Abstract
A Dutch traveller returning from Suriname in early March 2017, presented with fever and severe acute liver injury. Yellow fever was diagnosed by (q)RT-PCR and sequencing. During hospital stay, the patient’s condition deteriorated and she developed hepatic encephalopathy requiring transfer to the intensive care. Although yellow fever has not been reported in the last four decades in Suriname, vaccination is recommended by the World Health Organization for visitors to this country.
Keywords: The Netherlands, Suriname, vector-borne infections, viral infections, yellow fever, yellow fever virus, travel, clinic, laboratory
Yellow fever virus (YFV) is known to be enzootic in South America, causing periodic outbreaks of disease in monkeys and humans in some countries. In Brazil, there has been an outbreak of yellow fever ongoing since December 2016 with 1,500 cases as at 9 March [1,2]. Here we report an imported case of human infection with YFV in a traveller returning from Suriname, on the north-eastern coast of South America, from where the last case of yellow fever was reported 45 years ago.
Case description
In March 2017, a Dutch Caucasian female in her late 20s from the Netherlands was referred to the University Medical Center Groningen in the Netherlands because of high fever and signs of severe acute liver injury after returning from a two-week stay in Suriname. She had no co-morbidities apart from obesity (body mass index around 40 kg/m2, norm: 18.5–25 kg/m2). During her visit she stayed in the capital of Suriname, Paramaribo, and she made several daytrips by boat and car, of which two in the tropical rainforest (Figure).
Figure.
Timeline of events and diagnostic results, case of yellow fever in a traveller returning from Suriname to the Netherlands, March 2017
P: Paramaribo; RNA: ribonucleic acid; UMCG: University Medical Center Groningen; YFV: yellow fever virus.
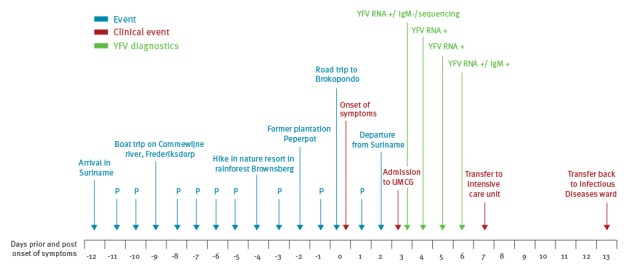
She recalled having been bitten by mosquitoes during her hike at Brownsberg, a nature resort in the rainforest with wildlife. Before her travel, she did not visit a travel clinic and did not receive yellow fever vaccination. On day 12 of her visit in Suriname, she experienced mild muscle pain, headache and nausea and she developed a high-grade fever. She returned to the Netherlands on day 15 and visited the emergency department of a secondary care centre, from where she was referred to our University hospital. At physical examination she was not icteric. Except for a temperature of 39.9 °C, vital parameters were normal. The results of the remaining physical examination were unremarkable. Laboratory testing revealed leukopenia (leukocytes 0.9x109/L, norm: 4.0–10.0x109/L) and massive liver injury (aspartate aminotransferase 5,787 U/L, norm: <31 U/L; alanine aminotransferase 4,910 U/L, norm: <34 U/L), with mildly elevated bilirubin levels (total bilirubin 20 µmol/L, norm: <17 µmol/L). Liver synthesis was impaired as revealed by increased clotting times (activated partial thromboplastin time (APTT): 49s, norm: 23–33s; prothrombin time (PT): 26.6s, norm: 9.0–12.0s) and reduced antithrombin (49%, norm: 80–120%). Fibrinogen was diminished suggestive of diffuse intravascular coagulation. Renal function was normal apart from severe albuminuria (up to 22.6 g/24h, norm: 0g/24h). Malaria, viral hepatitis (A, B, C, E, Epstein Barr virus, cytomegalovirus, herpes simplex virus), dengue, chikungunya and Zika were ruled out (Table). Diagnostic tests to exclude leptospirosis performed on day 6 post onset of symptoms (dps 6) were inconclusive (Table) and a convalescent serum was going to be tested at the time of publication. Because of the combination of fever, leukopenia, thrombocytopenia, liver injury and travel history, yellow fever was included in the differential diagnosis. Real-time reverse transcriptase PCR (qRT-PCR) was positive for YFV in serum taken on dps 3. On dps 7 the patient’s condition deteriorated due to hepatic encephalopathy (ammonia 149 µmol/L, norm: 15–45 µmol/L). Cerebral oedema and bleeding was ruled out by computed tomography (CT)-scan. The patient was transferred to the intensive care unit for close observation of vital parameters. Vitamin K was administered. Hepatic encephalopathy was treated with rifaximin and lactulose. Ceftriaxone (2g per day intravenously) was given for 7 days as antibiotic prophylaxis. Consequently, possible leptospirosis was also treated. Her neurological condition stabilised on dps 10 together with the coagulation parameters. On dps 13 the patient was transferred back to the ward.
Table. Pathogens for which laboratory tests were performed, yellow fever case, the Netherlands, March 2017.
| Pathogen | Blood (day 3 post onset of symptoms) |
|---|---|
| Plasmodium spp. | Thick smear negative, antigen test negative |
| Hepatitis A virus | IgM and IgG negative |
| Hepatitis B virus | Serological screening negative |
| Hepatitis C virus | Serological screening negative |
| Hepatitis E virus | PCR negative |
| Epstein Barr virus | IgM and IgG negative |
| Cytomegalovirus | IgM and IgG negative |
| Herpes simplex virus type 1 and 2 | PCR negative |
| Dengue virus | PCR negative, IgM and IgG negative |
| Chikungunya virus | PCR negative, IgM and IgG negative |
| Zika virus | PCR negative, IgM and IgG negativea |
| Leptospira spp. | PCR negative, microscopic agglutination test negative, IgM 1:80b |
a Performed on day 5 post onset of symptoms (dps 5).
b ELISA (in-house ELISA Dutch Leptospirosis Reference Center) performed on dps 6 showed IgM 1:80 (cut-off positive IgM ≥1:160). IgM results were negative on dps 3 and dps 7 using Leptocheck-WB (Zephyr Biomedicals, Goa, India).
Virology findings
qRT-PCR and/or pan-flavivirus RT-PCR on blood samples on dps 3 did not detect chikungunya virus (CHIKV), dengue virus (DENV), or Zika virus (ZIKV) (Table) [3,4]. In four consecutive samples of dps 3–6, YFV-RNA was detected (Figure) [4-6], with increasing Ct values (from 23 to 31 from dps 3 to dps 5 [5] and 39 on dps 6 [6]). Sequencing of a 176 bp pan-flavivirus hemi-nested RT-PCR product, targeting part of the NS5 genomic region confirmed YFV infection [4]. The sequence was deposited in the GenBank database under the following accession number: KY774973.
On dps 3, indirect immunofluorescence assays (IFA) was negative for IgM and IgG against YFV (Flavivirus Mosaic, Euroimmun AG, Luebeck, Germany). A convalescent sample of dps 6 was clearly positive for YFV IgM (titre 1:10, Figure), with non-reactive IgG. This anti-YFV IgM response on dps 6 is in line with literature stating that IgM antibodies usually appear during the first week of illness. Neutralising IgG antibodies are likely to appear towards the end of the first week after onset of illness and will be tested for in convalescent serum [7].
Background
YFV is a mosquito-borne virus in the genus Flavivirus, family Flaviviridae, related to DENV, ZIKV, tick-borne encephalitis virus and West Nile virus. YFV is maintained in a sylvatic cycle between non-human primates and so-called ‘jungle’-mosquitoes (Hemagogus and Sabethes spp. in South America) [8]. Sporadic infection of humans with sylvatic YFV can occur when unprotected humans are exposed while entering the habitats where the viruses circulate. Subsequent introduction of a viraemic human case to urban areas with high population densities and Aedes aegypti mosquitoes can initiate an urban transmission cycle [9]. YFV is endemic in (sub)tropical areas of South America and Africa. The risk for YFV infection in South America is the highest in tropical regions and during the rainy season (January–May) when mosquito population densities peak [10]. In 2011, Suriname was identified by the World Health Organization (WHO) as one of 14 South American countries at risk for YFV transmission based on current or historic reports of yellow fever, plus the presence of competent mosquito vectors and animal reservoirs [11].
Since December 2016, an outbreak of sylvatic YFV is ongoing in Brazil; as at 9 March 2017, there were 371 confirmed and 966 suspected human cases, while a total of 968 epizootics in non-human primates have been reported, of which 386 were confirmed [2]. So far, there has been no evidence for a change from sylvatic to an urban transmission cycle [1]. In addition, Bolivia, Colombia and Peru have reported suspected and confirmed yellow fever cases in 2017 [2].
A subclinical infection with YFV is believed to occur in most infected people. In symptomatic cases, symptoms of general malaise occur after an incubation period of 3–6 days (range 2–9 days), followed by remission of the disease in the majority of patients. However, 15-25% of symptomatic persons develop a complicated course of illness, in which symptoms recur after 24–48 hours, with a reported mortality of 20-60% [7,12]. This phase is characterised by fever, abdominal symptoms, severe hepatic dysfunction and jaundice, multi-organ failure and haemorrhagic diathesis. As no specific antiviral treatment is currently available, treatment consists of supportive care [7,12].
Discussion
Although Suriname is considered to be endemic for YFV, no human cases have been officially reported since 1971 [13]. With a population of ca 570,000 people, Suriname has a YFV vaccination coverage of 80–85% in infants [14]. Although WHO recommends vaccination for travellers to countries with risk of YFV transmission like Suriname, sporadic cases of imported yellow fever in returning travellers have been reported for example in Europe, the United States and Asia [15-17], with three reported cases related to the ongoing YFV outbreaks in South America in European travellers since 2016 [18,19]. The establishment of ongoing YFV circulation in Suriname extends the current YFV activity in South America to five countries [2]. However, despite the presence of competent Ae. albopictus mosquitoes in France [20] and Ae. aegypti in Madeira, the risk for YFV transmission in Europe is currently considered to be very low due to the lack of vector activity [18]. An effective, safe live-attenuated YFV vaccine is available for people aged ≥ 9 months and offers lifelong immunity [7]. Vaccination is advised by the WHO for all travellers to Suriname, for the coastal area as well as the inlands [21]. With regard to yellow fever, pre-travel health advice should take into account destination, duration of travel, season and the likelihood of exposure to mosquitoes (in rural areas, forests versus urban areas), and potential contraindications for vaccination with a live-attenuated vaccine.
The multi-country YFV activity might reflect current, wide-spread ecological conditions that favour elevated YFV transmissibility among wildlife and spill-over to humans. Thorough sequence analysis of currently circulating strains in Brazil, Bolivia, Colombia, Peru and Suriname should provide insight whether the human cases in these countries are epidemiologically linked or represent multiple, independent spill-over events without extensive ongoing community transmission. Because of its potential public health impact, our case of yellow fever was notified to the WHO and the European Union Early Warning and Response System on 9 March 2017, according to the international health regulations [22].
Conclusion
Clinicians in non-endemic countries should be aware of yellow fever in travellers presenting with fever, jaundice and/or haemorrhage returning from South America including Suriname. This case report illustrates the importance of maintaining awareness of the need for YFV vaccination, even for countries with risk of YFV transmission that have not reported cases for decades.
Acknowledgements
Bert Niesters and staff at clinical virology unit UMCG. Bas Oude Munnink, Robert Kohl and staff at clinical virology unit Erasmus MC. Alexander Schlaphof, Corinna Thome, Neele Neddersen, Insa Bonow, Petra Emmerich at Bernhard Nocht Institute for technical assistance. Aura Timen, final responsibility with respect to international notifications.
Funding: The Viroscience reference laboratory received funding from the EU FP7 and H2020 programs PREPARE (grant agreement no. 602525), COMPARE (no. 643476) and EVAg (no. 653316).
Conflict of interest: None declared.
Authors’ contributions: MWB: treating physician of case, wrote initial manuscript. MK: consultant-virologist of case, co-wrote manuscript. APvdB: consultant-hepatologist of case. CHGvK: consultant-virologist of case, serology, data interpretation. MPGK: reference diagnostics, expertise. CvLB: consultant-virologist of case. BOV: treating physician of case on Intensive Care Unit. SDP: molecular diagnostics, data interpretation. WLMR: coordination WHO notification. JSC: confirmatory diagnostics. SGSV: consultant-infectious diseases Suriname. TSvdW: treating physician, expertise. CBEMR: reference diagnostics and expertise, sequencing, data interpretation, co-wrote manuscript. WFWB: consultant-infectious diseases of case, co-wrote manuscript. All authors reviewed, provided comments and approved the final manuscript.
References
- 1.Paules CI, Fauci AS. Yellow Fever - Once Again on the Radar Screen in the Americas. N Engl J Med. 8 Mar 2017;NEJMp1702172. [Epub ahead of print]. [DOI] [PubMed]
- 2.Pan American Health Organization / World Health Organization (PAHO/WHO). Epidemiological Update Yellow Fever 9 March. Washington, D.C. PAHO/WHO; 2017. Available from: http://www.paho.org/hq/index.php?option=com_topics&view=readall&cid=2194&Itemid=40784&lang=en
- 3. Panning M, Grywna K, van Esbroeck M, Emmerich P, Drosten C. Chikungunya fever in travelers returning to Europe from the Indian Ocean region, 2006. Emerg Infect Dis. 2008;14(3):416-22. 10.3201/eid1403.070906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scaramozzino N, Crance JM, Jouan A, DeBriel DA, Stoll F, Garin D. Comparison of flavivirus universal primer pairs and development of a rapid, highly sensitive heminested reverse transcription-PCR assay for detection of flaviviruses targeted to a conserved region of the NS5 gene sequences. J Clin Microbiol. 2001;39(5):1922-7. 10.1128/JCM.39.5.1922-1927.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charlier N, Molenkamp R, Leyssen P, Paeshuyse J, Drosten C, Panning M, et al. Exchanging the yellow fever virus envelope proteins with Modoc virus prM and E proteins results in a chimeric virus that is neuroinvasive in SCID mice. J Virol. 2004;78(14):7418-26. 10.1128/JVI.78.14.7418-7426.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drosten C, Göttig S, Schilling S, Asper M, Panning M, Schmitz H, et al. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J Clin Microbiol. 2002;40(7):2323-30. 10.1128/JCM.40.7.2323-2330.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Monath TP. Yellow fever: an update. Lancet Infect Dis. 2001;1(1):11-20. 10.1016/S1473-3099(01)00016-0 [DOI] [PubMed] [Google Scholar]
- 8. Pinto CS, Confalonieri UE, Mascarenhas BM. Ecology of Haemagogus sp. and Sabethes sp. (Diptera: Culicidae) in relation to the microclimates of the Caxiuanã National Forest, Pará, Brazil. Mem Inst Oswaldo Cruz. 2009;104(4):592-8. 10.1590/S0074-02762009000400010 [DOI] [PubMed] [Google Scholar]
- 9. Dhawan R, Kumar M, Mohanty AK, Dey G, Advani J, Prasad TS, et al. Mosquito-Borne Diseases and Omics: Salivary Gland Proteome of the Female Aedes aegypti Mosquito. OMICS. 2017;21(1):45-54. 10.1089/omi.2016.0160 [DOI] [PubMed] [Google Scholar]
- 10.Cavalcante KR, Tauil PL. Epidemiological characteristics of yellow fever in Brazil, 2000-2012. Epidemiol Serv Saude. 2016 Jan-Mar;25(1):11-20 [DOI] [PubMed]
- 11. Jentes ES, Poumerol G, Gershman MD, Hill DR, Lemarchand J, Lewis RF, et al. Informal WHO Working Group on Geographic Risk for Yellow Fever The revised global yellow fever risk map and recommendations for vaccination, 2010: consensus of the Informal WHO Working Group on Geographic Risk for Yellow Fever. Lancet Infect Dis. 2011;11(8):622-32. 10.1016/S1473-3099(11)70147-5 [DOI] [PubMed] [Google Scholar]
- 12. Monath TP, Vasconcelos PF. Yellow fever. J Clin Virol. 2015;64:160-73. 10.1016/j.jcv.2014.08.030 [DOI] [PubMed] [Google Scholar]
- 13. De Haas RA, Oostburg BF, Sitalsing AD, Bellot SM. Isolation of yellow fever virus from a human liver obtained by autopsy in Surinam. Trop Geogr Med. 1971;23(1):59-63. [PubMed] [Google Scholar]
- 14.World Health Organization (WHO). WHO and UNICEF Estimates of National Infant Immunization Coverage. Geneva: WHO; [Accessed 13 March 2017]. Available from: http://www.who.int/immunization/monitoring_surveillance/routine/coverage/en/index4.html
- 15. Colebunders R. Imported case of confirmed yellow fever detected in Belgium. Euro Surveill. 2001;5(47):2058. [Google Scholar]
- 16. Centers for Disease Control and Prevention (CDC) Fatal yellow fever in a traveler returning from Venezuela, 1999. MMWR Morb Mortal Wkly Rep. 2000;49(14):303-5. [PubMed] [Google Scholar]
- 17. ProMED Mail Yellow fever - China (03): ex Angola. Archive. 2016;4106312(: 20160319):19 Available from: http://www.promedmail.org/post/4106312 [Google Scholar]
- 18.European Centre for Disease Prevention and Control (ECDC). Rapid risk assessment; outbreak of yellow fever in Brazil –25 January 2017 Stockholm: ECDC; 2017. Available from: http://ecdc.europa.eu/en/publications/Publications/Risk-assessment-yellow-fever-outbreak-Brazil-25-jan-2017.pdf
- 19.European Centre for Disease Prevention and Control (ECDC). Communicable disease threats report -18 Feb 2017 Stockholm: ECDC. Available from: http://ecdc.europa.eu/en/publications/Publications/communicable-disease-threats-report-18-feb-2017.pdf
- 20. Amraoui F, Vazeille M, Failloux AB. French Aedes albopictus are able to transmit yellow fever virus. Euro Surveill. 2016;21(39):30361. 10.2807/1560-7917.ES.2016.21.39.30361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization (WHO). WHO International travel and health, annex 1 – update – as of 16 Feb 2017. Geneva: WHO. Available from: http://www.who.int/ith/2017-ith-annex1.pdf?ua=1&ua=1
- 22.World Health Organization (WHO). International Health Regulations (2005). Geneva: WHO. Available from: http://www.who.int/topics/international_health_regulations/en/


