Abstract
Lymphadenopathy is a common clinical finding in a patient seeking oral health care. It may be in a localized, limited, or generalized form. Malignancies, infections, autoimmune disorders, iatrogenic, and other miscellaneous conditions are considered as the causes for cervical lymphadenopathy. Unexplained cervical lymphadenopathy is a cause of concern for physician and patient because sometimes it could be the manifestation of an underlying malignancy. However, a methodological approach to lymphadenopathy can disclose the accurate diagnosis causing minimal discomfort for the patient and in a short time. This paper reports the significance of cervical lymph node examination and ensuing investigations, which led to a diagnosis of non-Hodgkins lymphoma.
Keywords: Lymphadenopathy, lymphoma, non-Hodgkin's lymphoma, positron emission tomography/Computed tomography
INTRODUCTION
Lymph nodes are oval-shaped organs of immune system, distributed throughout the body and linked by lymphatic vessels. The body has about 600 lymph nodes of which approximately 60–70 nodes are situated in the head and neck region.[1] Any abnormality in the size, consistency, and number of lymph nodes is defined as lymphadenopathy, which is caused by the invasion or propagation of either inflammatory or neoplastic cells into the lymph node.[1,2] Malignancies, infections, autoimmune disorders, iatrogenic, and miscellaneous conditions are regarded as the causes for cervical lymphadenopathy.[2] Lymphadenopathy is broadly classified into localized, generalized, and dermatopathic. According to its duration, it can be acute (2 weeks duration), subacute (4–6 weeks duration) and chronic (does not resolve by 6 weeks duration).[2,3] Differentiating localized, and generalized lymphadenopathy is very essential for formulating a diagnosis. Thus, a patient reporting with palpable lymph node in the neck is a serious diagnostic and therapeutic problem. In most of the cases, lack of appropriate examination and investigation result in delay in correct diagnosis and may result in iatrogenic complication due to improper diagnosis. Here, we are reporting a case of non-Hodgkin's lymphoma (NHL) presented only as cervical lymphadenopathy and diagnosis is made by an extensive investigative work up. Sometimes, cervical lymphadenopathy may be the solitary sign of underlying condition as in our case.
CASE REPORT
A 62-year-old male patient presented with the chief complaints of swelling on the left side of face and neck region for 2 months, which was of sudden onset. Swelling started as a discomfort on the left side of the neck followed by the appearance of swelling, which gradually increased in size and associated pain for 1 month. Had multiple medical consultations and multiple courses of antibiotics and a course of antifilarial over the past 1 month, with no reduction in symptoms. There were no other associated signs or symptoms.
He was under medication for hypothyroidism and diabetes mellitus. History of filariasis 10 years back followed by 3–4 episodes of lymphadenitis and had antifilarial therapy during these episodes. He was a cigarette smoker for 20 years, smoking two cigarettes per day which he quit 3 years back.
Extraoral examination revealed facial asymmetry of the left side. Multiple lymph nodes were palpable, including left submandibular and level II cervical lymph node with the largest one measuring approximately 4 cm × 5 cm in size. Lymph nodes were non-tender, firm in consistency, mobile, and some were matted together. Skin over the lymph nodes appeared normal without any signs of inflammation or infection. Patient consent was obtained for taking photograph and using it for study and publication purposes [Figure 1a and b].
Figure 1.
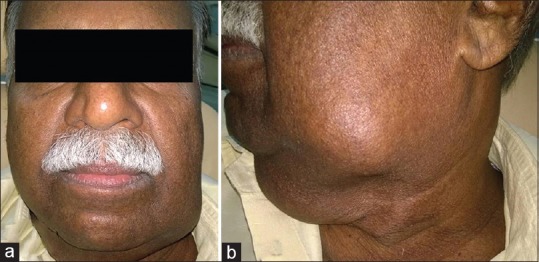
(a) A diffuse extraoral swelling in the lower one-third of face extending to submandibular region and (b) lateral view of the swelling.
Intraoral examination revealed poor oral hygiene with the presence of local factors and signs of periodontitis. On hard tissue examination, tooth number 36 had a crown with tenderness on vertical percussion. Thus by history and clinical examination, a differential diagnosis of cervical lymphadenopathy due to locoregional infection, filariasis, tuberculosis, NHL was given. Patient advised to discontinue all antibiotics and antifilarial drugs.
Panoramic radiograph revealed generalized horizontal bone loss and endodontically treated 36 with crown and periapical bone density [Figure 2a]. Posterior-anterior chest radiograph was noncontributory [Figure 2b]. All hematological investigations were within the normal limit except for lactate dehydrogenase (307.8 U/L, normal range 0–248 U/L). Blood smear was negative for filarial microbes, and Mantoux test was negative. Computed tomography (CT) of the neck with contrast showed multiple discrete and conglomerate lymph nodes in left level Ib, II, III, and V. Largest node measured 3.5 cm × 4.4 cm [Figure 2c]. The nodes did not show any enhancement/necrosis and were pushing the submandibular gland to one side. However, the gland appeared normal and was separated from the nodal mass [Figure 2d]. The adjacent bones did not show any erosion/lytic change. The right side also showed level Ib, II, and V nodes, but they were discrete and showed no evidence of necrosis or any other mass lesion [Figure 2e]. A tru-cut nodal biopsy taken from cervical lymph node, microscopic features were suggestive of lymphoproliferative disorder. Immunohistochemistry (IHC) was performed to categories the lesion; cells were positive for leukocyte common antigen, CD20, some for CD30 and Epstein–Barr virus latent membrane protein-1 (EBV-LMP-1) and were negative for CD3, CD10, cyclin D1, B-cell lymphoma-6 (BCL-6), CD23, anaplastic lymphoma kinase-1 and terminal deoxynucleotidyl transferase. IHC confirmed NHL, that is, suggestive of EBV-positive diffuse large B-cell lymphoma (DLBL) of elderly. Bone aspiration from pelvic bone ruled out the bone marrow involvement. Fluoro-2-deoxyD-glucose (FDG) positron emission tomography/CT (PET/CT), showed extensive FDG avid supra and infra-diaphragmatic lymph nodal lesions indicating metabolically active NHL. Focal FDG avid within spleen were present [Figure 3a]. Thus, according to Ann Arbor staging of primary lymphoma, he had Stage III S.
Figure 2.
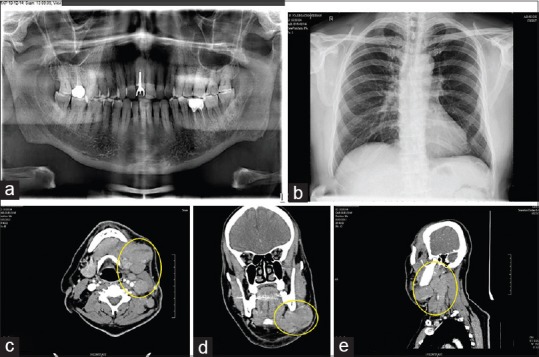
(a) Panoramic radiograph showing generalized bone loss, (b) posterior-anterior chest radiograph, showing a normal study, (c) computed tomography of neck with contrast, axial section demonstrating enlarged lymph nodes without any enhancement or necrosis, (d) computed tomography, coronal section demonstrating enlarged lymph node pushing the submandibular gland to one side, (e) computed tomography of neck with contrast, sagittal section demonstrating lymph node enlargement at level Ib, II, III and V.
Figure 3.
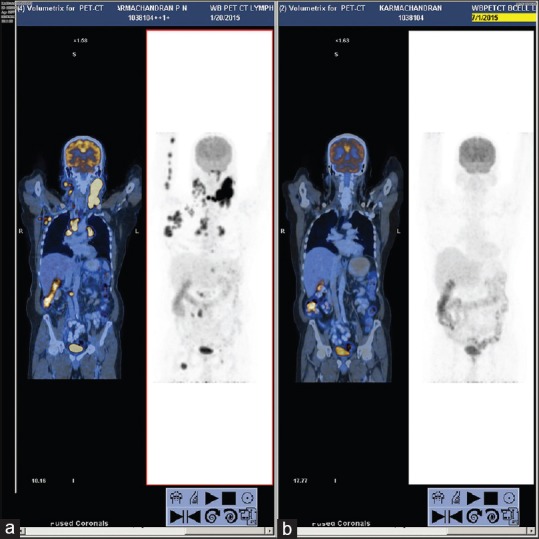
(a) Positron emission tomography/computed tomography images showing fluoro-2-deoxyD-glucose avid supra and infra diaphragmatic lymph nodes and focal fluoro-2-deoxyD-glucose avid in spleen and (b) positron emission tomography/computed tomography images after chemotherapy showing complete metabolic and near complete anatomical resolution of supra/infra diaphragmatic lymph nodes and splenic deposits.
A final diagnosis of NHL of DLBL was made based on the clinical presentations and investigations. The patient received six cycles of standard R-CHOP chemotherapy regimen which included rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2, and prednisone 50 mg/m2. On the 1st day of each cycle, the patient received rituximab, doxorubicin, vincristine, and cyclophosphamide and a 5-day course of prednisolone. At the end of the 21 days, patient started with second cycle of the R-CHOP chemotherapy, he completed six cycles of chemotherapy regimen. Whole-body PET/CT was done after six cycles of chemotherapy to assess the response to treatment, which showed complete metabolic and near complete anatomical resolution of supra/infra-diaphragmatic lymph nodes and splenic deposits [Figure 3b]. No new FDG avid lymph nodal/extranodal lymphomatous deposits were evident. Thus, a Deauville Score I was made suggesting an overall good response to chemotherapy and indicating no need of further chemotherapy at that level.
DISCUSSION
Head and neck region is considered as the most common site for lymphadenopathy. Infection is the most common reason for lymphadenopathy of which locoregional infections, tuberculosis, and filariasis are predominant in India.[3,4,5] Iqbal et al. showed that 70.45% of cervical lymphadenopathy is due to tuberculosis, 13.63% due to reactive lymphadenitis, 11.36% cases due to metastases, 4.54% cases due to lymphoma, and 2.27% of cases due to chronic nonspecific lymphadenitis.[5] Filariasis has a wide spectrum of clinical manifestations, which include asymptomatic filariemia, recurrent lymphadenitis, chronic lymphadenitis with swelling of dependent limbs, scrotal swelling and even as generalized cervical lymphadenopathy.[4]
Lymphadenopathy in a primary outpatient care setting is typically explained by an identifiable infection or regional injury. The most concern for a physician about the possibility of underlying malignancy is when there is no regional cause for lymphadenopathy.
In a primary care set up, the prevalence of malignancy in a patient with unexplained lymphadenopathy is thought to be quite low, as low as 1.1%.[6] However, in referral centers, the prevalence of malignancy is found to be 40%–60%.[7] Lymphomas represent malignant lymphoproliferative diseases, and they are generally classified as Hodgkin's (HL) or non-Hodgkin malignant lymphomas according to a difference in clinical course, site of involvement and histopathology. NHL is considered as the fourth common worldwide malignancy in males with a frequency of 6.1%.[8]
In our case, the asymptomatic cervical lymphadenopathy turned to being a manifestation of NHL, and that was the only presentation. The clinical symptoms of NHL include fever, drenching night sweat and weight loss more than 10% in 6 months were initially absent in our case. Features such as fever and night sweat are also seen in filariasis, thus the intake antifilarial drug might have masked these symptoms in our patient which reappeared on stoppage of medication. There is no literature regarding whether filariasis can lead to lymphoma, which need further clarification.
Diagnosis of NHL (DLBL) in our case was confirmed by extensive multidisciplinary diagnostic workup, which included blood investigations, Mantoux test, plain radiography, ultrasonography, tru-cut biopsy and IHC, CT, bone aspiration and PET/CT. In the case of lymphoma accurate diagnosis, correct staging and proper therapy are essential for a successful outcome. Plain radiography was noncontributory in our case. Ultrasonography is an established method for the assessment of cervical lymphadenopathy. On grayscale ultrasound, lymphomatous nodes tend to be round, well-defined, appear hypoechoic and are usually without an echogenic hilus. Intranodal reticulation (micronodular echo pattern) is commonly found in lymphomatous nodes and seldom shows cystic necrosis. On power Doppler ultrasound, lymphomatous lymph nodes tend to have both hilar and peripheral vessels (62%–90%).[9] This help to differentiate it from metastatic lymph node. CT findings of multiple large, bilateral lymph nodes without necrosis and matted appearance are features suggesting NHL.[10] CT also excluded any extranodal involvement because 40% of NHL is associated with extranodal sites.
By tru-cut lymph node biopsy, sufficient tissue sample was obtained for histopathological examination and IHC. IHC is used for the diagnosis of lymphoma and various subtypes of lymphoma. IHC positive for CD20 confirms DLBL, constitute 31% of NHL followed by follicular (22%).[11] Savage et al. in 2008 conducted an audit to make a protocol for the investigation of a patient with lymph node in the neck. Thay collected data from neck node of 112 lymphoma patients, and found out that definitive method of diagnosis was excision biopsy in 97 (87%) individuals of the patients, core biopsy in 14 (12%) patients and FNAC in only 1 (1%) patient.[12] In our case, core biopsy combined with IHC helped in diagnosis.
In diffuse large BCL (DLBCL), PET-CT is more sensitive than bone marrow biopsy (BMB) but has been reported to miss low-volume diffused involvement of 10%–20% of the marrow.[13] Berthet et al. conducted a study in DLBCL and found out that 27% of patients had marrow involvement (94% by PET-CT and only 40% by BMB).[14] Pelosi et al. concluded that the sensitivity of PET and BMB is similar (69% and 60%, respectively) and the integration of PET findings with BMB increases the diagnostic accuracy.[15] In our case, bone marrow aspiration was negative.
PET is a functional imaging modality. 18F-FDG is the most commonly used radiotracer in PET imaging. FDG is an analog of glucose; the uptake is directly proportional to the glucose metabolism of tumor tissue. Malignant tumors with high glucose metabolism show preferential uptake of FDG than normal cells. FDG is phosphorylated by hexokinase into FDG-6-phosphate in tumor cells, which is not a substrate for enzyme glucose-6-phosphate isomerase. As a result, 18F-FDG-6-phosphate is not involved in glycolysis and gets trapped within the cell. 18F-FDG-PET is now an established standard in the initial staging, monitoring the response to the therapy, and restaging after treatment of patients with HL and high-grade NHL.[16]
The patient was staged as Stage III S according to Ann Arbor staging of lymphoma by PET/CT [Table 1].[17] According to the International Prognostic Index (IPI), patient had a score of 3 suggesting intermediate high-risk group [Table 2].[18]
Table 1.
Ann Arbor staging of lymphoma
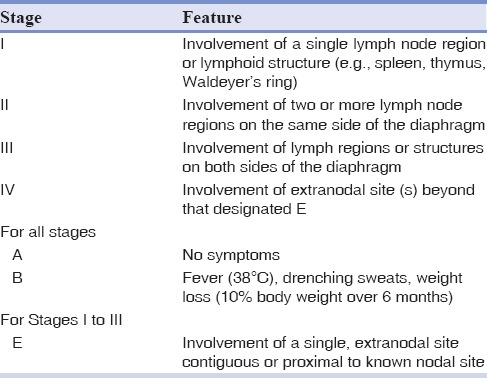
Table 2.
Five adverse prognostic risk factors for International Prognosis Index

Wilder et al. found out that IPI of 3–4 had a 5-year progression-free and overall survival rate were 37% and 32%, respectively.[18] DLBL has an aggressive course and 30%–60% can be cured by intensive chemotherapy and rituximab. R-CHOP chemotherapy has been the standard treatment for patients with later stages of aggressive NHLs, which includes 6–7 cycles of rituximab 375 mg/m2 d1, cyclophosphamide 750 mg/m2 d1, doxorubicin 50 mg/m2 d1, vincristine 1.4 mg/m2 d1, and prednisone 50 mg/m2 d1-5.[19] The patient had undergone six cycles of the standard regimen, and interim PET-CT was done to assess the treatment response. The Deauville criteria, also known as the London criteria, were first used for interpretation of interim PET scans in Hodgkin's lymphoma, which is a five-point visual scale [Tables 3 and 4]. In 2009, at the annual international workshop for interim PET in DLBCL and Hodgkin's lymphoma held in Deauville, France, this 5-point visual scale was proposed for use in DLBCL in addition to Hodgkin's lymphoma. The Deauville criteria depend on a visual comparison of FDG uptake in regions of interest to that of the liver, which generally shows higher FDG uptake.[20] According to this criteria, patient had a Deauville Score I, showing good response to therapy.
Table 3.
The International Prognostic Index
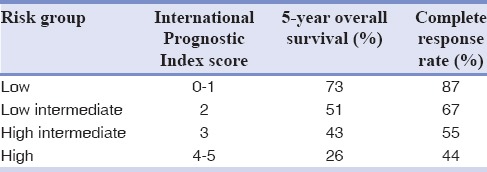
Table 4.
The International Prognostic Index

CONCLUSION
This case highlights the importance of proper examination, especially of lymph node and use of different diagnostic modalities for the exact diagnosis of disease. As oral physicians, we have to gatekeep the oral health and thereby medical health of the patient; hence, it is our responsibility to detect early manifestations of various systemic diseases and to provide the appropriate patient care.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Mohseni S, Shojaiefard A, Khorgami Z, Alinejad S, Ghorbani A, Ghafouri A. Peripheral lymphadenopathy: Approach and diagnostic tools. Iran J Med Sci. 2014;39(2 Suppl):158–70. [PMC free article] [PubMed] [Google Scholar]
- 2.Upadhyay N, Chaudhary A, Alok A. Cervical lymphadenopathy. J Dent Sci Oral Rehabil. 2012;3:30–3. [Google Scholar]
- 3.Bazemore AW, Smucker DR. Lymphadenopathy and malignancy. Am Fam Physician. 2002;66:2103–10. [PubMed] [Google Scholar]
- 4.Dhameja N, Bhatia BD. Filariasis presenting as massive diffuse cervical swelling in child. Am J Trop Med Hyg. 2014;90:5. doi: 10.4269/ajtmh.13-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iqbal M, Subhan A, Aslam A. Frequency of tuberculosis in cervical lymphadenopathy. J Surg Pak Int. 2010;15:107–9. [Google Scholar]
- 6.Fijten GH, Blijham GH. Unexplained lymphadenopathy in family practice. An evaluation of the probability of malignant causes and the effectiveness of physicians’ workup. J Fam Pract. 1988;27:373–6. doi: 10.1080/09503158808416945. [DOI] [PubMed] [Google Scholar]
- 7.Lee Y, Terry R, Lukes RJ. Lymph node biopsy for diagnosis: A statistical study. J Surg Oncol. 1980;14:53–60. doi: 10.1002/jso.2930140108. [DOI] [PubMed] [Google Scholar]
- 8.Kolokotronis A, Konstantinou N, Christakis I, Papadimitriou P, Matiakis A, Zaraboukas T, et al. Localized B-cell non-Hodgkin's lymphoma of oral cavity and maxillofacial region: A clinical study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:303–10. doi: 10.1016/j.tripleo.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Dudea SM, Lenghel M, Botar-Jid C, Vasilescu D, Duma M. Ultrasonography of superficial lymph nodes: Benign vs. malignant. Med Ultrason. 2012;14:294–306. [PubMed] [Google Scholar]
- 10.Harnsberger HR, Bragg DG, Osborn AG, Smoker WR, Dillon WP, Davis RK, et al. Non-Hodgkin's lymphoma of the head and neck: CT evaluation of nodal and extranodal sites. AJR Am J Roentgenol. 1987;149:785–91. doi: 10.2214/ajr.149.4.785. [DOI] [PubMed] [Google Scholar]
- 11.Hunt KE, Reichard KK. Diffuse large B-cell lymphoma. Arch Pathol Lab Med. 2008;132:118–24. doi: 10.5858/2008-132-118-DLBL. [DOI] [PubMed] [Google Scholar]
- 12.Savage SA, Wotherspoon HA, Fitzsimons EJ, MacKenzie K. Cervical lymphadenopathy resulting in a diagnosis of lymphoma. Scott Med J. 2008;53:13–6. doi: 10.1258/RSMSMJ.53.3.13. [DOI] [PubMed] [Google Scholar]
- 13.Carr R, Barrington SF, Madan B, O’Doherty MJ, Saunders CA, van der Walt J, et al. Detection of lymphoma in bone marrow by whole-body positron emission tomography. Blood. 1998;91:3340–6. [PubMed] [Google Scholar]
- 14.Berthet L, Cochet A, Kanoun S, Berriolo-Riedinger A, Humbert O, Toubeau M, et al. In newly diagnosed diffuse large B-cell lymphoma, determination of bone marrow involvement with 18F-FDG PET/CT provides better diagnostic performance and prognostic stratification than does biopsy. J Nucl Med. 2013;54:1244–50. doi: 10.2967/jnumed.112.114710. [DOI] [PubMed] [Google Scholar]
- 15.Pelosi E, Penna D, Douroukas A, Bellò M, Amati A, Arena V, et al. Bone marrow disease detection with FDG-PET/CT and bone marrow biopsy during the staging of malignant lymphoma: Results from a large multicentre study. Q J Nucl Med Mol Imaging. 2011;55:469–75. [PubMed] [Google Scholar]
- 16.Dhanapathi H, Kumar R. F-18 FDG PET/PET-CT in the management of lymphoma. Indian J Med Paediatr Oncol. 2007;28:17–23. [Google Scholar]
- 17.Armitage JO. Staging non-Hodgkin lymphoma. CA Cancer J Clin. 2005;55:368–76. doi: 10.3322/canjclin.55.6.368. [DOI] [PubMed] [Google Scholar]
- 18.Wilder RB, Rodriguez MA, Medeiros LJ, Tucker SL, Ha CS, Romaguera JE, et al. International prognostic index-based outcomes for diffuse large B-cell lymphomas. Cancer. 2002;94:3083–8. doi: 10.1002/cncr.10583. [DOI] [PubMed] [Google Scholar]
- 19.Zelenetz AD, Advani RH, Buadi F, Gordon LI, Wierda WG, Abramson JS, et al. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Non-Hodgkin's lymphoma. 4. 2009 doi: 10.6004/jnccn.2010.0021. Available at: https://www.nccn.org/about/nhl.pdf . [DOI] [PubMed] [Google Scholar]
- 20.Coughlan M, Elstrom R. The use of FDG-PET in diffuse large B cell lymphoma (DLBCL): Predicting outcome following first line therapy. Cancer Imaging. 2014;14:34. doi: 10.1186/s40644-014-0034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


