Abstract
Background and aims
Children living in the inner city are particularly vulnerable to asthma. While we know much about factors that affect near-term outcomes in inner-city children, there is little evidence to guide clinicians on what to expect in the coming years, especially in preschool children. The purpose of our study was to determine which clinical and environmental factors are predictive of poor long-term asthma control in preschool inner-city children.
Materials and methods
Baseline characteristics determined to be potential predictors of asthma severity were examined: demographics, asthma symptoms, medication use, healthcare utilization, early life medical history, family history, allergen exposure and allergic disease, and pollutant exposure. Bivariate and multivariate analyses were performed using logistic regression to examine the association of predictors of asthma severity with healthcare utilization at 2 years.
Results
Of the 150 children at baseline, the follow-up rate was 83% at 2 years; therefore, 124 children were included in final analyses. At baseline, the mean age was 4.4 years and participants were predominantly African-American (90%). Most of the children were atopic and 32.5% reported using inhaled corticosteroids. Nighttime awakening from asthma and a history of pneumonia were predictive of future poor control.
Conclusion
Preschool children with nighttime awakening from asthma and a history of pneumonia may deserve closer monitoring to prevent future asthma morbidity.
Keywords: asthma, inner city, preschool children
Introduction
Asthma is one of the most common chronic illnesses of childhood, affecting an estimated 6 million children in the United States alone (1). We know that children living in the inner city are particularly vulnerable to asthma, including higher asthma prevalence and poor disease outcomes, with worse disease severity, increased hospitalizations, and increased mortality (2–6). While we know much about factors that affect near-term outcomes in inner-city children, there is little evidence to guide clinicians on what to expect in the coming years, especially in preschool children. Parents of young children with asthma want to know if the disease will stabilize, improve, or worsen, and healthcare practitioners need to know which children will require more intensive follow-up and medical services. It would be helpful to identify clinical factors that could distinguish which children will develop or continue to have troublesome symptoms, especially as they prepare to enter school.
Cross-sectional studies have shown that certain factors are associated concurrently with asthma control. For example, a recent study (7) investigated children and adolescents aged 4–17 years and found that uncontrolled asthma was linked to self-reported asthma severity and to reported history of recent cold, flu or sinus infections, history of exacerbation, and caregiver unemployment. In another cross-sectional study of children aged 4–18 years, race/ethnicity, lower lung function, and immunoglobulin E (IgE) sensitization to cockroach were concurrently linked to asthma control. In one of the few studies looking exclusively at preschool age children (2–5 years), factors found to precede exacerbations by only 1 day included nighttime use of a short-acting bronchodilator (8). To date and to our knowledge, most studies have been cross-sectional or focused on very near-term outcomes; asthma morbidity predictors have varied from study to study; and none has examined long-term predictors of asthma severity in a group confined to preschool-aged children in the inner city.
Pediatricians commonly collect information from parents about the child’s early life history, current asthma health, medication use, environmental exposures, and family health history. The predictive value of such information for longer term prognosis has not been sufficiently studied. The purpose of our study was to determine which, if any, clinical factors are predictive of poor long-term asthma control.
Methods
Study Participants
The study population was composed of children who participated in the observational study: Baltimore Indoor Environment Study of Asthma in Kids (9–11). At enrollment, participants were between 2 and 6 years of age and resided in one of nine contiguous zip codes in Baltimore, MD, USA. The participants were identified from a sample of children with healthcare encounters within the previous 12 months at Johns Hopkins Community Physicians or Bayview Pediatrics, the medical centers that provide the majority of care to children living in these inner-city zip codes. Eligibility criteria for children included: (1) doctor-diagnosed asthma, (2) symptoms or use of asthma medications within the last 6 months, and (3) at least one healthcare encounter for asthma within the preceding 12 months (ICD-9 493.xx). Participants were recruited between September 2001 and December 2003, and written informed consent was obtained from parents or legal guardians, and the Johns Hopkins Medical Institutional Review Board approved the study protocol.
Baseline Data Collection
Baseline data were collected using an interviewer-administered survey with closed-ended questions. Caregivers reported on household demographics, indoor environmental exposures (including allergens and pollutants), family history (asthma, hay fever, and eczema), the child’s respiratory symptoms during the previous 2 weeks, and medication use (long-term controller [LTC] use and rescue medications use within the previous 2 weeks). The use of the following LTC medications was assessed: inhaled corticosteroids (ICSs), cromolyn and nedocromil, oral leukotriene modifiers, long-acting beta-agonists, and oral theophylline.
Asthma severity was categorized as intermittent, mild persistent, moderate persistent, and severe persistent based on reported daytime symptoms, nighttime symptoms, reliever medication use, and activity limitation over the prior 2 weeks, as recommended by the National Asthma Education and Prevention Program (NAEPP) guidelines (1).
Allergic sensitization status was assessed by skin prick testing (Multi-Test II, Lincoln Diagnostics, Decatur, IL, USA) to 14 common aeroallergens and 2 controls (histamine and glycerine) (Hollister-Stier Laboratories, Spokane, WA, USA and Greer Laboratories, Lenoir, NC, USA), as previously described (10,11). A positive skin test was defined as a net wheal diameter of at least 2 mm, and a child was considered atopic if he/she had at least one positive skin test in the panel of allergens tested. Subjects also underwent blood draws, with serum samples analyzed for total IgE (kU/l) by CAP-RAST (Pharmacia Diagnostics, Uppsala, Sweden).
Environmental assessments included an inspection of the entire dwelling, and dust and air sampling were conducted in the child’s bedroom. This location was chosen as the indoor monitoring site as it represents where the child spends a substantial portion of time while indoors. Household dust samples were collected from the child’s bedroom as previously described (11) and assayed for the allergens of cat (Fel d 1), dog (Can f 1), and cockroach (Bla g 1) using standard methods (12–16). The limits of detection of the assay were 50 ng/g for Fel d 1 and Can f 1, and 1 U/g for Bla g 1.
Air samples were collected following previously described procedures (11,17). Briefly, integrated air sampling of particulate matter (PM) was performed over 3 days using PM10 (PM with an aerodynamic size less than 10 μm) and PM2.5 (PM with an aerodynamic size less than 2.5 μm) and 4 l/min MSP™ impactors (St. Paul, MN, USA) loaded with 37 mm diameter, 2.0 μm pore size, PALL Teflo™ polytetrafluoroethylene (PTFE) membrane filters with polypropylene support rings (Pall Corporation, Ann Arbor, MI, USA). Samples were collected using battery operated pumps plugged into house electrical service to assure 72 h of operation. Nitrogen dioxide (NO2) samples were collected for the same period using passive Ogawa badges (11,18).
2-Year Follow-Up Data Collection
Participants were contacted after 2 years to complete an interviewer-administered survey with closed-ended questions. Asthma severity after 2 years of follow-up was the primary outcome of interest; as with baseline asthma severity, it was determined by parent/guardian report of the child’s symptoms over the previous 2 weeks. Categories of asthma severity were dichotomized as intermittent/mild persistent versus moderate/severe persistent. The secondary outcome of interest was acute healthcare utilization (HCU) at 2 years, defined as any unscheduled doctor’s visit, emergency department visit, or hospitalization for asthma in the previous 3 months and reported at the 2-year follow-up; responses were dichotomized as yes or no.
Statistical Analysis
We used descriptive statistics to characterize the patient sample, with proportions or means with standard deviations applied where appropriate. Student’s t-test and Fisher’s exact test were also used, where appropriate, to determine differences between subjects lost to follow-up and those remaining in the study.
Baseline characteristics that were determined to be potential predictors of future asthma morbidity were identified (Figure 1) and categorized as (1) demographics (age, race, gender, and caregiver education); (2) asthma symptoms (symptoms of wheeze and cough during the previous 2 weeks); (3) medication use (ICSs and short-acting beta-agonist); (4) healthcare utilization (HCU); (5) early-life medical history (birth weight and history of pneumonia, bronchitis, prematurity [born 3 weeks early], being breast fed, being on a ventilator, and antibiotic use in first year of life, or previous 12 months); (6) family history (mother and/or father with a history of atopy, hay fever, or asthma); (7) allergen exposure and allergic disease (symptoms of rhinitis, symptoms of eczema); (8) exposure and/or sensitization to common household allergens – mouse, cat, dog, cockroach); and (9) pollutant exposure (PM10, PM2.5, NO2, and total cigarettes smoked in the household).
Figure 1. Potential baseline predictors of asthma severity at 2 years.
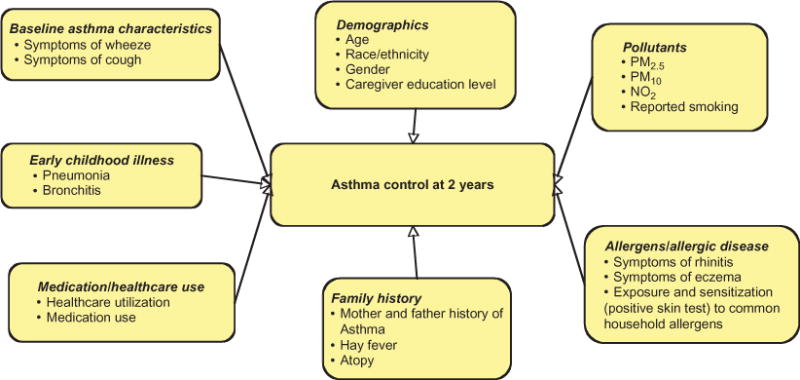
Note: PM2.5, PM with an aerodynamic size less than 2.5 μm; PM10, PM with an aerodynamic size less than 10 μm; NO2, nitrogen dioxide.
To address covariates with missing data, we used multivariate imputation by chained equations (19). Bivariate analysis was performed using logistic regression to examine the association of predictors shown in Figure 1 with asthma severity and healthcare utilization at 2 years. Variables with a p-value <.1 in bivariate analysis were included in the multivariable models. Results were reported as odds ratios (ORs) with 95% confidence intervals (CIs). All analyses were performed using StataSE 10.1 (Stata Corporation, College Station, TX, USA), and a two-tailed p-value < .05 was used to detect statistically significant differences.
Results
Study Population
Of the 150 children at baseline, 124 (83%) returned for the 2-year follow-up visit and were included in final analyses. The mean age of these 124 children at baseline was 4.4 years (range 2–6 years); participants were predominantly boys (61%) and African American (90%). Most of the children were atopic and 32.5% reported using ICSs (Table 1). The use of other LTC medication use was infrequent: cromolyn and nedocromil (3%), and leukotriene modifiers (7%). Nine children reported taking oral corticosteroids during the past 2 weeks. Median baseline pollutant concentrations were 33.5 mcg/m3 for PM2.5, 47.5 mcg/m3 for PM10, and 24 ppb for NO2.
Table 1.
Baseline participant characteristics.
| n = 124 | |
|---|---|
| Mean age (range) | 4.44 (2–6) |
| Gender | |
| Percentage of male (%) | 61.3 |
| Race (%) | |
| African-American | 90.3 |
| Other | 9.7 |
| Inhaled corticosteroid (ICS) use | 32.5 |
| Educational attainment of caregiver (%) | |
| 8th Grade/some high school | 42.7 |
| High school graduate | 42.0 |
| Some college or more | 15.3 |
| Recent healthcare utilization (HCU) | |
| (previous 3 months) (%) | |
| Urgent doctor visit | 17.7 |
| Emergency department visit | 25.8 |
| Hospitalization | 3.2 |
| Atopic (%) | 67.6 |
| Baseline asthma control (%) | |
| Mild intermittent/persistent | 55.6 |
| Moderate/severe persistent | 44.4 |
Characteristics between subjects included in the final analyses and those lost to follow-up were not significantly different, except male subjects (61 vs. 38%, p = .04) and those with less educated caregivers were more likely to attend the follow-up visit and therefore be included in the final analyses. There was no significant difference in the proportion of children with mild asthma or moderate/severe disease between the two visits. Almost half of the children were classified as having moderate/severe asthma at both baseline and the 2-year follow-up (44% and 43%, respectively). Despite minimal change in the proportion of children with mild or severe disease, 48 children (38.7%) showed a change in their asthma severity (improvement or worsening) at the follow-up visit. Specifically, 25 (20.2%) children had improved and 23 (18.5%) children had worsened with regard to disease severity at 2 years (Table 2).
Table 2.
Asthma severity at baseline and 2 years.
| 2-Year asthma severity
|
|||
|---|---|---|---|
| Intermittent/mild persistent | Moderate/severe persistent | Total | |
| Baseline asthma severity | |||
| Intermittent/mild persistent | 46 | 23 | 69 |
| Moderate/severe persistent | 25 | 30 | 55 |
| Total | 71 | 53 | 124 |
Baseline Predictors of Asthma Severity at 2-Year Follow-Up Visit
In bivariate analyses, children with recent HCU at base- line were three times more likely (OR = 3.06; 95% CI = 1.43–6.53) to have moderate/severe persistent asthma symptoms at 2-year follow-up, while children with a reported history of ever having pneumonia were four times more likely (OR = 4.44; 95% CI = 1.32–14.86) (Table 3). Reported antibiotic use in the 12 months prior to the baseline visit was borderline statistically associated (p = .052) with worse asthma severity. Similarly, baseline medication use (both short-acting beta-agonist and ICS use) and daytime and nighttime symptoms of wheeze predicted increased asthma severity at the 2-year follow-up. Specifically, ICS use (OR = 2.45; 95% CI = 1.14–5.31), beta-agonist use (OR = 1.10, 95% CI = 1.02–1.18), wheeze without a cold (OR = 2.52; 95% CI = 1.21–5.26), and nighttime awakening due to wheeze (OR = 3.18; 95% CI = 1.29–7.57) were associated with having moderate/persistent asthma symptoms at follow-up. Demographic factors, other measured early life exposures, markers of allergy/allergic disease, family history, and in-home allergen and pollutant exposures including PM and NO2 were not predictive of worse asthma symptoms at 2 years.
Table 3.
Baseline predictors of asthma symptoms at 2-year follow-up.
| Asthma symptoms (moderate/severe vs. intermittent/mild symptoms) Unadjusted | Asthma symptoms (moderate/severe vs. intermittent/mild symptoms) Adjusteda | |
|---|---|---|
| Baseline severity | 1.34 (0.99–1.81) | 1.09 (0.71–1.66) |
| Acute healthcare visit | 3.06 (1.43–6.53) | 1.72 (0.65–4.57) |
| Inhaled corticosteroids (ICSs) |
2.45 (1.14–5.31) | 1.39 (0.52–3.72) |
| Beta-agonist use | 1.10 (1.02–1.18) | 0.99 (0.88–1.10) |
| Wheeze without cold | 2.52 (1.21–5.26) | 1.32 (0.53–3.29) |
| Woken with wheeze | 3.18 (1.29–7.57) | 2.69 (1.00–7.21) |
| Pneumonia | 4.44 (1.32–14.86) | 5.39 (1.43–20.28) |
| Antibiotic use | 2.05 (0.99–4.25) | 1.38 (0.59–3.20) |
| Rhinitis | 1.97 (0.96–4.06) | 1.31 (0.54–3.20) |
| Mother/father hay fever | 1.94 (0.92–4.09) | 1.37 (0.56–3.35) |
Note: Bold values are those statistically significant by P < 0.05.
Adjusted for all variables shown in table (these include all variables associated with asthma symptoms in bivariate analyses with p < .1).
In the multivariable models, after adjusting for factors associated with higher asthma severity in bivariate analyses, a reported history of pneumonia (OR = 4.91; 95% CI = 1.14–21.15) and nocturnal wheeze (OR = 2.65; 95% CI = 1.02–6.93) were independently associated with risk of having moderate/severe persistent asthma symptoms at 2 years. Medication use and baseline healthcare utilization were no longer significant predictors of asthma symptoms.
Baseline Predictors of Healthcare Utilization at the 2-Year Follow-Up Visit
Children with acute healthcare visits and children who reported wheeze without a cold at baseline were almost three times as likely to require healthcare utilization 2 years later. Similarly, those with markers of allergic disease, including a history of eczema (OR = 2.46; 95% CI = 1.07–5.66), rhinitis symptoms (OR = 3.35; 95% CI = 1.45–7.76), and a family history of mother with hay fever (OR = 2.91; 95% CI = 1.27–6.69) were more likely to report healthcare utilization at 2 years. Baseline demographic factors, history of pneumonia or bronchitis, and in-home allergen and pollutant exposures were not predictive of HCU at 2 years.
After adjusting for factors associated with healthcare utilization in bivariate analyses, all tested factors continued to show a trend toward predicting healthcare utilization at 2 years, though none met statistical significance (Table 4).
Table 4.
Baseline predictors of healthcare utilization (HCU) at 2-year follow-up.
| HCU
|
HCU
|
|
|---|---|---|
| unadjusted | adjusteda | |
| Acute healthcare visit | 2.84 (1.25–6.46) | 1.83 (0.70–4.77) |
| Inhaled corticosteroids (ICSs) | 2.19 (0.96–5.00) | 1.46 (0.53–4.06) |
| Wheeze without cold | 2.84 (1.25–6.44) | 1.53 (0.56–4.21) |
| Woken with wheeze | 1.60 (0.68–3.79) | 1.14 (0.43–3.05) |
| Rhinitis | 3.35 (1.45–7.76) | 1.64 (0.61–4.44) |
| Mother hay fever | 2.91 (1.27–6.69) | 2.39 (0.91–6.26) |
| Atopic | 2.31 (0.88–6.01) | 2.28 (0.77–6.79) |
| Eczema ever | 2.46 (1.07–5.66) | 2.22 (0.85–5.83) |
| Caregiver education | 1.63 (0.93–2.84) | 1.06 (0.54–2.09) |
Note: Bold values are those statistically significant by P < 0.05.
Adjusted for all variables shown in table (these include all variables associated with HCU in bivariate analyses with p < .1).
Discussion
Although poor, inner-city children suffer disproportionately from asthma morbidity, few studies have investigated which clinical and environmental factors predict future asthma morbidity in inner-city preschool children. Several studies have shown that children with asthma have more lost days from school, activity restriction, and missed sleep which may lead to worse school performance (20). Furthermore, it has been shown that beginning school with asthma independently predicts low achievement (21). This has several implications for children with asthma in the inner city who suffer high levels of asthma severity and may have poor school performance (20). Thus understanding which preschool children will have worse asthma morbidity upon entering school age may help target those at higher risk of worse outcomes. To our knowledge, no study has examined long-term predictors of asthma severity in a group confined to preschool-aged children in the inner city. In this study, we determined that certain information readily available to clinicians is a strong predictor of worse asthma symptoms and healthcare utilization 2 years later in inner-city preschool children. These clinical factors, namely nighttime awakening from asthma and a history of pneumonia, were predictive of which children would have more severe disease 2 years later. Therefore, children with these clinical risk factors may deserve closer monitoring to prevent future asthma morbidity.
The NAEPP guidelines recommend assessing nocturnal awakening due to asthma symptoms when assessing a child’s history and selecting an asthma treatment plan (1). Prior studies have highlighted the importance of nocturnal asthma symptoms by demonstrating that nocturnal symptoms were associated with children’s decreased school attendance and performance, and parents’ lower work attendance, independent of daytime symptoms (22). In our study, children who awakened at night due to asthma symptoms had worse asthma symptoms 2 years later, independent of baseline daytime symptoms or overall asthma severity. The finding that the presence of nocturnal symptoms was more predictive of future disease severity than overall baseline asthma severity supports the theory that the presence of nocturnal symptoms is an important prognostic indicator. Not only does this symptom require short-term management changes but it also necessitates more intensive, longer term follow-up.
A clinician can easily obtain a child’s clinical history during a routine office visit, including whether he or she has had pneumonia. Our results indicate that, in addition to recent nocturnal symptoms, children with a previous occurrence of pneumonia had worse asthma symptoms 2 years after enrollment. Whether having had pneumonia previously predisposes one to worse asthma symptoms through an altered immune response or structural lung changes, or whether those at risk for worse asthma are more likely to develop pneumonia or a lower respiratory illness remains unclear. However, the importance of early life infection has been previously implicated as a risk factor for persistent wheeze in children and adults (23–25). Based on our results, early life pneumonia is an important clinical factor associated with children developing wheeze, and with the presence of worse symptom severity in those with already established asthma. The above findings show that nocturnal symptoms and previous pneumonia are predictive of worse asthma symptoms, regardless of baseline atopic status and environmental exposures.
Numerous studies have suggested that markers of allergic disease and environmental exposures are associated with concurrent worse asthma symptoms and may be predictive of future asthma symptoms in children (26–29). Specifically, the Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study revealed that patients with three or more allergic triggers were two times more likely to have a future exacerbation (30,31). The Childhood Asthma Management Program retrospectively compared children with prior hospitalization with those without prior hospitalization over 12 months and noted that markers of atopy, such as greater number of positive allergy skin tests, higher serum IgE level, and greater peripheral blood eosinophilia, were strongly associated with hospitalization in children with mild-to-moderate asthma (32). In our study, the presence of rhinitis, along with family history of atopic disease, tended to be associated with worse asthma symptoms at 2 years. Similarly, children with atopy, allergic symptoms including rhinitis and eczema, and a history of mother with hay fever showed an overall trend toward increased HCU at 2 years, after adjusting for other factors, including baseline healthcare utilization. Although allergic markers did not have a statistically significant association with worse outcomes after adjustment for other factors, the overall trends support prior longitudinal studies demonstrating that markers of allergic disease tend to be predictive of future asthma symptoms in children (26–29,33). This information may help identify children at risk, and therefore studies investigating the effect of early treatment of allergies on the natural history of allergies and asthma are warranted.
We have previously demonstrated that inner-city children are exposed to high levels of indoor pollutants and allergens (11,17,34). We have also shown that increasing concentrations of PM and NO2 measured over 72 h at 3-month intervals, as well as exposure to high levels of mouse allergen in mouse-sensitized individuals, were associated with increased asthma symptoms at the time of higher pollutant concentrations in the home (9,11,17,35). Similarly, previous studies have demonstrated allergen exposure such as mouse and cockroach are independent risk factors for increased asthma morbidity in inner-city children (36). Gruchalla et al. (37) showed that school age children, aged 5–11 years, in the inner city with exposure and sensitization to allergens – in particular to cockroaches – had worse 2-year asthma morbidity in the Inner City Asthma Study. Similarly, Illi et al. showed that sensitization and exposure to perennial allergens in early life predisposed children to loss of lung function and asthma at school age (33). The current results, however, show that baseline pollutant and allergen concentrations were not predictive of future asthma severity or HCU at 2 years in inner-city preschool children. Pollutant levels measured over a 72-h period or allergen exposure measured at a single time point may not be an accurate reflection of chronic exposure over a 2-year period, and measuring exposure concentrations repeatedly over longer periods may be warranted to assess long-term risk of exposure. Furthermore, given our smaller sample size, we were limited in power for subgroup analysis to investigate whether sensitized individuals exposed to a particular allergen may be more predisposed to poor future asthma morbidity.
Our comprehensive baseline evaluation, including baseline measures of asthma severity, medication use, and allergen and pollutant environmental exposures, allowed for a thorough evaluation of multiple factors which may contribute to disease progression. All of the data regarding symptoms, medication use, and HCU were collected by caregiver report. Therefore, reported LTC use may differ from verified use, and medication compliance was not assessed. In addition, a reported history of pneumonia and early life infections may be particularly subject to recall bias; however, this recall bias is likely minimized by the young age of the children at enrollment and the prospective design of the study where parents reported history of pneumonia at baseline without knowing if their children would continue to develop significant asthma morbidity at 2 years of follow-up. Objective measures of airway inflammation (i.e., exhaled nitric oxide) and lung were not available; therefore, we were unable to assess their importance or additional contribution to predicting future asthma severity and HCU. In addition, culture data were not available, therefore we were unable to distinguish whether the association of pneumonia with increased disease severity was due to a particular organism of infection. Lastly, the demographic homogeneity of our poor, mostly African-American, inner-city population limits generalizability, but is in fact a strength of our study, as we are able to identify baseline clinical characteristics that are particularly relevant in a population at high risk of suffering significant asthma morbidity.
In summary, inner-city preschool children with asthma who report a history of pneumonia and nighttime symptoms of wheeze have worse asthma symptoms 2 years later. Findings from this study may help to identify children who will continue to contribute to the high asthma burden that exists among school-aged minority children with asthma and those residing in the inner city. These findings suggest that by using information readily available to them, clinicians may identify preschool age inner-city children who may be at risk for high asthma morbidity in the future and who may require closer monitoring and asthma management.
Acknowledgments
The study was supported by grants from the US Environmental Protection Agency (R82672401), NIEHS (ES09606), and NHLBI (HL04266, HL076322, and HL67850). None of the authors has any financial, consulting, or personal relationship with other people or organizations that could have influenced the work described in this article.
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.National Asthma Education and Prevention Program. NIH Publication No. 08–4051. National Heart Lung and Blood Institute; 2007. Expert Panel Report III: Guidelines for the Diagnosis and Management of Asthma. [Google Scholar]
- 2.Stevenson LA, Gergen PJ, Hoover DR, Rosenstreich D, Mannino DM, Matte TD. Sociodemographic correlates of indoor allergen sensitivity among United States children. J Allergy Clin Immunol. 2001;108(5):747–752. doi: 10.1067/mai.2001.119410. [DOI] [PubMed] [Google Scholar]
- 3.Togias A, Fenton MJ, Gergen PJ, Rotrosen D, Fauci AS. Asthma in the inner city: the perspective of the National Institute of Allergy and Infectious Diseases. J Allergy Clin Immunoll. 2010;125(3):540–4. doi: 10.1016/j.jaci.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Aligne CA, Auinger P, Byrd RS, Weitzman M. Risk factors for pediatric asthma. Contributions of poverty, race, and urban residence. Am J Respir Crit Care Med. 2000;162(3 Pt 1):873–877. doi: 10.1164/ajrccm.162.3.9908085. [DOI] [PubMed] [Google Scholar]
- 5.Busse WW, Mitchell H. Addressing issues of asthma in inner-city children. J Allergy Clin Immunol. 2007;119(1):43–49. doi: 10.1016/j.jaci.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Carr W, Zeitel L, Weiss K. Variations in asthma hospitalizations and deaths in New York City. Am J Public Health. 1992;82(1):59–65. doi: 10.2105/ajph.82.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanford RH, Gilsenan AW, Ziemiecki R, Zhou X, Lincourt WR, Ortega H. Predictors of uncontrolled asthma in adult and pediatric patients: analysis of the Asthma Control Characteristics and Prevalence Survey Studies (ACCESS) J Asthma. 2010;47(3):257–262. doi: 10.3109/02770900903584019. [DOI] [PubMed] [Google Scholar]
- 8.Swern AS, Tozzi CA, Knorr B, Bisgaard H. Predicting an asthma exacerbation in children 2 to 5 years of age. Ann Allergy Asthma Immunol. 2008;101(6):626–30. doi: 10.1016/S1081-1206(10)60226-8. [DOI] [PubMed] [Google Scholar]
- 9.Matsui EC, Eggleston PA, Buckley TJ, Krishnan JA, Breysse PN, Rand CS, Diette GB. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. Ann Allergy Asthma Immunol. 2006;97(4):514–520. doi: 10.1016/S1081-1206(10)60943-X. [DOI] [PubMed] [Google Scholar]
- 10.Hansel NN, Eggleston PA, Krishnan JA, Curtin-Brosnan J, Rand CS, Patino CM, Diette GB. Asthma-related health status determinants of environmental control practices for inner-city preschool children. Ann Allergy Asthma Immunol. 2006;97(3):409–417. doi: 10.1016/S1081-1206(10)60809-5. [DOI] [PubMed] [Google Scholar]
- 11.Diette GB, Hansel NN, Buckley TJ, Curtin-Brosnan J, Eggleston PA, Matsui EC, McCormack MC, Williams DL, Breysse PN. Home indoor pollutant exposures among inner-city children with and without asthma. Environ Health Perspect. 2007;115(11):1665–1669. doi: 10.1289/ehp.10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman MD, Aalberse RC, Brown MJ, Platts-Mills TA. Monoclonal antibodies to the major feline allergen Fel d I. II. Single step affinity purification of Fel d I, N-terminal sequence analysis, and development of a sensitive two-site immunoassay to assess Fel d I exposure. J Immunol. 1988;140(3):812–818. [PubMed] [Google Scholar]
- 13.Ohman JL, Jr, Hagberg K, MacDonald MR, Jones RR, Jr, Paigen BJ, Kacergis JB. Distribution of airborne mouse allergen in a major mouse breeding facility. J Allergy Clin Immunol. 1994;94(5):810–817. doi: 10.1016/0091-6749(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 14.Pollart SM, Smith TF, Morris EC, Gelber LE, Platts-Mills TA, Chapman MD. Environmental exposure to cockroach allergens: analysis with monoclonal antibody-based enzyme immunoassays. J Allergy Clin Immunol. 1991;87(2):505–510. doi: 10.1016/0091-6749(91)90009-d. [DOI] [PubMed] [Google Scholar]
- 15.Wood RA, Eggleston PA, Lind P, Ingemann L, Schwartz B, Graveson S, Terry D, Wheeler B, Adkinson NF., Jr Antigenic analysis of household dust samples. Am Rev Respir Dis. 1988;137(2):358–363. doi: 10.1164/ajrccm/137.2.358. [DOI] [PubMed] [Google Scholar]
- 16.Wood RA, Eggleston PA, Rand C, Nixon WJ, Kanchanaraksa S. Cockroach allergen abatement with extermination and sodium hypochlorite cleaning in inner-city homes. Ann Allergy Asthma Immunol. 2001;87(1):60–64. doi: 10.1016/S1081-1206(10)62324-1. [DOI] [PubMed] [Google Scholar]
- 17.Hansel NN, Breysse PN, McCormack MC, Matsui EC, Curtin-Brosnan J, Williams DL, Moore JL, Cuhran JL, Diette GB. A longitudinal study of indoor nitrogen dioxide levels and respiratory symptoms in inner-city children with asthma. Environ Health Perspect. 2008;116(10):1428–1432. doi: 10.1289/ehp.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breysse PN, Buckley TJ, Williams D, Beck CM, Jo SJ, Merriman B, Kanchanaraksa S, Swartz LJ, Callahan KA, Butz AM, Rand CS, Diette GB, Krishnan JA, Moseley AM, Curtin-Brosnan J, Durkin NB, Eggleston PA. Indoor exposures to air pollutants and allergens in the homes of asthmatic children in inner-city Baltimore. Environ Res. 2005;98(2):167–176. doi: 10.1016/j.envres.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Moons KG, Donders RA, Stijnen T, Harrell FE., Jr Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59(10):1092–1101. doi: 10.1016/j.jclinepi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell DK, Adams SK, Murdock KK. Associations among risk factors, individual resources, and indices of school-related asthma morbidity in urban, school-aged children: a pilot study. J Sch Health. 2005;75(10):375–383. doi: 10.1111/j.1746-1561.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- 21.Liberty KA, Pattemore P, Reid J, Tarren-Sweeney M. Beginning school with asthma independently predicts low achievement in a prospective cohort of children. Chest. 2010;138(6):1349–1355. doi: 10.1378/chest.10-0543. [DOI] [PubMed] [Google Scholar]
- 22.Diette GB, Markson L, Skinner EA, Nguyen TT, Algatt-Bergstrom P, Wu AW. Nocturnal asthma in children affects school attendance, school performance, and parents’ work attendance. Arch Pediatr Adolesc Med. 2000;154(9):923–928. doi: 10.1001/archpedi.154.9.923. [DOI] [PubMed] [Google Scholar]
- 23.Rooney JC, Williams HE. The relationship between proved viral bronchiolitis and subsequent wheezing. J Pediatr. 1971;79(5):744–747. doi: 10.1016/s0022-3476(71)80385-2. [DOI] [PubMed] [Google Scholar]
- 24.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354(9178):541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 25.Galobardes B, McCarron P, Jeffreys M, Davey SG. Association between early life history of respiratory disease and morbidity and mortality in adulthood. Thorax. 2008;63(5):423–429. doi: 10.1136/thx.2007.086744. [DOI] [PubMed] [Google Scholar]
- 26.Bahceciler NN, Barlan IB, Nuhoglu Y, Basaran MM. Risk factors for the persistence of respiratory symptoms in childhood asthma. Ann Allergy Asthma Immunol. 2001;86(4):449–455. doi: 10.1016/S1081-1206(10)62494-5. [DOI] [PubMed] [Google Scholar]
- 27.Clough JB, Keeping KA, Edwards LC, Freeman WM, Warner JA, Warner JO. Can we predict which wheezy infants will continue to wheeze? Am J Respir Crit Care Med. 1999;160(5 Pt 1):1473–1480. doi: 10.1164/ajrccm.160.5.9807019. [DOI] [PubMed] [Google Scholar]
- 28.Limb SL, Brown KC, Wood RA, Wise RA, Eggleston PA, Tonascia J, Hamilton RG, Adkinson NF., Jr Adult asthma severity in individuals with a history of childhood asthma. J Allergy Clin Immunol. 2005;115(1):61–66. doi: 10.1016/j.jaci.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 29.Matricardi PM, Illi S, Gruber C, Keil T, Nickel R, Wahn U, Lau S. Wheezing in childhood: incidence, longitudinal patterns and factors predicting persistence. Eur Respir J. 2008;32(3):585–592. doi: 10.1183/09031936.00066307. [DOI] [PubMed] [Google Scholar]
- 30.Miller MK, Lee JH, Miller DP, Wenzel SE. Recent asthma exacerbations: a key predictor of future exacerbations. Respir Med. 2007;101(3):481–489. doi: 10.1016/j.rmed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Haselkorn T, Zeiger RS, Chipps BE, Mink DR, Szefler SJ, Simons FE, Massanari M, Fish JE. Recent asthma exacerbations predict future exacerbations in children with severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2009;124(5):921–927. doi: 10.1016/j.jaci.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Bacharier LB, Dawson C, Bloomberg GR, Bender B, Wilson L, Strunk RC. Hospitalization for asthma: atopic, pulmonary function, and psychological correlates among participants in the Childhood Asthma Management Program. Pediatrics. 2003;112(2):e85–e92. doi: 10.1542/peds.112.2.e85. [DOI] [PubMed] [Google Scholar]
- 33.Illi S, von Mutius E, Lau S, Niggemann B, Grueber C, Wahn U, Multicentre Allergy Study (MAS) group Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368(9542):1154. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 34.McCormack MC, Breysse PN, Hansel NN, Matsui EC, Tonorezos ES, Curtin-Brosnan J, Williams DL, Buckley TJ, Eggleston PA, Diette GB. Common household activities are associated with elevated particulate matter concentrations in bedrooms of inner-city Baltimore pre-school children. Environ Res. 2008;106(2):148–155. doi: 10.1016/j.envres.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCormack MC, Breysse PN, Matsui EC, Hansel NN, Williams D, Curtin-Brosnan J, Eggleston P, Diette GB, Center for Childhood Asthma in the Urban Environment In-home particle concentrations and childhood asthma morbidity. Environ Health Perspect. 2009;117(2):294–298. doi: 10.1289/ehp.11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, Malveaux F. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336(19):1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 37.Gruchalla RS, Pongracic J, Plaut M, Evans R, III, Visness CM, Walter M, Crain EF, Kattan M, Morgan WJ, Steinbach S, Stout J, Malindzak G, Smartt E, Mitchell H. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115(3):478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]


