Abstract
In 2008, a clinical practice guideline (CPG) was developed for the prevention of infections among combat casualties and was later revised in 2011. We evaluated utilization of antimicrobials within 48 hours following injury in the combat zone over a five-year period (June 2009–May 2014) with regard to number of regimens, type of antimicrobial, and adherence to the 2011 CPG. The study population consisted of 5196 wounded military personnel. Open fractures and skin and soft-tissue injuries were the most frequent injuries. Closed injuries had the highest overall compliance (83%), while open fractures and maxillofacial injuries had significant improvement in compliance from 2009–2010 (34% and 50%, respectively) to 2013–2014 (73% and 76%; p<0.05). Part of the improvement with open fractures was a significant reduction of expanded Gram-negative coverage (61% received it in 2009–2010 compared to 7% in 2013–2014; p<0.001). Use of Gram-negative coverage with maxillofacial injuries also significantly declined (37% to 12%; p=0.001). Being injured during 2011–2014 compared to 2009–2010 was associated with CPG compliance (p<0.001), while high injury severity scores (≥10) and admission to the intensive care unit in Germany were associated with noncompliance (p<0.001). Our analysis demonstrates an increasing trend toward CPG compliance with significant reduction of expanded Gram-negative coverage.
Keywords: clinical practice guidelines, antimicrobial prophylaxis, guideline adherence, combat-related infections
INTRODUCTION
The administration of antimicrobials as part of the immediate care of trauma patients is a standard practice to prevent subsequent infections. In general, the majority of clinical practice guidelines (CPGs) related to infectious complications resulting from traumatic injuries have been developed in the civilian setting.1–8 Furthermore, review of the effectiveness of these guidelines has also been focused on civilian population.2,9–11 As combat trauma presents with differences in injury mechanism, severity of injury, and timing of treatment, the Department of Defense (DoD) Joint Trauma System (JTS) convened an expert consensus panel to adapt these civilian guidelines for the military setting. The first CPG for the prevention of infections associated with combat-related injuries was published in March 2008.12 The CPG outlined appropriate post-injury antimicrobial prophylaxis choices as determined by injury pattern and specifically recommended cefazolin or clindamycin (i.e., Gram-positive coverage) for open fractures (including Type IIIb and IIIc) and maxillofacial injuries. Shortly thereafter, the JTS released internal guidance in March 2010 based on civilian guidelines,1,4 which was contradictory to the 2008 published CPG as it recommended expanded Gram-negative coverage (levofloxacin, aminoglycosides, or other Gram-negative coverage) in addition to the Gram-positive coverage for contaminated fractures or certain maxillofacial injuries.
In an attempt to standardize recommendations and incorporate evidence-based findings related to dosing regimens, a revised CPG was published in 2011 by an expert panel of DoD trauma and infectious disease physicians,13 which was later validated by a corresponding JTS document in 2012.14 In this revised CPG, addition of Gram-negative coverage for post-injury prophylaxis in the setting of open fractures and maxillofacial injuries was not recommended and the number of choices for antibiotic prophylaxis with penetrating abdominal injuries was significantly curtailed (i.e., only cefazolin/metronidazole or ertapenem were recommended as appropriate prophylactic antibiotic choices).13 The goal of the expert consensus panel was to significantly decrease broad-spectrum antibiotics use in the post-injury antibiotic prophylaxis timeframe because of concern that use of overly broad antibiotics for prophylaxis may increase the risk of multidrug-resistant organism acquisition and infection, unnecessarily expose patients to potential toxicities (e.g., aminoglycoside-induced kidney injury), and possibly lead to less emphasis on quality surgical debridement.15,16 In two prior publications, we evaluated antimicrobial prophylaxis compliance with the 2008 CPG over a six-month and one-year period among injured United States (U.S.) military personnel medically evacuated to Landstuhl Regional Medical Center (LRMC).17,18 Although the six-month analysis was comprehensive at the time, methodological limitations restricted applicability of the results. Therefore, a revised methodology based on Abbreviated Injury Scale (AIS) codes for injury categorization, rather than International Classification of Disease (9th edition) diagnostic codes, and improved antibiotic regimen coding were applied to a subsequent one-year cohort, resulting in rates of overall CPG compliance of 75%.
Despite the improved methodology, the determination of “CPG compliant” in both publications allowed for subjects who had received expanded Gram-negative coverage for open fracture and maxillofacial injuries because of the conflicting recommendations of the 2008 CPG and 2010 JTS guidance. For example, in the one-year analysis, 48% of patients with open fractures who were considered “compliant” with the 2008 CPG received Gram-negative coverage in addition to the standard Gram-positive regimen. Similarly, 27% of “compliant” maxillofacial injury patients received Gram-negative coverage.18 Furthermore, in both the six-month and one-year analyses,17,18 vancomycin was allowed as a substitution for Gram-positive prophylaxis despite not being recommended by either the 2008 CPG or 2010 JTS guidance.
Examination of data from the one-year analysis suggested that a longer term assessment of antimicrobial use trends was needed. Currently, the overarching observational cohort project, the DoD – Department of Veterans Affairs Trauma Infectious Disease Outcomes Study (TIDOS), has collected data for over five years. During this period, use of broad-spectrum antibiotics for prophylaxis was discouraged as embodied in the 2008 and 2011 CPGs. Nevertheless, the antibiotic stewardship goal of reducing the use of Gram-negative coverage in the setting of post-combat-trauma antibiotic prophylaxis was hindered by varied clinical practice patterns of trauma surgeons, high turnover of trauma team members, and the fast-moving practice environment unique to trauma care in a war zone (e.g., several transfers of care within the first 72 hours post-injury). In the analysis herein, we evaluate the administration of post-injury prophylactic antibiotics vis-à-vis the 2011 CPG recommendations, which embody the antibiotic stewardship goals throughout the study period. Additionally, we examine the use of expanded Gram-negative coverage across injury types and duration of prophylactic antibiotic use over a five-year period.
METHODS
Study Population and Data Collection
Trauma patients were eligible for inclusion in the analysis if they were active-duty personnel or DoD beneficiaries, at least 18 years of age, and sustained deployment-related injuries in either the Iraq or Afghanistan combat theaters requiring medical evacuation to LRMC (Germany) between June 1, 2009 and May 31, 2014. These data were collected as part of TIDOS, which is an ongoing observational cohort study assessing the short- and long-term infectious complications related to deployment-related traumatic injuries.19 For the analysis, data were obtained from the DoD Trauma Registry (DoDTR)20 and supplemented by the TIDOS infectious disease module. This study was approved by the Infectious Disease Institutional Review Board of the Uniformed Services University of the Health Sciences (Bethesda, MD).
Injury Characterization and Classification
Injury data obtained from the DoDTR were standardized into AIS-defined codes21 using an injury coding software system, Tri-Code (Digital Innovations, Inc., Forest Hill, MD). In brief, AIS is an anatomically-based injury severity scoring system, which allows for the categorization of distinct injury types (e.g., blunt force and penetrating trauma) by specific body regions. Trauma patients were classified into one of the five established injury categories18 based on their pattern of injury as well as their antibiotic prophylaxis requirement in a stepwise fashion using the highest level of antibiotic coverage required. Patients not meeting an injury criteria requiring antibiotic prophylaxis were placed in the “closed” injury category. Due to limited numbers, patients meeting the criteria for a penetrating central nervous system injury were excluded from the analysis.
Antimicrobial Prophylaxis Compliance
As previously described,18 antibiotic use was determined via prospective review of the medical records. Compliance was defined as receipt of approved antibiotics in accordance with the 2011 CPG recommendations.13 Unlike the previous two publications which evaluated compliance over six and twelve months,17,18 expanded Gram-negative coverage in open fracture and maxillofacial injuries was not deemed compliant. Antibiotic regimens, including broad-spectrum Gram-negative coverage, were classified as previously described18 and a new class (expanded Gram-negative coverage) was developed, which included regimens with (e.g., piperacillin/tazobactam and meropenem) and without (i.e., levofloxacin) anaerobic coverage. Although not available in the United States, intravenous amoxicillin-clavulanate was the first-line choice for post-injury antimicrobial prophylaxis by the British military, in accordance with their published guidelines.22 As a portion of wounded U.S. military personnel were treated by coalition forces in the combat zone, use of amoxicillin-clavulanate as a substitute for cefazolin and cefazolin/metronidazole (in the case of penetrating abdomen injury) was deemed to be compliant. Furthermore, use of vancomycin with any injury category was also not considered compliant. This more stringent evaluation was chosen in order to better assess trends toward compliance with the 2011 CPG, which embody the expert panel’s antibiotic stewardship goals.
As in our previous publications,17,18 adherence was assessed in the immediate period following injury out to 48 hours to account for the potential of documentation omissions and multiple transitions of care associated with combat trauma care/medical evacuation. Our analysis also examines duration of antimicrobial use specific to injury patterns and use of specific antimicrobials (i.e., ciprofloxacin/levofloxacin, vancomycin, meropenem/imipenem, and aminoglycosides).
Statistical Analysis
Categorical variables across the study years were assessed using Fisher’s exact and Chi square tests for trends. Non-parametric tests were used to compare overall continuous variable distributions. Data were also examined on a per study year basis: 2009–2010, 2010–2011, 2011–2012, 2012–2013, and 2013–2014 (June through May of subsequent year). Logistic regression was also used to examine potential predictors for antimicrobial prophylaxis compliance (related to 2011 CPG) in a univariate and multivariate analysis. Statistical analysis was performed with SAS® version 9.3 (SAS, Cary, NC). Significance was defined as p<0.05.
RESULTS
Study Population
A total of 5196 military personnel sustained injuries during the study period (June 2009 – May 2014). The patients were predominantly men (98%) who sustained blast injuries (55%) in support of military operations in Afghanistan (90%). The proportion of blast injuries remained fairly consistent during the first three years of the analysis (56–59%); however, as military operations slowed during the last two years of the analysis, it declined to 48% during 2012–2013 and 39% in 2013–2014 (p<0.001). The proportion of wounded personnel with gunshot wounds also increased from 16% during the first year to 21% in 2013–2014 (p=0.005). Similarly, injury severity score (ISS) varied over the analysis period with the highest scores during 2010–2011 (median: 10; interquartile range: 5–22) and the lowest during the last year of the analysis (median: 8; interquartile range: 4–17; p<0.001). Furthermore, the proportion of patients with a shock index >1.5 (i.e., heart rate/systolic blood pressure) significantly increased from 2.2% in 2009–2010 to 3.5% in 2011–2012 and then declined to 2.8% in 2013–2014 (p=0.015). There was no significant difference in the proportion of patients admitted to the LRMC intensive care unit (ICU) over the study period (26–31%; p=0.194).
Infections were observed in 5% of wounded personnel admitted to LRMC. As with injury severity, the proportion of patients with infections varied over the years with an increase from 3.4% in 2009–2010 to 7.1% in 2011–2012, followed by a decline to 2.3% in the final year (p<0.001). Furthermore, 2445 (47%) patients transferred to a participating military hospital in the United States, of which 30.0% had an infectious complication. There was a significant difference in the proportion of patients with an infection at the U.S. hospitals over the study period with an increase from 27% in 2009–2010 to 36% in 2012–2013 (p=0.001).
Injury Patterns
Injuries requiring prophylaxis for open fractures were the most common injury pattern accounting for 37% of the study cohort followed by patients with skin and soft-tissue (25%) and closed (24%) injury patterns (Table 1). There was a significant difference in the pattern of injuries across the study years (p<0.001). Open fractures peaked during 2010–2011 (42%) and then declined to 30% in the last study year. In contrast, closed injuries declined from 24% in the first year to 19% during 2010–2011 and then increased to 33% in the last year of the analysis. Penetrating abdominal injuries increased from 4% in 2009–2010 to 5% between 2010 and 2013, whereas maxillofacial injuries declined from 11% in the first year to 7% in the last year of the analysis. Lastly, the proportion of skin and soft-tissue injuries remained fairly constant across the years, ranging from 23% to 27%.
Table 1.
Pattern of Injury for Wounded Military Personnel Admitted to Landstuhl Regional Medical Center (2009–2014). All indicated time periods begin in June and end in May. Data are expressed as number (percentage). Injury patterns were significantly different across the years; p<0.001.
| Injury Patterna | Total | 2009–2010 | 2010–2011 | 2011–2012 | 2012–2013 | 2013–2014 |
|---|---|---|---|---|---|---|
| Open Fractures | 1922 (37.0) | 401 (34.9) | 631 (41.8) | 499 (36.3) | 285 (35.1) | 106 (30.0) |
| Skin and Soft-Tissue | 1306 (25.1) | 306 (26.7) | 376 (24.9) | 322 (23.4) | 215 (26.5) | 87 (24.7) |
| Closedb | 1252 (24.1) | 275 (24.0) | 290 (19.2) | 361 (26.3) | 208 (25.7) | 118 (33.4) |
| Maxillofacial | 470 (9.1) | 123 (10.7) | 134 (8.9) | 123 (9.0) | 65 (8.0) | 25 (7.1) |
| Penetrating Abdomenc | 246 (4.7) | 43 (3.8) | 78 (5.2) | 70 (5.1) | 38 (4.7) | 17 (4.8) |
| Total | 5196 | 1148 | 1509 | 1375 | 811 | 353 |
Injury patterns based on Abbreviated Injury Scale (2005-Military) codes.
Defined by no evidence of open wounds.
Defined by injury to a hollow viscus except for hematomas, anal injuries, and ‘no perforation partial thickness’.
Antimicrobial Use Patterns
Between 2009 and 2011, 58–61% of patients with open fractures received expanded Gram-negative coverage; however, the proportion significantly decreased to 12%, 8%, and 7% in 2011–2012, 2012–2013, and 2013–2014, respectively (p<0.001; Figure 1). A similar pattern was observed for maxillofacial injuries with the proportion of expanded Gram-negative coverage decreasing from 36–37% between 2009–2011 to 12–25% in the later time period (p=0.001).
Figure 1.
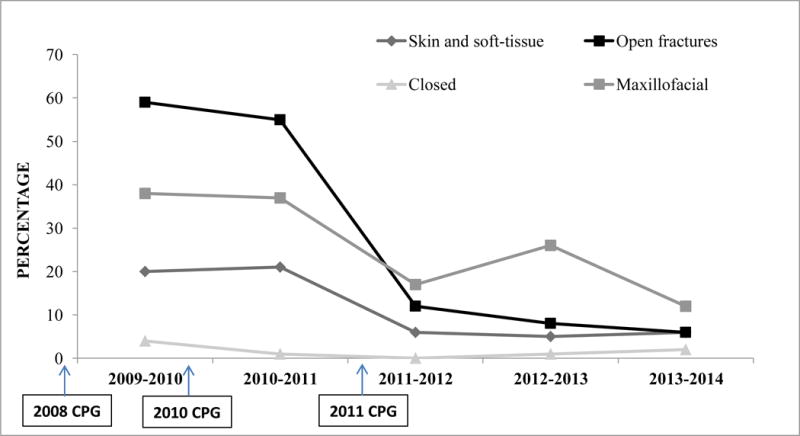
Proportion of wounded military personnel who received expanded Gram-negative coverage by injury pattern. Each time period began in June and ended in May. Overall total patients who received expanded Gram-negative coverage per injury pattern was: open fractures = 703; skin and soft-tissue injuries = 175; closed = 18; and maxillofacial = 133. All injury patterns had significant difference in proportion across the study years with p-values of <0.001 for skin and soft-tissue injuries and open fractures, p=0.007 for closed injuries, and p=0.001 for maxillofacial injuries. The 2008 CPG12 did not recommend use of Gram-negative agents in open fractures of maxillofacial injuries. The 2010 JTS guidance document recommended use of Gram-negative agents in contaminated open fractures and some maxillofacial injuries. The 2011 CPG13 recommended against use of Gram-negative coverage in all injuries except for penetrating abdomen where cefazolin/metronidazole or ertapenem was recommended
Along with use of expanded Gram-negative coverage, specific antibiotics evaluated included vancomycin, carbapenems, and fluoroquinolones. In general, very little vancomycin was prescribed during the study period. Patients with penetrating abdominal injuries received the largest proportion (7%) during 2009–2010. With regard to injury patterns, there were no significant differences in the use of vancomycin over the study years. The largest proportion of carbapenem usage (6%) was associated with penetrating abdominal injuries during 2013–2014; however, there was no statistical difference in the receipt of the antibiotic over the study years for any of the injury patterns. Use of levofloxacin and ciprofloxacin did significantly decrease for each injury pattern over the study period (p<0.001) with the largest reduction being from 51–55% with open fractures (2009–2011) to 2–5% (2011–2014). For penetrating abdominal injuries, 42% were prescribed levofloxacin/ciprofloxacin in 2010–2011, which decreased to 8% in 2012–2013 and zero in 2013–2014.
Cefazolin use in open fractures and soft-tissue injuries requiring prophylaxis varied widely during the study years (Table 2). Specifically, 95% of patients with open fractures received cefazolin; however, the proportion that received the antibiotic for more than five days significantly reduced from 51% in 2009–2010 to 23–28% between 2011 and 2014 (p<0.001). Duration of cefazolin use in soft-tissue injuries also significantly decreased during the study years (p=0.018), with 16% receiving the antibiotic for more than five days in 2009–2010 compared to 8–10% between 2011 and 2014.
Table 2.
Usage of Cefazolin by Injury Pattern with Regards to Duration, No. (%).
| Cefazolin Usage | Open Fracturesa (N=1922) | Skin/Soft-Tissue (N=1306) |
|---|---|---|
| Total Received Cefazolin | 1825 (95) | 1054 (81) |
| 2009–2010b | ||
| Total Injuries | 401 | 306 |
| Duration of cefazolin Use | ||
| ≤48 hours | 94 (23) | 112 (37) |
| 3–5 days | 87 (22) | 92 (30) |
| >5 days | 205 (51) | 48 (16) |
| 2010–2011b | ||
| Total Injuries | 631 | 376 |
| Duration of cefazolin Use | ||
| ≤48 hours | 199 (32) | 152 (40) |
| 3–5 days | 154 (24) | 118 (31) |
| >5 days | 254 (40) | 45 (12) |
| 2011–2012b | ||
| Total Injuries | 499 | 322 |
| Duration of cefazolin Use | ||
| ≤48 hours | 173 (35) | 148 (46) |
| 3–5 days | 182 (36) | 88 (27) |
| >5 days | 115 (23) | 26 (8) |
| 2012–2013b | ||
| Total Injuries | 285 | 215 |
| Duration of cefazolin Use | ||
| ≤48 hours | 109 (38) | 88 (41) |
| 3–5 days | 82 (29) | 54 (25) |
| >5 days | 73 (26) | 20 (9) |
| 2013–2014b | ||
| Total Injuries | 106 | 87 |
| Duration of cefazolin Use | ||
| ≤48 hours | 38 (36) | 33 (38) |
| 3–5 days | 30 (28) | 21 (24) |
| >5 days | 30 (28) | 9 (10) |
Duration of cefazolin use is significantly different across the study years (p<0.001 for open fractures and p=0.018 for open skin and soft-tissue).
Time period starts in June and ends in May.
Adherence to Antimicrobial Prophylaxis Recommendations
Using the 2011 CPG as the reference, antimicrobial adherence ranged from 34% with penetrating abdominal injuries to 83% with closed injuries (Table 3). Compliance with guidelines related to closed injuries significantly improved over the study years from 78% in the first year to 90% in the last (p=0.006). In addition, maxillofacial injuries showed improvement with an increase from 50% compliant in 2009–2010 to 76% in 2013–2014 (p=0.042). Open fracture compliance also improved from 34% in the first year of the analysis to 77% in 2012–2013 and then declined slightly to 73% in the final year (p<0.001). Furthermore, skin and soft-tissue injuries had an increase in compliance from 58% in 2009–2010 to 69% in 2011–2012 and then decreased to 61% in 2013–2014 (p=0.013). Lastly, penetrating abdominal injuries improved from 16% in 2009–2010 to 47% in 2011–2012 and then declined to 34–35% for the final two years of the analysis (p=0.019). The overwhelming reason for non-compliance related to penetrating abdominal injuries was administration of antimicrobial regimens not consistent with the CPG rather than not prescribing prophylaxis.
Table 3.
Antimicrobial Prophylaxis in Combat-Related Trauma – Adherence to Published Clinical Practice Guideline (CPG).a
| InjuryPattern | Total Injuries | Total Compliant | Compliant with CPG, No (%)b | |||||
|---|---|---|---|---|---|---|---|---|
| 2009–2010 | 2010–2011 | 2011–2012 | 2012–2013 | 2013–2014 | P-valuec | |||
| OpenFractures | 1922 | 1027 (53.4) | 138 (34.4) | 214 (33.9) | 379 (76.0) | 219 (76.8) | 77 (72.6) | <0.001 |
| Skin and Soft Tissue | 1306 | 810 (62.0) | 177 (57.8) | 217 (57.7) | 221 (68.6) | 142 (66.1) | 53 (60.9) | 0.013 |
| Closed | 1252 | 1035 (82.7) | 213 (77.5) | 232 (80.0) | 302 (83.7) | 182 (87.5) | 106 (89.8) | 0.006 |
| Maxillofacial | 470 | 264 (56.2) | 62 (50.4) | 68 (50.8) | 78 (63.4) | 37 (56.9) | 19 (76.0) | 0.042 |
| Penetrating Abdomen | 246 | 83 (33.7) | 7 (16.3) | 24 (30.8) | 33 (47.1) | 13 (34.2) | 6 (35.3) | 0.019 |
Predictors of Antimicrobial Prophylaxis Compliance
Time period (injuries sustained during 2011–2014 as compared to the earlier years), admission to LRMC ICU, volume of blood product transfusion within 24 hours, age at injury, shock index, and ISS were examined for an association with CPG antimicrobial prophylaxis compliance in a univariate analysis (Table 4). Due to high correlation with injury severity and LRMC ICU (p<0.001), injury pattern was not included in the model. Admission to the LRMC ICU, shock index (>1), and ISS (≥10) were significantly associated with noncompliance (p<0.001), while age at injury and time period were significantly associated with compliance (p=0.032 and <0.001, respectively). Using stepwise selection, only ISS, LRMC ICU admission, and time period were retained in the final multivariate analysis (Table 4). As with the univariate analysis, ISS (≥10) and LRMC ICU admission were associated with noncompliance (p<0.001), while being injured in the time period of 2011–2014 was associated with antimicrobial prophylaxis compliance (p<0.001).
Table 4.
Examination of Predictive Factors Related to Antimicrobial Prophylaxis Compliance.a
| Predictive Factor | Univariate Odds Ratio (95% confidence interval) | P-value | Multivariate Odds Ratio (95% confidence interval) | P-value |
|---|---|---|---|---|
| Time period | <0.001 | <0.001 | ||
| 2009–2010 | Reference | Reference | ||
| 2010–2014 | 0.37 (0.33–0.42) | 0.40 (0.35–0.45) | ||
| Intensive care unit admission at LRMC | 1.92 (1.70–2.17) | <0.001 | 1.51 (1.27–1.81) | <0.001 |
| Age at injury | 0.99 (0.98–1.00) | 0.032 | – | |
| Blood product requirements 24 hours post-injury | 0.509 | |||
| 1–9 units | Reference | – | ||
| 10–20 units | 0.98 (0.75–1.27) | – | ||
| >20 units | 1.17 (0.88–1.55) | – | ||
| Shock indexb | <0.001 | |||
| 0–1 | Reference | – | ||
| 1–1.5 | 1.46 (1.19–1.79) | – | ||
| >1.5 | 1.77 (1.28–2.46) | – | ||
| Injury severity scorec | <0.001 | <0.001 | ||
| 0–9 | Reference | Reference | ||
| 10–15 | 1.87 (1.59–2.20) | 1.66 (1.38–1.98) | ||
| 16–25 | 1.71 (1.45–2.03) | 1.32 (1.08–1.62) | ||
| ≥26 | 2.40 (2.07–2.79) | 1.68 (1.36–2.08) |
LRMC = Landstuhl Regional Medical Center
Using injury severity as a predictor, statistical power is 0.547
Shock index is defined as heart rate/systolic blood pressure.
Injury severity score is an overall score of severity based on anatomical regional values.28
DISCUSSION
In the years leading up to the publication of the 2011 CPG, DoD combat-related antibiotic prophylaxis recommendations (e.g., 2008 CPG and 2010 JTS guidance) were contradictory. This discord was a reflection of not only disagreement in clinical practice between the JTS trauma team members, but also the controversy that existed in the civilian literature regarding the use of Gram-negative coverage in the setting of Type III open fractures.1,2,16,22–25 The primary goal of the 2011 CPG and follow-on internal 2012 JTS guidance was to standardize care and reduce variability in practice in the fast moving and austere environment which complicates combat trauma care.13,14 Overall, our analysis of antimicrobial usage patterns over a five-year period highlights an increasing trend toward compliance (in accordance with the 2011 published CPG) from 2009–2010 to 2013–2014. Notably, there was a marked reduction in the administration of expanded Gram-negative coverage and broad-spectrum antibiotics that coincided with the release of the CPG, which was published and disseminated downrange to MTFs, Landstuhl Regional Medical Center, and U.S. facilities caring for combat casualties. Furthermore, the participation of senior DoD trauma leaders in the development of the guidelines, along with infectious disease physicians, most likely led to improved acceptance of the recommendations.
Over the study period, compliance with the 2011 CPG improved in the period around June 2010 to June 2012, and these improvements were sustained throughout the study period. Additionally, and even though injury severity (which has been shown to be a negative predictor of CPG compliance) declined over the years, the multivariate analysis identified time period as a significant predictor of compliance (odds ratio: 0.40; 95% confidence interval: 0.35–0.45). Use of broad-spectrum antibiotics such as meropenem was very low, as was the use of vancomycin. These results provide further evidence of the effectiveness of the CPG and add to the medical literature supporting the use of standardized guidelines to enhance antibiotic stewardship efforts. Of note, a recent assessment of compliance with recommendations at trauma centers found that for every 10% increase in compliance, there was an associated 14% decrease in mortality risk.26 Furthermore, adherence to surgical antibiotic prophylaxis guidelines among trauma patients was associated with a lower proportion of surgical site infections, duration of antibiotic usage, and total hospitalization.27 Therefore, a continued focus on improving compliance with antibiotic stewardship goals and decreasing variability is justified.
Our study is limited to an extent by our methodology, which relies on retrospective review of prospective data abstracted from charts by trauma research nurses and populated into the DoDTR. Although we mitigated this issue in our last analysis by using AIS codes to improve injury characterization,17 there is still a possibility that a small number of subjects may have had their injuries misclassified. Methodology constraints notwithstanding, in this analysis, we applied more stringent assessments of adherence and still showed dramatic improvements in CPG compliance over the course of five years. In addition, we have further improved our methodology by delineating antibiotic regimens as those containing expanded Gram-negative coverage, which will allow us to take our research to the next logical step – studying infectious outcomes in those that did and did not receive expanded Gram-negative coverage.
Acknowledgments
We are indebted to the Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study team of clinical coordinators, microbiology technicians, data managers, clinical site managers, and administrative support personnel for their tireless hours to ensure the success of this project.
Funding Sources: Support for this work (IDCRP-024) was provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense program executed through the Uniformed Services University of the Health Sciences. This project has been funded by the National Institute of Allergy and Infectious Diseases, National Institute of Health, under Inter-Agency Agreement Y1-AI-5072, and the Department of the Navy under the Wounded, Ill, and Injured Program.
Footnotes
Guarantor: Col Bradley A. Lloyd
A portion of this data was presented at the 2015 Military Health System Research Symposium, 17–20 August 2015, Fort Lauderdale, FL.
Disclaimer: The views expressed are those of the authors do not necessarily reflect the official views of the Uniformed Services University of the Health Sciences, Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the National Institute of Health or the Department of Health and Human Services, Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of Defense, or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organization does not imply endorsement by the U.S. Government.
References
- 1.Hauser CJ, Adams CA, Jr, Eachempati SR, Council of the Surgical Infection Society Surgical Infection Society guideline: prophylactic antibiotic use in open fractures: an evidence-based guideline. Surg Infect. 2006;7(4):379–405. doi: 10.1089/sur.2006.7.379. [DOI] [PubMed] [Google Scholar]
- 2.Hoff WS, Bonadies JA, Cachecho R, Dorlac WC. East Practice Management Guidelines Work Group: update to practice management guidelines for prophylactic antibiotic use in open fractures. J Trauma. 2011;70(3):751–4. doi: 10.1097/TA.0b013e31820930e5. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg SR, Anand RJ, Como JJ, Dechert T, Dente C, Luchette FA, et al. Prophylactic antibiotic use in penetrating abdominal trauma: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5 suppl 4):S321–5. doi: 10.1097/TA.0b013e3182701902. [DOI] [PubMed] [Google Scholar]
- 4.Luchette FA, Bone LB, Born CT, DeLong WG, Jr, Hoff WS, Mullins D, et al. East Practice Management Guidelines Work Group. Practice Management Guidelines for Prophylactic Antibiotic Use in Open Fractures. Eastern Association for the Surgery of Trauma. 2000 Available at: https://www.east.org/content/documents/openfrac.pdf. Accessed August 5, 2015.
- 5.Luchette FA, Borzotta AP, Croce MA, O’Neill PA, Whittmann DH, Mullins CD, et al. Practice management guidelines for prophylactic antibiotic use in penetrating abdominal trauma: the EAST practice management guidelines work group. J Trauma Acute Care Surg. 2000;48(3):508–18. doi: 10.1097/00005373-200003000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Jaeger M, Maier D, Kern WV, Sudkamp NP. Antibiotics in trauma and orthopedic surgery – a primer of evidence-based recommendations. Injury. 2006;37(Suppl 2):S74–80. doi: 10.1016/j.injury.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Hadzikadic-Gusic L, Agarwal S. Current prophylactic perioperative antibiotic guidelines in trauma: a review of the literature and outcome data. Bosn J Basic Med Sci. 2009;9(Suppl 1):46–53. doi: 10.17305/bjbms.2009.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez L, Jung HS, Goulet JA, Cicalo A, Machado-Aranda DA, Napolitano LM. Evidence-based protocol for prophylactic antibiotics in open fractures: improved antibiotic stewardship with no increase in infection rates. J Trauma Acute Care Surg. 2014;77(3):400–8. doi: 10.1097/TA.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 9.Kirton OC, O’Neill PA, Kestner M, Tortella BJ. Perioperative antibiotic use in high-risk penetrating hollow viscus injury: a prospective randomized, double-blind, placebo-control trial of 24 hours versus 5 days. J Trauma Acute Care Surg. 2000;49(5):822–32. doi: 10.1097/00005373-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Obremskey W, Molina C, Collinge C, Nana A, Tornetta P, III, Sagi C, et al. Current practice in the management of open fractures among orthopaedic trauma surgeons. Part A: initial management. a survey of orthopaedic trauma surgeons. J Orthop Trauma. 2014;28(8):e198–202. doi: 10.1097/BOT.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 11.Miles BA, Potter JK, Ellis E., 3rd The efficacy of postoperative antibiotic regimens in the open treatment of mandibular fractures: a prospective randomized trial. J Oral Maxillofac Surg. 2006;64(4):576–82. doi: 10.1016/j.joms.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Hospenthal DR, Murray CK, Andersen RC, Blice JP, Calhoun JH, Cancio LC, et al. Guidelines for the prevention of infection after combat-related injuries. J Trauma Acute Care Surg. 2008;64(3):S211–20. doi: 10.1097/TA.0b013e318163c421. [DOI] [PubMed] [Google Scholar]
- 13.Hospenthal DR, Murray CK, Andersen RC, Bell RB, Calhoun JH, Cancio LC, et al. Guidelines for the prevention of infections associated with combat-related injuries: 2011 update: endorsed by the Infectious Diseases Society of America and the Surgical Infection Society. J Trauma Acute Care Surg. 2011;71(2 Suppl 2):S210–34. doi: 10.1097/TA.0b013e318227ac4b. [DOI] [PubMed] [Google Scholar]
- 14.Joint Theater Trauma System. Guidelines to prevent infection in combat-related injuries (Approved April 2, 2012) United States Army Institute of Surgical Research; Available at: http://www.usaisr.amedd.army.mil/cpgs/Infection_Control_2_Apr_12.pdf. Accessed August 18, 2015. [Google Scholar]
- 15.Hospenthal DR, Murray CK. Preface: Guidelines for the prevention of infections associated with combat-related injuries: 2011 update. J Trauma Acute Care Surg. 2011;71(2 Suppl 2):S197–201. doi: 10.1097/TA.0b013e318227ac23. [DOI] [PubMed] [Google Scholar]
- 16.Murray CK, Obremskey WT, Hsu JR, Andersen RC, Calhoun JH, Clasper JC, et al. Prevention of infections associated with combat-related extremity injuries. J Trauma Acute Care Surg. 2011;71(2 Suppl 2):S235–57. doi: 10.1097/TA.0b013e318227ac5f. [DOI] [PubMed] [Google Scholar]
- 17.Tribble DR, Lloyd B, Weintrob A, Ganesan A, Murray CK, Li P, et al. Antimicrobial prescribing practices following publication of guidelines for the prevention of infections associated with combat-related injuries. J Trauma Acute Care Surg. 2011;71(2 Suppl 2):S299–306. doi: 10.1097/TA.0b013e318227af64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lloyd BA, Weintrob AC, Hinkle MK, Fortuna GR, Murray CK, Bradley W, et al. Adherence to published antimicrobial prophylaxis guidelines for wounded service members in the ongoing conflicts in southwest Asia. J Mil Med. 2014;179(3):324–8. doi: 10.7205/MILMED-D-13-00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tribble DR, Conger NG, Fraser S, Gleeson TD, Wilkins K, Antonille T, et al. Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: trauma infectious disease outcome study. J Trauma Acute Care Surg. 2011;71(Suppl 1):S33–42. doi: 10.1097/TA.0b013e318221162e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eastridge BJ, Jenkins D, Flaherty S, Schiller H, Holcomb JB. Trauma system development in a theater of war: experiences from Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma Acute Care Surg. 2006;61(6):1366–73. doi: 10.1097/01.ta.0000245894.78941.90. [DOI] [PubMed] [Google Scholar]
- 21.Champion HR, Holcomb JB, Lawnick MM, Kelliher T, Spott MA, Galarneau MR, et al. Improved characterization of combat injury. J Trauma Acute Care Surg. 2010;68(5):1139–50. doi: 10.1097/TA.0b013e3181d86a0d. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher TE, Hutley E, Adcock CJ, Martin N, Wilson DR. Deployed antimicrobial stewardship: an audit of antimicrobial use at Role 3. J R Army Med Corps. 2013;159(3):237–9. doi: 10.1136/jramc-2013-000116. [DOI] [PubMed] [Google Scholar]
- 23.Lane JC, Mabvuure NT, Hindocha S, Khan W. Current concepts of prophylactic antibiotics in trauma: a review. Open Orthop J. 2012;6:511–7. doi: 10.2174/1874325001206010511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United Kingdom Ministry of Defence. Clinical Guidelines for Operations. Joint Service Publication 999. 2012 Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/79106/20121204-8-AVB-CGO_Online_2012.pdf. Accessed August 5, 2015.
- 25.Merens A, Rapp C, Delaune D, Danis J, Berger F, Michel R. Prevention of combat-related infections: Antimicrobial therapy in battlefield and barrier measures in French military medical treatment facilities. Travel Med Infect Dis. 2014;12(4):318–29. doi: 10.1016/j.tmaid.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Shafi S, Barnes SA, Rayan N, Kudyakov R, Foreman M, Cryer HG, et al. Compliance with recommended care at trauma centers: association with patient outcomes. J Am Coll Surg. 2014;219(2):189–98. doi: 10.1016/j.jamcollsurg.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Smith BP, Fox N, Fakhro A, LaChant M, Pathak AS, Ross SE, et al. “SCIP”ping antibiotic prophylaxis guidelines in trauma: The consequences of noncompliance. J Trauma Acute Care Surg. 2012;73(2):452–6. doi: 10.1097/TA.0b013e31825ff670. [DOI] [PubMed] [Google Scholar]
- 28.Linn S. The injury severity score–Importance and uses. Ann Epidemiol. 1995;5(6):440–6. doi: 10.1016/1047-2797(95)00059-3. [DOI] [PubMed] [Google Scholar]


