Abstract
Background:
Antibacterial activity is one of the important characteristics of an ideal root canal sealer. The aim of this study was to investigate the antibacterial activity of five different sealers against Enterococcus faecalis using two different methods.
Materials and Methods:
The mineral trioxide aggregate (MTA) Fillapex, Tg-sealer, Endomethasone, AH-26, and RoekoSeal sealers were placed into the brain heart infusion (BHI) culture medium containing E. faecalis (PTCC1393). The diameter of the bacterial zone of inhibition was measured. In the direct contact test, a suspension containing grinded set sealers and E. faecalis bacteria was cultured in BHI after 6, 15, and 60 min. The number of colonies in milliliter was calculated. Data were subjected to one-way ANOVA and Tukey's multiple comparisons test (P < 0.05).
Results:
In the agar diffusion test, Endomethasone had the highest antibacterial activity against E. faecalis compared to other sealers (P < 0.001). In the direct test, the antibacterial effect of MTA Fillapex was significantly higher than that of all other sealers (P < 0.001).
Conclusion:
The technique and components of the tested sealers affect the antibacterial activity results. This study showed that all of sealers had antimicrobial effect.
Keywords: Anti-Bacterial agents, Enterococcus faecalis, root canal filling materials, root canal therapy
INTRODUCTION
The ultimate aim of the root canal therapy is elimination or reduction of microorganisms in dental root canal.[1] Enterococcus faecalis with an incidence rate of 22%–77% is known to be resistant to treatment and most common cause of root canal treatment failure.[2] E. faecalis can penetrate into the dentinal tubules, survive in high pH, and endure starvation, which leads to the secondary infection.[3,4]
Root canal sealers can be useful in reducing the remaining microorganisms in the root canal due to their antibacterial effect.[5] The most well-known sealers are zinc-oxide eugenol-based sealers (Tg-sealer), calcium hydroxide-based sealers (Apexit), glass ionomers (Ketac-endo), resins (AH26), silicone sealers (RoekoSeal), and sealers containing pharmaceutical materials (Endomethasone).[6] In recent years, a sealer containing mineral trioxide aggregate (MTA) Fillapex has also been introduced into dentistry.[7] One important step in root canal therapy is choosing an antibacterial canal sealer. Ahangari et al.[8] evaluated the antibacterial activity of AH26, Dorifill, and Apexit on Peptostreptococcus microorganism using agar diffusion test (ADT) in an in vitro condition. Based on their results, both AH26 and Dorifill had higher antibacterial activity with no significant differences, and Apexit showed the lowest antibacterial activity. Gürkan et al.[9] evaluated the antibacterial activity of Endomethasone, AH26, AH Plus, Sultan, Kerr pulp canal, Seal Apex, and RoekoSeal using ADT and concluded that all sealers had antibacterial activity except for RoekoSeal. Using direct contact test (DCT), Pizzo et al.[10] indicated that Vcanalare kept its antibacterial activity even after 1 week.
Morgental et al.[7] evaluated the antibacterial activity of the sealers using DCT and ADT. ADT was used for evaluating the antibacterial activity of nonset sealers, and DCT was used for after setting. The results of their study indicated that in ADT, the zones of growth inhibition were seen only in MTA Fillapex and Endofill. However, MTA Fillapex and Endofill did not show any antibacterial effect in DCT.
Based on the mentioned studies, there is little information on the antibacterial activity of the MTA Fillapex sealer in comparison with other common sealers.[7] Therefore, the aim of the present study was to investigate the antibacterial activity of MTA Fillapex sealer against E. faecalis prior and subsequent to setting as well as to compare their effects with those of conventional sealers such as AH26, RoekoSeal, and Endomethasone.
MATERIALS AND METHODS
In this in vitro study, the Iranian standard strain of E. faecalis bacterium (PTCC1393) was used. The sealers used in this study were RoekoSeal (Coltene/Whaledent, Langenau, Germany), AH26(DENTSPLY DETREY, GmbH, Konstanz, Germany), Tg-sealer (Technical and General Ltd., London, United Kingdom), Endomethasone (Septodont, Saint-Maur-des-Fosses, France), and MTA Fillapex (Angelus, Londrina, Parana, Brazil). Plates contained fresh agar medium (BioMerieux, Marcy-l’Etoile, France) cultured with E. faecalis. Sealers were prepared according to the manufactures’ instructions. The contaminated mediums with any kind of microorganisms except the E. faecalis were excluded from the study.
Agar diffusion test
Bacterial suspension was prepared with 0.5 McFarland standard densities of E. faecalis (PTCC1393), which contained 1.5 × 108 bacteria in 1 ml brain heart infusion medium (Difco, MA, USA). Ten Petri dishes (Asahi Glass Co. Ltd., Tokyo, Japan) which contained 5 mm thick Mueller-Hinton agar (BioMerieux, Marcy-l’Etoile, France) were inoculated. An amount of 0.1 ml of the bacteria was distributed uniformly on the media surfaces using a sterile loop. Five cylinder-shaped cavities with the diameter of 6 mm, depth of 5 mm, and minimum distance of 1.5 cm from the plate rim and 2.5 cm from each other were created for each of the sealer samples. Cavity ends were sealed using agar suspension to prevent the sealer permeation into the interface between the plate and the medium. Freshly mixed sealers, in accordance with the instructions of the manufacturing company, were placed in the cavities. The dishes containing cavities filled with one type of sealer were kept in the laboratory environment temperature for 2h. After 2 h, samples were placed in an incubator (Farazmehr, Isfahan, Iran) in aerobic conditions at 37°C for 48 h.[10,11,12,13] After 48 h, the diameter of the bacterial inhibition zone around each cavity was measured in 4 different directions using a ruler (Juya, Isfahan, Iran) (with the precision of 0.5 mm). To provide a positive control group, the sealers were placed in the cavities in an agar-containing plate without adding the bacterial suspension so that the sterile condition of the sealers could be measured. In addition, in the negative control group, to ensure the sterile condition of the agar environment, cavity free plates containing the medium were placed in an incubator without adding either the sealer or the bacterial suspension. All the experiments were conducted three times for the sealers and control groups (positive and negative).
Direct contact test
The DCT was used to evaluate the antibacterial properties of the endodontic sealers based on turbidimetric assessment of bacterial growth in microtiter plates. All sealers were mixed based on manufacturer's instructions and were placed in sterile cylinder-shaped plastic blocks with the diameter of 5 mm and the depth of 5 mm. The samples were placed in an incubator at the temperature of 37°C and the humidity of 100% for 7 days. The obtained sealer blocks were grinded and pulverized using a ceramic mixer (CoorsTek, Golden Co, USA). The powder was placed in special sterile packs and sterilized with ethylene oxide gas. Fifty milligrams of each sealer powder was weighed by Digital scale (Bell Engineering, Monza, Lombardy, Italy), and 1 ml of sterilized saline suspension was added to each powdered sealer using sterile pipettes so that a suspension with the density of 50 mg/ml produced. The bacterial suspension with the standard density of 0.5 McFarland (1.5 × 108/ml) was prepared. Equal volumes of bacterial suspension and the sealer suspension (1 ml) were mixed using an IkaVibro fix mixer (IKA Werke, Hamburg, Germany). The sealer-free saline suspension was considered as the positive control group. 6, 15, and 60 min after mixing the suspensions, the density of the suspensions was diluted 10,000 times, and 0.01 ml of the diluted suspension was cultivated on the already-provided Muller-Hinton agar media using a sampler. After incubation at 37°C for 48 h, the colonies formed on the agar plates were counted.[13] Then, the number of colonies formed in the volume unit colony-forming unit (CFU/ml) was calculated for each of the sealers in different times of the experiment with a NRL Contact Angle Goniometer (Rame-hart, Netcong, NJ, USA). These experiments were repeated forsix times.
Data were analyzed by SPSS software version 14 (SPSS Inc., Chicago, Illinois, USA) software using one-way ANOVA and Tukey's multiple comparisons test. P < 0.05 was considered statistically significant.
RESULTS
In the ADT, there were significant differences between the mean diameters of the bacterial inhibition zone for the freshly produced sealers of Endomethasone, AH26, and Tg-sealer with each other and also other sealers (P < 0.05). Furthermore, the mean diameter of the bacterial inhibition zone for MTA Fillapex sealer showed no significant difference compared to RoekoSeal sealer (P = 0.99).
The antibacterial activity of the Endomethasone sealer against E. faecalis was stronger than the others, and AH26 and Tg-sealer sealers presented less antibacterial activity, respectively. Subsequently, the RoekoSeal and MTA Fillapex sealers revealed no antibacterial activity [Table 1].
Table 1.
Diameter (mean±standard deviation) of Enterococcus faecalis zones of growth inhibition in different groups

In the DCT, the MTA Fillapex sealer showed least means of the logarithm of the CFU/ml [Table 2]. Therefore, the antibacterial activity of this sealer was significantly higher than other sealers and control group (P < 0.05) in the three times evaluated[Table 2].
Table 2.
Log colony-forming unit/ml (mean±standard deviation) of different groups in direct contact with Enterococcus faecalis in the three times periods
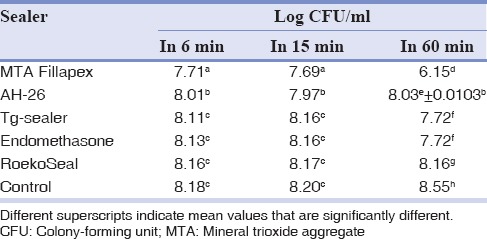
However, in 6 and 15 min, AH-26 sealer had more antibacterial activity after the MTA Fillapex sealer, and this different was significant and then endomethasone, RoekoSeal, and Tg-sealer (P < 0.05). In 6 and 15 min, antibacterial activity of the endomethasone, Tg-sealer, and RoekoSeal sealers demonstrated nonsignificant differences with control group (P > 0.05) [Table 2].
There were significant differences between each group with other groups in 60 min, except between Tg-sealer and endomethasone sealers [Table 2].
DISCUSSION
Studies have shown that microorganisms remain intact in inaccessible parts of root canal after mechanical and chemical cleaning.[2] Antibacterial activity of root canal sealers on these microorganisms may help decrease residual microorganisms. E. faecalis has a special ability in invading into the dentinal tubules.[2,12] Therefore, for evaluating the antibacterial effects of root canal sealers, E. faecalis was preferred in this study and this property was assessed by two methods (ADT and DCT).[7,13] The ADT has been widely used to investigate the antimicrobial activity of dental materials and is one of the most common and simplest methods. However, it has some limitations such as lack of standardization of inoculum density, adequate culture medium, agar viscosity, plate-storage condition, size and number of specimens per plate, time and temperature of incubation, and dependency on the solubility and diffusion characteristic of both the test material and media.[14] Thus, only water-soluble materials can be tested using ADT method.[4,14] The DCT method is used for evaluating antimicrobial activity of nonsoluble materials. This method is based on measuring the effect of physical direct contact between test bacteria and the tested material and has been suggested for solving the limitations of the ADT method.[13] The DCT has several advantages such as reproducibility, quantitative assay, contemporary testing of fifty samples, and continuous measurements of bacterial outgrowth with over 2400 measurements per plate.[7,14] Therefore, both methods have their own specific characteristics, and it is difficult to compare their results.
In ADT, Endomethasone showed a higher level of antibacterial activity followed by AH-26, Tg-sealer. RoekoSeal and MTA Fillapex sealers did not show any antibacterial activity. However, based on the DCT, MTA Fillapex sealer presented the highest antibacterial activity that followed byAH-26, Tg-sealer, Endomethasone, RoekoSeal in 6 and 15 first min, Tg-sealer, Endomethasone, AH-26, RoekoSeal after 60 min. Different tests evaluate different properties of the antibacterial components. ADT results indicate the antibacterial activity of freshly mixed sealers and the existence of diffusible factors while DCT shows the activity of insoluble antibacterial factors.
The ADT results showed that the Endomethasone sealer has a higher level of antibacterial activity against E. faecalis. The reasons for this can be attributed to the existence of components such as paraformaldehyde, thymol iodide, and zinc oxide in the structure of this sealer.[15] The AH-26 sealer was ranked second in terms of antibacterial activity, which is probably due to the release of paraformaldehyde from the sealer during the first 48 h of its combination as well as the existence of antibacterial components in epoxy resin.[6,16,17] The results obtained from these two sealers in this study are similar to those of other studies.[18] Tg-sealer, which was ranked in the third place in terms of antibacterial activity, belongs to the family of zinc oxide-based sealers. Its antibacterial activity is probably because of the existence of zinc oxide and thymol iodide. The results of this study are comparable with the findings of Ahangari et al.[8] It should be noted that the level of permeation for this sealer in the agar medium (17 mm) was higher than the formed bacterial inhibition zone. Therefore, it can be deduced that a sealer's antibacterial activity does not depend on the permeation ability in agar medium, but it depends on the antibacterial components of the sealer. The findings of our study indicate that RoekoSeal and MTA Fillapex sealers did not show any inhibition activity on E. faecalis bacterium. The reasons for this inability can be attributed to the absence of appropriate medium and permeation ability of these sealers.[18] This is in line with previous research findings like those of Gürkan et al.[9] The result of antibacterial activity of the MTA Fillapex sealer in this research was in line with those of Yasuda et al.,[19] but in contrast with the results of Morgental et al.[7] RoekoSeal and MTA Fillapex set more quickly than other sealers. Therefore, it seems that setting time is one of the factors that affect the sealer's permeability and also antibacterial activity.
To evaluate the antibacterial activity of the sealers through DCT method, the time of 60 min was suggested, because in 6 and 15 min, the sealers have no sufficient time to affect resisting bacteria such as E. faecalis. Owing to the dynamics of the antibacterial activity of each sealer, the control group was considered separately through the course of the experiment. Hence, the study was conducted in three consecutive time periods of 6, 15, and 60 min. In the period of 60 min after sealers’ direct contact with E. faecalis bacteria, the MTA Fillapex sealer presented the highest antibacterial activity in comparison with other sealers. This is probably due to the existence of effective antibacterial components in its structure (MTA base) as well as the high pH of the powdered sealer set in the suspension (pH >11).[20,21] One of the advantages of this study is that each sealer has been evaluated using two different methods. In DCT method, sealers were in the form of powder, and they were placed in suspension. Therefore, they were able to easily diffuse, but in ADT method, due to the bulky nature of sealers, they were not able to easily diffuse and this can affect their antibacterial effects.[6]
Morgental et al.[7] reported that MTA Fillapex did not present any antibacterial activity. The reason for this difference can be the difference in density and the kind of bacteria used in the produced suspension. Thus, further studies are needed to be conducted on MTA Fillapex. Tg-sealer and Endomethasone were ranked after MTA Fillapex in terms of antibacterial activity. These two sealers had no significant statistical difference. The reason for this similarity can be attributed to the similarity of their composition after setting (thymol iodide and zinc oxide),[22] and because a significant part of the other antibacterial components in Endomethasone (paraformaldehyde) is excluded from its composition after 1 week.
Inhibition effect of AH-26 sealer can be attributed to the antibacterial components in the epoxy resin structure and also little amount of formaldehyde, which remains in the composition of the sealer even after 2 weeks.[23] This was similar to Eldeniz et al.[24] results. The RoekoSeal sealer presented the least antibacterial activity against E. faecalis in this period. This can be because of the lack of antibacterial components[17] and the unique physical characteristics of RoekoSeal after setting. Although RoekoSeal possessed no clear antibacterial activity, it significantly prevented the growth and multiplication of the bacteria during observation in comparison with the control group. It can be concluded that this sealer at least does not cause bacterial growth in the medium.[25] The MTA Fillapex sealer presented the highest level of inhibition activity in all three-time periods and its antibacterial activity increased by the time. Endomethasone and Tg-sealer had their highest level of antibacterial activity in the period of 60 min probably due to the necessity of a longer period to overcome E. faecalis. Antibacterial activity of AH-26 decreased by time, which can be explained with the minimal amount of formaldehyde released over time. This is in agreement with Slutzky-Goldberg et al.[26] study. The number of the E. faecalis colonies formed in the control group increased. This can show that the bacteria have a tendency for growth and multiplication in a neutral environment. It must be noted that a sealer may present different antibacterial activity in its freshly produced and set states (like MTA Fillapex). This study did not address the question of whether these antibacterial properties might be effective with other microorganisms. It should be considered that in choosing sealers beside its antibacterial effect, their biocompatibility is also important.
CONCLUSION
In the ADT, Endomethasone sealer showed maximum antibacterial activity and in the DCT the MTA Fillapex sealer had maximum antibacterial activity. Therefore, the technique and components of the tested sealers affect the antibacterial activity results.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Hargreaves KM, Cohen S, Berman LH. Cohen's pathways of the pulp. 10th ed. St. Louis, MO: Mosby Elsevier; 2011. [Google Scholar]
- 2.Siqueira JF, Jr, Rôças IN. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:85–94. doi: 10.1016/s1079-2104(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 3.Pinheiro ET, Gomes BP, Ferraz CC, Sousa EL, Teixeira FB, Souza-Filho FJ. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J. 2003;36:1–11. doi: 10.1046/j.1365-2591.2003.00603.x. [DOI] [PubMed] [Google Scholar]
- 4.Rôças IN, Siqueira JF, Jr, Santos KR. Association of Enterococcus faecalis with different forms of periradicular diseases. J Endod. 2004;30:315–20. doi: 10.1097/00004770-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Hancock HH, 3rd, Sigurdsson A, Trope M, Moiseiwitsch J. Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:579–86. doi: 10.1067/moe.2001.113587. [DOI] [PubMed] [Google Scholar]
- 6.Siren EK, Haapasalo MP, Ranta K, Salmi P, Kerosuo EN. Microbiological findings and clinical treatment procedures in endodontic cases selected for microbiological investigation. Int Endod J. 1997;30:91–5. [PubMed] [Google Scholar]
- 7.Morgental RD, Vier-Pelisser FV, Oliveira SD, Antunes FC, Cogo DM, Kopper PM. Antibacterial activity of two MTA-based root canal sealers. Int Endod J. 2011;44:1128–33. doi: 10.1111/j.1365-2591.2011.01931.x. [DOI] [PubMed] [Google Scholar]
- 8.Ahangari Z, Ashraf H, Oskooi M, Soltani S, Naser M. Antibacterial activity of three endodontic sealers with various bases. Beheshti Univ Dent J. 2005;22:1–6. [Google Scholar]
- 9.Gürkan G, Semra S, Aykut M. Antimicrobial activity of various root canal sealers. Balkan J Stomatol. 2002;6:18–22. [Google Scholar]
- 10.Pizzo G, Giammanco GM, Cumbo E, Nicolosi G, Gallina G. In vitro antibacterial activity of endodontic sealers. J Dent. 2006;34:35–40. doi: 10.1016/j.jdent.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Lewinstein I, Matalon S, Slutzkey S, Weiss EI. Antibacterial properties of aged dental cements evaluated by direct-contact and agar diffusion tests. J Prosthet Dent. 2005;93:364–71. doi: 10.1016/j.prosdent.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Orstavik D. Materials used for root canal obturation: Technical, biological and clinical testing. Endod Topics. 2006;12:25–38. [Google Scholar]
- 13.Love RM. Enterococcus faecalis – A mechanism for its role in endodontic failure. Int Endod J. 2001;34:399–405. doi: 10.1046/j.1365-2591.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 14.Cobankara FK, Altinöz HC, Ergani O, Kav K, Belli S. In vitro antibacterial activities of root-canal sealers by using two different methods. J Endod. 2004;30:57–60. doi: 10.1097/00004770-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Whitworth J. Methods of filling root canals: Principles and practices. Endod Topics. 2005;12:2–24. [Google Scholar]
- 16.Leonardo MR, Bezerra da Silva LA, Filho MT, Santana da Silva R. Release of formaldehyde by 4 endodontic sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:221–5. doi: 10.1016/s1079-2104(99)70119-8. [DOI] [PubMed] [Google Scholar]
- 17.Sousa CJ, Loyola AM, Versiani MA, Biffi JC, Oliveira RP, Pascon EA. A comparative histological evaluation of the biocompatibility of materials used in apical surgery. Int Endod J. 2004;37:738–48. doi: 10.1111/j.1365-2591.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 18.Kayaoglu G, Erten H, Alaçam T, Ørstavik D. Short-term antibacterial activity of root canal sealers towards Enterococcus faecalis. Int Endod J. 2005;38:483–8. doi: 10.1111/j.1365-2591.2005.00981.x. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda Y, Kamaguchi A, Saito T. In vitro evaluation of the antimicrobial activity of a new resin-based endodontic sealer against endodontic pathogens. J Oral Sci. 2008;50:309–13. doi: 10.2334/josnusd.50.309. [DOI] [PubMed] [Google Scholar]
- 20.Bystrom A, Claesson R, Sundqvist G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol. 1985;1:170–5. doi: 10.1111/j.1600-9657.1985.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 21.Tanomaru JM, Tanomaru-Filho M, Hotta J, Watanabe E, Ito IY. Antimicrobial activity of endodontic sealers based on calcium hydroxide and MTA. Acta Odontol Latinoam. 2008;21:147–51. [PubMed] [Google Scholar]
- 22.Poggio C, Lombardini M, Colombo M, Dagna A, Saino E, Arciola CR, et al. Antibacterial effects of six endodontic sealers. Int J Artif Organs. 2011;34:908–13. doi: 10.5301/ijao.5000055. [DOI] [PubMed] [Google Scholar]
- 23.Mohammadi Z, Giardino L, Palazzi F, Shalavi S. Antibacterial activity of a new mineral trioxide aggregate-based root canal sealer. Int Dent J. 2012;62:70–3. doi: 10.1111/j.1875-595X.2011.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eldeniz AU, Erdemir A, Hadimli HH, Belli S, Erganis O. Assessment of antibacterial activity of EndoREZ. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:119–26. doi: 10.1016/j.tripleo.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Grossman LI. An improved root canal cement. J Am Dent Assoc. 1958;56:381–5. doi: 10.14219/jada.archive.1958.0055. [DOI] [PubMed] [Google Scholar]
- 26.Slutzky-Goldberg I, Slutzky H, Solomonov M, Moshonov J, Weiss EI, Matalon S. Antibacterial properties of four endodontic sealers. J Endod. 2008;34:735–8. doi: 10.1016/j.joen.2008.03.012. [DOI] [PubMed] [Google Scholar]


