burden and quality of life: literature review. Am J Ther. 2005;12(5):460–466. doi: 10.1097/01.mjt.0000104489.93653.0f. [DOI] [PubMed] [Google Scholar]
- 5.Holtzer-Goor KM, Schaafsma MR, Joosten P, et al. Quality of life of patients with chronic lymphocytic leukaemia in the Netherlands: results of a longitudinal multicentre study. Qual Life Res. 2015;24(12):2895–2906. doi: 10.1007/s11136-015-1039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang XS, Mendoza TR, Broadway JB, et al. Symptom Profiles in Patients with Chronic Lymphocytic Leukemia: Validation and Application of the M.D. Anderson Symptom Inventory. Blood. 2009;114(22):1393–1393. [Google Scholar]
- 7.Fung FY, Li M, Breunis H, Timilshina N, Minden MD, Alibhai SM. Correlation between cytokine levels and changes in fatigue and quality of life in patients with acute myeloid leukemia. Leuk Res. 2013;37(3):274–279. doi: 10.1016/j.leukres.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8(11):887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 9.Kleppe M, Kwak M, Koppikar P, et al. JAK-STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response. Cancer Discov. 2015;5(3):316–331. doi: 10.1158/2159-8290.CD-14-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29(10):1356–1363. doi: 10.1200/JCO.2010.32.9490. [DOI] [PubMed] [Google Scholar]
- 11.Rozovski U, Keating MJ, Estrov Z. Targeting inflammatory pathways in chronic lymphocytic leukemia. Crit Rev Oncol Hematol. 2013;88(3):655–666. doi: 10.1016/j.critrevonc.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan XJ, Dozmorov I, Li W, et al. Identification of outcome-correlated cytokine clusters in chronic lymphocytic leukemia. Blood. 2011;118(19):5201–5210. doi: 10.1182/blood-2011-03-342436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rozovski U, Wu JY, Harris DM, et al. Stimulation of the B-cell receptor activates the JAK2/STAT3 signaling pathway in chronic lymphocytic leukemia cells. Blood. 2014;123(24):3797–3802. doi: 10.1182/blood-2013-10-534073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeiser R, Burchert A, Lengerke C, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062–2068. doi: 10.1038/leu.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurwitz HI, Uppal N, Wagner SA, et al. Randomized, Double-Blind, Phase II Study of Ruxolitinib or Placebo in Combination With Capecitabine in Patients With Metastatic Pancreatic Cancer for Whom Therapy With Gemcitabine Has Failed. J Clin Oncol. 2015;33(34):4039–4047. doi: 10.1200/JCO.2015.61.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cleeland CS, Zhao F, Chang VT, et al. The symptom burden of cancer: Evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns study. Cancer. 2013;119(24):4333–4340. doi: 10.1002/cncr.28376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118(2):401–408. doi: 10.1182/blood-2011-01-328955. [DOI] [PubMed] [Google Scholar]
- 20.Wang XS, Giralt SA, Mendoza TR, et al. Clinical factors associated with cancer-related fatigue in patients being treated for leukemia and non-Hodgkin's lymphoma. J Clin Oncol. 2002;20(5):1319–1328. doi: 10.1200/JCO.2002.20.5.1319. [DOI] [PubMed] [Google Scholar]
- 21.Mascarenhas J, Hoffman R. Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res. 2012;18(11):3008–3014. doi: 10.1158/1078-0432.CCR-11-3145. [DOI] [PubMed] [Google Scholar]
- 22.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 23.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazan-Halevy I, Harris D, Liu Z, et al. STAT3 is constitutively phosphorylated on serine 727 residues, binds DNA, activates transcription in CLL cells. Blood. 2010;115(14):2852–2863. doi: 10.1182/blood-2009-10-230060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geyer HL, Dueck AC, Scherber RM, Mesa RA. Impact of Inflammation on Myeloproliferative Neoplasm Symptom Development. Mediators Inflamm. 2015;2015:284706. doi: 10.1155/2015/284706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galli S, McLornan D, Harrison C. Safety evaluation of ruxolitinib for treating myelofibrosis. Expert Opin Drug Saf. 2014;13(7):967–976. doi: 10.1517/14740338.2014.916273. [DOI] [PubMed] [Google Scholar]
- 27.Ghoreschi K, Gadina M. Jakpot! New small molecules in autoimmune and inflammatory diseases. Exp Dermatol. 2014;23(1):7–11. doi: 10.1111/exd.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyerinas B, Zafrir M, Yesilkanal AE, Price TT, Hyjek EM, Sipkins DA. Adhesion to osteopontin in the bone marrow niche regulates lymphoblastic leukemia cell dormancy. Blood. 2013;121(24):4821–4831. doi: 10.1182/blood-2012-12-475483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson PA, O'Brien SM, Xiao L, et al. beta2 -microglobulin normalization within 6 months of ibrutinib-based treatment is associated with superior progression-free survival in patients with chronic lymphocytic leukemia. Cancer. 2016;122(4):565–573. doi: 10.1002/cncr.29794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cascio G, Martin-Cofreces NB, Rodriguez-Frade JM, et al. CXCL12 Regulates through JAK1 and JAK2 Formation of Productive Immunological Synapses. J Immunol. 2015;194(11):5509–5519. doi: 10.4049/jimmunol.1402419. [DOI] [PubMed] [Google Scholar]
- 31.Montresor A, Toffali L, Mirenda M, Rigo A, Vinante F, Laudanna C. JAK2 tyrosine kinase mediates integrin activation induced by CXCL12 in B-cell chronic lymphocytic leukemia. Oncotarget. 2015;6(33):34245–34257. doi: 10.18632/oncotarget.5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellado M, Rodriguez-Frade JM, Aragay A, et al. The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J Immunol. 1998;161(2):805–813. [PubMed] [Google Scholar]
- 33.Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123(12):1810–1817. doi: 10.1182/blood-2013-09-527853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain P, O'Brien S. Chronic Lymphocytic Leukemia. Targeted Therapy in Translational Cancer Research: John Wiley & Sons, Inc; 2015. pp. 130–144. [Google Scholar]
- 36.Zucha MA, Wu AT, Lee WH, et al. Bruton's tyrosine kinase (Btk) inhibitor ibrutinib suppresses stem-like traits in ovarian cancer. Oncotarget. 2015;6(15):13255–13268. doi: 10.18632/oncotarget.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z, Hazan-Halevy I, Harris DM, et al. STAT-3 activates NF-kappaB in chronic lymphocytic leukemia cells. Mol Cancer Res. 2011;9(4):507–515. doi: 10.1158/1541-7786.MCR-10-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rozovski U, Harris DM, Li P, et al. Stimulation of the B-Cell Receptor (BCR) Induces Successive Activation of STAT3 and Nuclear Factor-Kappa-B in CLL Cells. Blood. 2015;126(23):4125–4125. [Google Scholar]
- 39.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol. 2012;30(9):1005–1014. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicolas CS, Amici M, Bortolotto ZA, et al. The role of JAK-STAT signaling within the CNS. JAKSTAT. 2013;2(1):e22925. doi: 10.4161/jkst.22925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spaner DE, Wang G, McCaw L, et al. Activity of the janus kinase inhibitor Ruxolitinib in chronic lymphocytic leukemia: results of a phase II trial. Haematologica. 2016 doi: 10.3324/haematol.2015.135418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.