Abstract
Background
In Ohio the infant mortality rate is above the national average and the black infant mortality rate is more than twice the white infant mortality rate. Having a short interpregnancy interval has been shown to correlate with preterm birth and low birth weight, but the effect of short interpregnancy interval on infant mortality is less well established.
Objective
To quantify the population impact of interpregnancy interval on the risk of infant mortality.
Study Design
This was a statewide population-based retrospective cohort study of all births (n=1,131,070) and infant mortalities (n=8,152) using linked Ohio birth and infant death records from 1/2007 through 9/2014. For this study we analyzed 5 interpregnancy interval categories: 0 to < 6 months, 6 to < 12 months, 12 to < 24 months, 24 to < 60 months, and ≥ 60 months. The primary outcome for this study was infant mortality. During the study period, 3701 infant mortalities were linked to a live birth certificate with an interpregnancy interval available. We calculated the frequency and relative risk (RR) of infant mortality for each interval compared to a referent interval of 12 to < 24 months. Stratified analyses by maternal race were also performed. Adjusted risks were estimated after accounting for statistically significant and biologically plausible confounding variables. Adjusted relative risk was utilized to calculate the attributable risk percent of short interpregnancy intervals on infant mortality.
Results
Short interpregnancy intervals were common in Ohio during the study period. 20.5% of all multiparous births followed an interval of < 12 months. The overall infant mortality rate during this time was 7.2 per 1000 live births (6.0 for white mothers and 13.1 for black mothers). Infant mortalities occurred more frequently for births that occurred following short intervals of 0 to < 6 months (9.2 per 1000) and 6 to < 12 months (7.1 per 1000) compared to 12 to < 24 months (5.6 per 1000), (p= <0.001 and <0.001). The highest risk for infant mortality followed interpregnancy intervals of 0 to < 6 months, adjRR 1.32 (95% CI 1.17–1.49) followed by interpregnancy intervals of 6 to < 12 months, adjRR 1.16 (95% CI 1.04–1.30). Analysis stratified by maternal race revealed similar findings. Attributable risk calculation showed that 24.2% of infant mortalities following intervals of 0 to < 6 months and 14.1% with intervals of 6 to < 12 months are attributable to the short interpregnancy interval. By avoiding short interpregnancy intervals of 12 months or less we estimate that in the state of Ohio 31 infant mortalities (20 white and 8 black) per year could have been prevented and the infant mortality rate could have been reduced from 7.2 to 7.0 during this time frame.
Conclusion
An interpregnancy interval of 12–60 months (1–5 years) between birth and conception of next pregnancy is associated with lowest risk of infant mortality. Public health initiatives and provider counseling to optimize birth spacing has the potential to significantly reduce infant mortality for both white and black mothers.
Keywords: interpregnancy interval, infant mortality
INTRODUCTION
Infant mortality (IM) is defined as a death of a baby in the first year of life and the infant mortality rate (IMR) is the number of infant deaths for every 1,000 live births (Eunice Kennedy Shriver National Institute of Child Health and Human Development, 2014; Centers for Disease Control and Prevention, 2016). IM is often used as an indicator of the overall health of a community or population because it is a reflection of a combination factors including maternal health, quality and access to medical care, and socioeconomic status (MacDorman, Mathews, Mohangoo, & Zeitlin, 2014). The United States’ IMR has improved in recent years but remains one of the highest amongst developed countries (MacDorman, Mathews, Mohangoo, & Zeitlin, 2014). Unfortunately in our home state of Ohio the IMR is higher than the national average. In 2014 there were 955 infant deaths with an IMR of 6.8 which is 13% higher than the national average of 6.0 (Ohio Department of Health, 2014). In the same year in our home city of Cincinnati the IMR was even higher at 8.8 with a 10-year average IMR of 10.3 (Ohio Department of Health, 2014). Even more striking is the disparity in the IMR by race with the Black IMR remaining more than twice the White IMR (14.3 vs 5.3 in 2014) (Ohio Department of Health, 2014).
The leading causes of IM are well documented and have been shown to be related to preterm birth, small for gestational age (SGA) births, sleep-related deaths, and birth defects (Ohio Collaborative to Prevent Infant Mortality, 2015). There are a multitude of risk factors that contribute to these leading causes of infant mortality, some like smoking may contribute to more than one of the leading causes. Smoking throughout pregnancy results in an increased risk of preterm birth (Moore, Blatt, Chen, Van Hook, & DeFranco, 2016) and fetal growth restriction (Blatt, Moore, Chen, Van Hook, & DeFranco, 2015). Additionally, Dietz et al estimate that 5.3–7.7% of preterm deliveries, 23.2–33.6% of sudden infant death syndrome (SIDS), and 5.0–7.3% of preterm-related deaths are directly attributable to prenatal smoking (Dietz, et al., 2010). Other major risk factors that contribute to IM include maternal health status and social determinants of health including racism, poverty, poor nutrition, and poor education (Ohio Collaborative to Prevent Infant Mortality, 2015). An additional risk factor that has consistently been correlated with many of the adverse perinatal outcomes associated with infant mortality is having a short interpregnancy interval (IPI) (World Health Organization, 2005). Specifically, a short IPI carries an increased risk of preterm birth, SGA births, and birth defects (Conde-Agudelo, Belizan, Norton, & Rosas-Bermudez, 2005; Chen, Jhangri, & Chandra, 2014). Similarly to smoking, having a short IPI likely has a complex multifactorial effect on IM.
The exact mechanism by which having a short IPI leads to adverse perinatal outcomes is not entirely understood, but it has been shown that short IPI increases the risk of uterine rupture in women attempting vaginal birth after cesarean, premature rupture of membranes, endometritis, third trimester bleeding, placenta previa, placental abruption, maternal death, and anemia (Conde-Agudelo, Rosas-Bermudez, & Kafury-Goeta, Effects of birth spacing on maternal health: a systematic review, 2007; Conde-Agudelo & Belizan, Maternal morbidity and mortality associated with interpregnancy interval: cross sectional study, 2000). One popular explanation in the literature that may lead to these adverse outcomes is the possibility of maternal nutritional depletion syndrome (Conde-Agudelo, Rosas-Bermudez, & Kafury-Goeta, Birth Spacing and Risk of Adverse Perinatal Outcomes A Meta-analysis, 2006). This hypothesis proposes that a closely spaced pregnancies worsen the maternal nutritional status because of the limited recovery time after the stress of the first pregnancy and subsequent post-partum breastfeeding. This impaired maternal nutritional status then increases the risk of adverse perinatal outcomes (Winkvist, Rasmussen, & Habicht, 1992).
A short IPI may subsequently lead to an increased risk of IM via the adverse outcomes that place infants at an increased risk of death or via another unidentified mechanism. Regardless, reduction and prevention of short IPIs is a potentially achievable intervention to reduce the IMR especially in high risk groups. Many of the studies that have looked at the effect of IPI on IM thus far have looked at different IPIs, failed to control for appropriate confounding variables, had small sample sizes, are now out-of-date, or were either done outside of the United States or in United States with a notably different patient population from that seen in the Midwest (Hussaini, Ritenour, & Coonrod, 2013; Conde-Agudelo, Belizan, Norton, & Rosas-Bermudez, 2005). This study examines the effects of IPI on IM using linked recent Ohio Birth and Infant Death Certificate records.
MATERIALS and METHODS
We performed a statewide population-based retrospective cohort study of all births (n=1,131,070) and infant deaths (n=8,152) using linked Ohio birth and infant death records from January 2007 through September 2014. The protocol for this study was approved by the human subjects Institutional Review Board of the Ohio Department of Health and a de-identified data set extracted from both live-birth and infant death certificates was provided for this analysis. This study was exempt from review by the Institutional Review Board at the University of Cincinnati, Cincinnati, Ohio.
IPI was defined as the time from the most recent prior birth to conception of the index birth. To calculate the IPI, we first determined the birth-to-birth interval in weeks. Then we subtracted the gestational age of the current index birth (in weeks) from the birth-to-birth interval. For this study we stratified IPI into 5 categories: 0 to < 6 months, 6 to < 12 months, 12 to < 24 months, 24 to < 60 months, and ≥ 60 months. The 12 to < 24 month category was chosen as the referent group based on data showing that an IPI of 12 to <24 months is associated with the lowest risk of adverse outcomes including small for gestational age newborns and preterm birth (Atreya, Muglia, Greenberg, & DeFranco; Lengyel, Ehrlich, Iams, Muglia, & DeFranco, 2016; DeFranco, Seske, & Greenberg, 2015) – known risk factors for IM (Conde-Agudelo, Belizan, Norton, & Rosas-Bermudez, 2005). The primary outcome for this study was IM. IM was additionally stratified by maternal race which is documented on the live-birth record (National Center for Health Statistics, 2016).
During the study period, there were 1,131,070 live births and 8,152 infant deaths (Figure 1). These births were comprised of 443,182 primiparous women with 3,171 infant deaths and 687,888 multiparous women with 4,981 infant deaths. All births were used to compare baseline socio-demographic, maternal pregnancy related characteristics, and birth related outcomes between IPI categories and to primiparous women. Additionally, all births were utilized to compare those pregnancies that did or did not subsequently result in an infant death. When looking specifically at the impact of IPI on IM, we excluded births to primiparous women (n=443,182 with 3,171 infant deaths) and births missing the required data to calculate an IPI (n=83,671 with 1,280 infant deaths). After these exclusions there were 604,217 live births to multiparous women with 3,701 linked infant deaths available to examine the effect of IPI on IM. The women with missing data required to calculate an IPI comprised 12.2% of the births in multiparous women. We calculated the frequency and relative risk (RR) of infant mortality for each IPI compared to a referent IPI category of 12 to < 24 months. Stratified analyses by maternal race were also performed. Adjusted relative risks were estimated after accounting for statistically significant and biologically plausible confounding variables. For overall IM the final model included adjustment for marital status, Medicaid use, tobacco use, maternal age, and race. For white and black IM the model included adjustment for marital status, Medicaid use, tobacco use, and maternal age. Adjusted relative risk was utilized to calculate the attributable risk percent of IPI on infant mortality among births that occurred following each of the IPIs.
Figure 1.
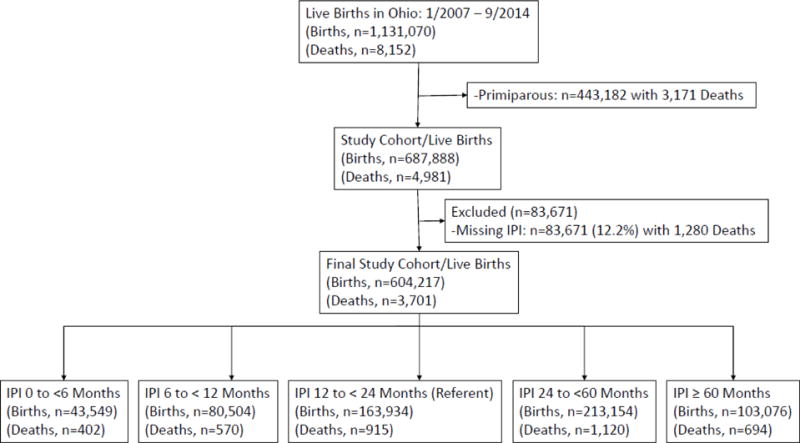
Flow diagram of the study population.
Births = lives births. Deaths = infant deaths/infant mortality.
Statistical analysis was performed using chi-square test for comparisons of dichotomous variables and ANOVA for continuous variables. Multivariate logistic regression analysis with backward selection of covariates was used to estimate adjusted relative risk. Significant differences were defined as comparisons with P value less than 0.05 and 95 percent confidence interval not inclusive of the null value 1.0. Statistical analyses were performed using STATA Release 12 Software (StataCorp, College Station, TX).
RESULTS
Short IPIs were common in Ohio during the study period with 20.5% of all multiparous births, 20.0% of births to multiparous white mothers, and 23.6% of births to multiparous black mothers following an IPI of < 12 months (Fig. 2). Although the percentage of births with a short IPI was high in our study, the percentage births with a short IPI among those that that resulted in an infant death was even higher at 34.3%.
Figure 2.
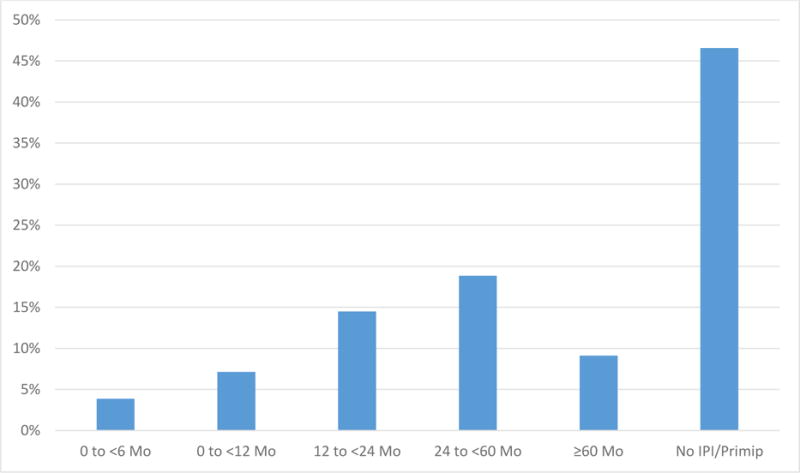
Frequency distribution of interpregnancy intervals
When comparing the socio-demographic characteristics of the women with very short (0 to <6months) and short (6 to <12months) IPIs to the referent group (12 to <24months), women with very short and short IPIs are less likely to be married and be a Non-Hispanic white female, but more likely to be younger, have less than a high school diploma, have Medicaid, be enrolled in WIC, use tobacco, and have less than 6 prenatal visits (Table 1).
Table 1.
Socio-demographic, maternal pregnancy, and birth related characteristics for multiparous births by interpregnancy interval and primiparous births.
| 0 to <6months (n=43,549) |
6 to <12months (n=80,504) |
12 to <24months (n=163,934) |
24 to <60months (n=213,154) |
≥60months (n=103,076) |
Primips | p Value | |
|---|---|---|---|---|---|---|---|
| Socio-Demographic Characteristics | |||||||
| Age in years Mean (SD) | 25.6 (5.2) | 27.2 (5.4) | 28.3 (5.3) | 28.9 (5.2) | 31.8 (4.9) | 25.0 (5.7) | p < 0.001 |
| Married | 47.7% | 63.3% | 70.2% | 64.2% | 53.5% | 49.6% | p < 0.001 |
| ≤ High School Education | 27.9% | 21.1% | 15.7% | 13.7% | 12.4% | 15.7% | p < 0.001 |
| Medicaid | 56.6% | 41.7% | 34.1% | 38.3% | 43.1% | 36.7% | p < 0.001 |
| WIC | 58.0% | 42.3% | 34.8% | 38.8% | 45.4% | 43.3% | p < 0.001 |
| Tobacco Use | 29.6% | 22.1% | 19.2% | 23.9% | 31.1% | 22.9% | p < 0.001 |
| ≤ 5 Prenatal Visits | 19.0% | 13.5% | 9.8% | 8.0% | 7.6% | 7.3% | p < 0.001 |
| Non-hispanic White | 69.4% | 76.6% | 80.5% | 76.5% | 70.6% | 77.5% | p < 0.001 |
| Non-hispanic Black | 23.1% | 17.0% | 13.3% | 15.9% | 20.7% | 15.2% | p < 0.001 |
| Hispanic | 5.7% | 4.4% | 3.9% | 4.8% | 5.9% | 4.0% | p < 0.001 |
| Other | 1.5% | 1.8% | 2.1% | 2.6% | 2.6% | 3.1% | p < 0.001 |
| Maternal Pregnancy Characteristics | |||||||
| Parity (Median, IQR) | 2 (0, 4) | 1 (0, 1) | 1 (0, 1) | 1 (0, 1) | 1 (0, 1) | 0 (0, 0) | p < 0.001 |
| Prior preterm birth | 8.4% | 6.7% | 6.0% | 6.3% | 6.8% | 0.0% | p < 0.001 |
| Underweight (BMI < 18.5) | 3.7% | 4.2% | 4.3% | 3.9% | 3.1% | 5.1% | p < 0.001 |
| Obese (BMI > 30) | 29.6% | 24.8% | 22.8% | 26.1% | 31.4% | 20.4% | p < 0.001 |
| Pre-pregnancy BMI (SD) | 27.4 (6.9) | 26.6 (6.7) | 26.2 (6.6) | 26.8 (6.9) | 27.8 (7.3) | 25.8 (6.5) | p < 0.001 |
| Pre-gestational diabetes | 0.7% | 0.7% | 0.7% | 0.9% | 1.5% | 0.8% | p < 0.001 |
| Chronic Hypertension | 1.8% | 1.6% | 1.6% | 2.1% | 3.9% | 1.8% | p < 0.001 |
| Gestational weight gain in pounds (SD) | 27.6 (17.2) | 28.9 (16.8) | 30.1 (16.6) | 30.2 (17.7) | 30.4 (19.0) | 34.4 (17.6) | p < 0.001 |
| Gestational diabetes | 5.2% | 5.0% | 5.1% | 6.5% | 9.7% | 5.1% | p < 0.001 |
| Preeclampsia | 3.7% | 3.6% | 3.6% | 4.4% | 6.3% | 5.8% | p < 0.001 |
| Birth Characteristics | |||||||
| Cesarean Delivery | 27.1% | 27.8% | 28.9% | 31.6% | 34.2% | 30.2% | p < 0.001 |
| Male infant gender | 51.0% | 51.1% | 51.0% | 51.2% | 51.0% | 51.3% | p = 0.635 |
| Gestational age Mean (SD) | 38.4 (2.84) | 38.6 (2.58) | 38.6 (2.35) | 38.5 (2.38) | 38.3 (2.69) | 38.7 (2.75) | p < 0.001 |
| Birth Weight in grams, Mean (SD) | 3210 (606) | 3319 (574) | 3357 (560) | 3320 (574) | 3239 (625) | 3226 (605) | p < 0.001 |
| SGA Births <10% | 11.3% | 8.7% | 7.8% | 8.8% | 11.0% | 12.4% | p < 0.001 |
| LGA Births >90% | 8.9% | 11.4% | 12.4% | 11.6% | 10.6% | 7.8% | p < 0.001 |
| Preterm Birth | 14.7% | 11.6% | 10.1% | 11.0% | 14.0% | 11.8% | p < 0.001 |
Women with very short and short IPIs also tended to have more maternal comorbidities during pregnancy including obesity, higher pre-pregnancy BMI, and pre-gestational diabetes. When looking at chronic hypertension the very short IPI group had a higher rate of preexisting hypertension at 1.75% but the short IPI and referent group were similar at 1.58% and 1.60% respectively. In addition to maternal comorbidities the maternal complications of pregnancy were also more prevalent in the very short and short IPI groups. Woman with very short and short IPIs were more likely to have a prior preterm birth, but only women with a very short IPI have higher incidence of gestational diabetes and preeclampsia when compared to women in the referent group (Table 1). A rise in maternal comorbidities and pregnancy complications can also be seen in women with longer IPIs which is thought to be related to the more advanced maternal age in these groups.
Evaluation of the birth outcomes of infants born after very short and short IPIs reveals that these infants are more likely to be preterm, small for gestational age, and have smaller birthweight. Those same infants are less likely to be born by cesarean delivery and large for gestational age (Table 1).
Similar findings can be seen when comparing the socio-demographic characteristics of the women with births resulting in infant mortalities to those without. Women with births that subsequently had infant mortalities are less likely to be married, but more likely to be younger, have less than a high school diploma, have Medicaid, be enrolled in WIC, use tobacco, have less than 6 prenatal visits, and of Non-Hispanic black race/ethnicity (Table 2). Maternal pregnancy characteristics such as pregestational diabetes, chronic hypertension, and preeclampsia, but not gestational diabetes also increase the risk of IM. The birth characteristics that were found to increase the risk of IM in the newborn were cesarean delivery, small for gestational age birthweight, and preterm birth. Large for gestational age birthweight appears to lower the risk of IM.
Table 2.
Socio-demographic, maternal pregnancy, and birth related characteristics comparing pregnancies that had an infant mortality to pregnancies without an infant mortality.
| No Infant Mortality | Infant Mortality | IMR | P value | |
|---|---|---|---|---|
| Socio-Demographic Characteristics | ||||
|
| ||||
| Age in years Mean (SD) | 27.3 (5.9) | 26.4 (6.2) | – | p < 0.001 |
| Married | 57.3% | 41.3% | 5.2 | p < 0.001 |
| ≤ High School Education | 15.9% | 23.8% | 10.4 | p < 0.001 |
| Medicaid | 38.6% | 50.6% | 9.4 | p < 0.001 |
| WIC | 41.9% | 43.8% | 7.3 | p = 0.001 |
| Tobacco Use | 23.7% | 31.2% | 9.3 | p < 0.001 |
| ≤ 5 Prenatal Visits | 8.9% | 42.5% | 29.7 | p < 0.001 |
| Non-Hispanic White | 78.3% | 65.2% | 6.0 | p < 0.001 |
| Non-Hispanic Black | 16.8% | 30.8% | 13.1 | p < 0.001 |
| Hispanic | 4.6% | 4.3% | 6.7 | p = 0.227 |
| Primip | 39.1% | 38.1% | 7.0 | p = 0.706 |
|
| ||||
| Maternal Pregnancy Characteristics | ||||
|
| ||||
| Pre-gestational diabetes | 0.9% | 1.7% | 13.9 | p < 0.001 |
| Chronic Hypertension | 2.0% | 3.3% | 11.3 | p < 0.001 |
| Gestational diabetes | 5.9% | 4.7% | 5.6 | p < 0.001 |
| Preeclampsia | 5.8% | 6.4% | 7.8 | p = 0.025 |
|
| ||||
| Birth Characteristics | ||||
|
| ||||
| Cesarean Delivery | 30.8% | 36.7% | 8.6 | p < 0.001 |
| SGA Births <10% | 10.7% | 26.6% | 16.5 | p < 0.001 |
| LGA Births >90% | 9.9% | 3.4% | 2.3 | p < 0.001 |
| Preterm Birth | 12.1% | 66.1% | 33.5 | p < 0.001 |
IMR-Infant mortality rate; WIC-Woman, Infants, and Children; SGA-small for gestational age; LGA-large for gestational age. The IMR is displayed as number of infant deaths per 1000 live births among women with the corresponding characteristic.
The overall IMR during this time period was 7.2/1000 (6.0 for white mothers and 13.1 for black mothers). Infant mortality occurred more frequently for births that occurred following short IPIs of 0 to < 6 months (9.2 per 1000) and 6 to < 12 months (7.1 per 1000) compared to 12 to < 24 months (5.6 per 1000), (p= <0.001 and <0.001) (Fig. 3). The highest risk for infant mortality followed short IPI of 0 to < 6 months, adjRR 1.32 (95% CI 1.17–1.49) followed by IPI of 6 to < 12 months, adjRR 1.16 (95% CI 1.04–1.30) (Fig. 4). Further analysis stratified by maternal race revealed similar findings. For infants born to white mothers a short IPI of 0 to < 6 months had the highest risk of infant mortality, adjRR 1.30 (95% CI 1.11–1.52) followed by a short IPI of 6 to < 12 months, adjRR 1.20 (95% CI 1.06, 1.37). For infants born to black mothers, birth following a short IPI of 0 to < 6 months had the highest risk of infant mortality, adjRR 1.29 (95% CI 1.05, 1.59) but an IPI of 6 to < 12 months was not found to increase risk of infant mortality, adjRR 1.05 (95% CI 0.86, 1.28) (Fig. 4). After adjustment a long IPI of ≥ 60 months did not increase the risk of overall infant mortality, adjRR 0.98 (95% CI 0.88, 1.09) or infant mortality when stratified by race. We did perform a separate analysis limited to term births (≥ 37 weeks gestation) using the same model. When looking only at term births the risk of infant mortality following a short IPI of 0 to < 6 months remains significantly elevated, adjRR 1.30 (95% CI 1.08–1.57). For an IPI of 6 to < 12 months a trend towards an increased risk of IM can be seen, but is not significant, adjRR 1.10 (95% CI 0.93–1.29). Additionally, to consider the potential influence of multiple gestations on the relationship between IPI and IMR, we included adjustment for multiples in the regression models. After adjusting for multiple gestation, the risk for IM following short IPI of 0 to <6months, adjRR 1.32 (95% CI 1.17–1.49) and following an IPI of 6 to <12 months, adjRR 1.17 (95% CI 1.05–1.31) was virtually unchanged and thus multiples were not included in the final adjusted model.
Figure 3.
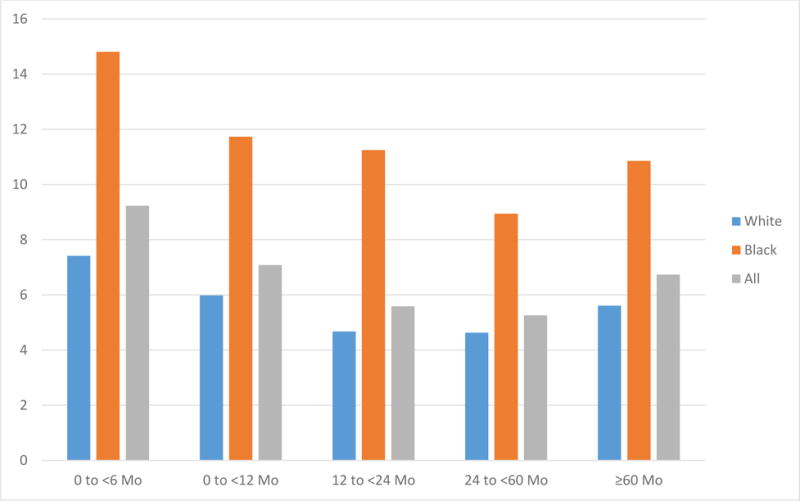
Infant Mortality Rate by interpregnancy intervals and race
Figure 4.
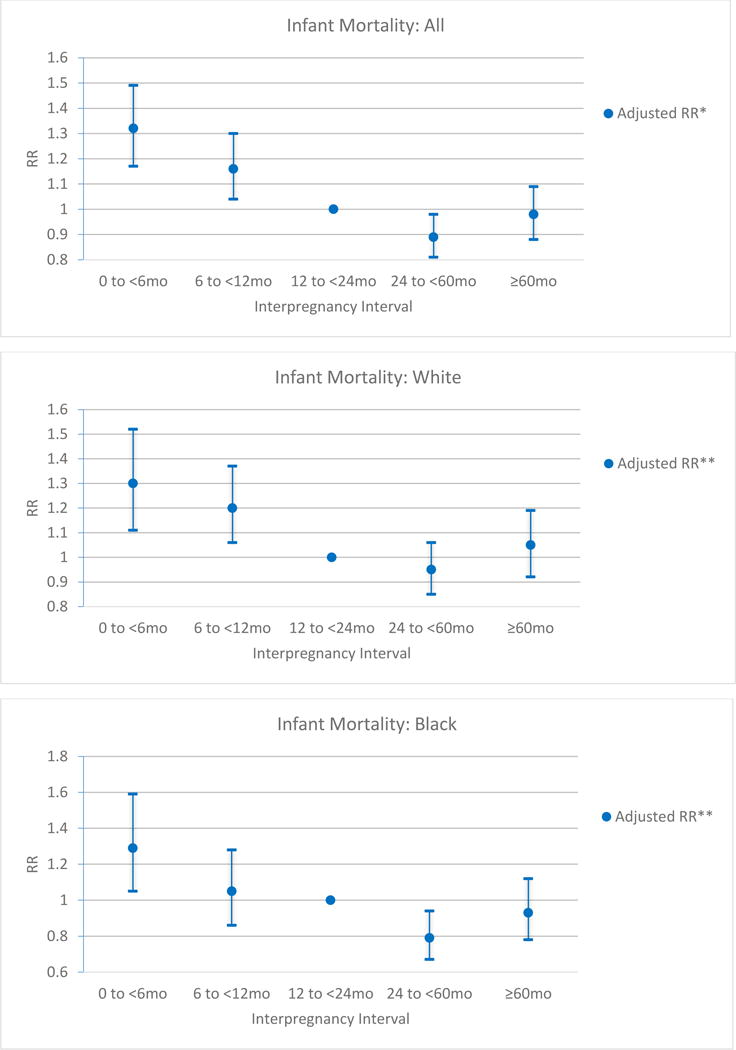
Adjusted RR for Infant Mortality among for all women, white women, and black women compared to referent IPI category of 12 to < 24 months.
*Adjusted for marital status, socioeconomic status (Medicaid), smoking, maternal age, and race.
**Adjusted for marital status, socioeconomic status (Medicaid), smoking, maternal age.
An attributable risk calculation was performed using the adjusted relative risks and revealed that overall 24.2% of infant mortalities with IPI of 0 to < 6 months and 14.1% of infant mortalities with IPI of 6 to < 12 months were attributable to short IPI. For white mothers 23.2% and 16.9% of infant mortalities were attributable to short IPI of 0 to < 6 months and 6 to < 12months, respectively. For black mothers 22.5% of infant mortalities were attributed to a short IPI of 0 to < 6 months. By avoiding short IPI less than 12 months we estimate that in the state of Ohio 31 infant mortalities (including 20 white infant deaths and 8 black infant deaths) per year could have been prevented and the IMR could have been decreased from 7.2 to 7.0 during this time frame.
DISCUSSION
In this population-based study spanning over 7 years and 9 months of births in the State of Ohio, we found that a short IPI of less than 12 months is associated with significantly increased risk of infant mortality even after adjustment for confounding socio-economic and maternal characteristics. This is especially true for very short IPIs of less than 6 months. Using attributable risk calculations we estimate that 240 infant deaths could have been prevented by eliminating short IPIs. This is an important finding that affects small changes such as provider practice patterns as well as larger changes such as implementation of state and federal programs that can help reduce short IPI as a means to combat infant mortality in Ohio and across the United States.
The likely underlying pathway by which short IPI leads to increased incidence of IM is by increasing the risk of preterm birth, SGA births, and birth defects (Conde-Agudelo, Belizan, Norton, & Rosas-Bermudez, 2005; Chen, Jhangri, & Chandra, 2014). All of these are known risk factors and leading causes of IM (Ohio Collaborative to Prevent Infant Mortality, 2015). The mechanisms by which short IPI lead to increased incidence of these complications of pregnancy are not entirely understood but have been proposed to be secondary to nutritional depletion and inadequate time to recover after the initial pregnancy and postpartum breast feeding (Conde-Agudelo, Rosas-Bermudez, & Kafury-Goeta, Birth Spacing and Risk of Adverse Perinatal Outcomes A Meta-analysis, 2006). Additionally, short IPIs may incur more social and psychological stress, which may contribute to an adverse pregnancy outcome. Rather than arbitrarily assigning a referent IPI for comparisons, in this study we identified the referent group of 12 to <24 months IPI by quantifying IMR frequency among a variety IPI categories and identifying the group with the lowest frequency of the outcome. The lowest risk IPI category of 12 to <24 months from birth to conception of the next pregnancy also has the lowest risk of complications known to be associated with infant mortality like preterm birth, low birth weight, and birth defects (Atreya, Muglia, Greenberg, & DeFranco; DeFranco, Seske, & Greenberg, 2015). (Schisterman, Cole, & Platt, 2009)
Other studies have examined the effect of IPI on preterm birth and infant mortality, but several limitations exist and differ from this study in a number of ways. One of the largest, most recent, comparable studies was published in 2013 by Hussaini et al. They performed a retrospective case control study using matched birth and death certificate data from Arizona that included 1,466 multiparous births that resulted in an IM and compared them to a random sample of 2000 multiparous births with surviving infants from the same time period. They published adjRR for IM from a variety of models but all showed that a short IPI (18 months or less) significantly increased the risk of infant mortality and the shortest IPI (<6 months) consistently predicted the highest infant mortality rates even after adjusting for confounders (Hussaini, Ritenour, & Coonrod, 2013). Ultimately the findings presented in our study are similar to the findings by Hussaini. However, we feel the Arizona based study has limited generalizability because the population in the study had a very low proportion of births to those at higher risk (only 4.5% Black women compared to 45% Hispanic and 37% White). This is distinctly different from our current study where Black women represent 17.6% of mothers and Hispanic women represent less than 3% of mothers. Interestingly in their study they found that IPI of 12 to 17 months also increased the risk of IM compared to the referent group (18–23 months). We did not find an increased IM risk with IPI of 12 to 17 months. In our analysis when stratifying the analysis of 12 to <24 month into 12 to <18 months and 18 to <24 months no increased risk of IM was seen in the 12 to <18 month group, adjRR 1.08 (95% CI 0.94 – 1.24). Therefore, we found that the optimal referent group was an IPI of 12 to <24months.
Another large study by Conde-Agudelo et al in 2005 examined the influence of IPI on perinatal outcomes in over 1.1 million pregnancies in Uruguay between 1985 and 2004. They reported that infants born to women following short IPI (<6 months) had increased risk of early neonatal death (aOR 1.49, 95% CI 1.06–1.96), fetal death (aOR 1.54, 95% CI 1.28–1.83), low birth weight (aOR 1.88, 95% CI 1.78–1.90), and preterm birth (aOR 1.80, 95% CI 1.71–1.89) when compared to infants with an IPI of 18–23 months (Conde-Agudelo, Belizan, Norton, & Rosas-Bermudez, 2005). They also found that IPIs of 6–11 months and > 60 months were associated with the above adverse perinatal outcomes. This study varies from our current study in that it did not examine the outcome of overall infant mortality. However they did confirm that short IPIs of less than 12 months were associated with many of the risk factors associated with IM, which is consistent with findings of our current study.
The only published report we identified that examined the effect of short IPIs independently for black and white women was published by Rawlings et al in 1995. They studied birth outcomes of 1992 white and black women with consecutive pregnancies and examined the rate of adverse perinatal outcomes in the second pregnancy including preterm delivery and SGA births. They found that short IPI was more frequent in black women. Additionally they found that for black women, an IPI of less than 9 months was associated with increases in preterm birth and SGA infants. For white women, an IPI of less than 3 months was associated with preterm birth or SGA infants (Rowlings, Rowlings, & Read, 1995). Again this study did not look at IM directly but rather risk factors for IM. Additionally, this study was limited by small sample size in comparison to our study. We feel it is this difference in same size that allowed us to have enough power to detect more subtle differences that exist in the slightly longer IPIs. Our study is the first we could identify that examines differences in the impact of short IPI on IM when stratified by maternal race.
Appropriate adjustment for confounding influences is important to optimize risk estimation in observational studies. A parsimonious model should balance thorough adjustment with avoidance of model over-fitting, or over-adjustment (Schisterman, Cole, & Platt, 2009). In this study we adjusted for a variety of statistically significant and biologically plausible confounding variables. The final model for overall IM included adjustment for marital status, Medicaid use, tobacco use, maternal age, and race. The model for IM for both black and white women was the same minus race. WIC and level of education were not included in the adjusted models due to collinearity with Medicaid use. Additionally, maternal complications of pregnancy such as preeclampsia or gestational diabetes were not considered confounders for inclusion in the adjusted models as these may be as least in part be caused by the short IPI and are along the pathway to infant mortality via preterm birth, altered birthweight, and other similar mechanisms. For this same reason we did not include birth related complications such as SGA, LGA, congenital birth defects, or preterm birth in adjusted models as over-adjustment by including variables on the pathway from exposure and outcome should be avoided as this may result in biased results (Schisterman, Cole, & Platt, 2009; Rothman, Greenland, & Lash, 2008). Birth defects were not included in the adjusted model as they are likely factors on the pathway from interpregnancy interval to infant mortality, and thus would not be statistically appropriate to consider as a confounder. However, even if considering congenital anomalies in the adjusted model, there was no significant change in the adjusted results, and interpretation of the findings were unchanged (data not shown). Additionally, if congenital anomalies were appropriate confounders to consider in the model, their underreporting in the birth certificate would not fully account for their influence. A separate analysis limited to term births (≥ 37 weeks gestation) was performed and the risk of infant mortality following a short IPI of 0 to < 6 months remained significantly elevated. For an IPI of 6 to < 12 months a trend towards an increased risk of IM can be seen but was not significant. This may be at least in part be a reflection of smaller sample size. This implies that prematurity contributes to IM in pregnancies following a short IPI, but cannot completely account for the increased risk of IM as term births with a short IPI are still at an increased risk for IM. Since multiple gestations are associated with many of the complications that increase the risk of IM in newborns such as preterm birth, low birth weight, and congenital birth defects, multiple gestations were considered as a potential confounder during adjusted analyses. The total number of multiples (twins and higher order) in our cohort of women was 42,191 (3.7%). Adjusted models including multiples as a covariate resulted in no change in risk estimates compared to without adjustment for multiples, and therefore through backward elimination multiples were not included in the final regression model for this study. We also performed a separate model that included the preexisting maternal conditions pregestational diabetes and chronic hypertension. However, due to minimal influence on the final adjusted risk of IPI on IM, both were also removed from the final model via backward elimination.
One of this study’s strengths is the large demographically diverse population-based sample of births and infant mortalities from a contemporary US population. The large sample size allowed for detection of more subtle differences that would not have been observed in smaller studies. The statistical methodology of this study, including proper covariate adjustment and calculation of population-based attributable risks, are additional study strengths.
This data utilized for this study was taken from the US vital statistics live-birth records, which have been shown to have good reliability and validity (Reichman & Hade, 2001; Dietz, et al., 2010). Despite this there are still several limitations to our study. These limitations include missing data on birth and death certificates, under-representation of maternal co-morbidities and congenital birth defects, and lack of knowledge about other pregnancies that did not receive a birth or death certificate. Although interpregnancy intervals following pregnancies that resulted in miscarriage or termination of pregnancy were not represented in this study, short periods of time following these early pregnancy losses are not associated with preterm birth or infant mortality in subsequent pregnancy. Therefore lack of this data should not substantially influence our study findings.
Our study has several important implications for women’s reproductive and newborn health. Pregnancy planning to optimize IPI is essential. Findings from our study suggest that an IPI of 1–2 years between the delivery of a previous child and conception of the next is associated with fewer cases of infant mortality, even after adjustment for other co-existent maternal risk factors. Nutritional counseling prior to conception may also help to reduce IM but, women and infants will benefit from the most from postpartum interventions that have been proven to reduce the IMR. Some of these important interventions include routine implementation of provider counselling about importance of pregnancy spacing and providing reliable contraception including long-acting reversible contraception during the post-partum period to reduce the number of unintended pregnancies with a short IPI. This study additionally builds on existing data to further the awareness and importance of pregnancy spacing amongst the general population and providers.
Table 3.
Risk of Infant Mortality by interpregnancy interval and race.
| 0 to <6months (n=43,549) |
6 to <12months (n=80,504) |
12 to <24months (n=163,934) |
24 to <60months (n=213,154) |
≥60months (n=103,076) |
|
|---|---|---|---|---|---|
| Overall Infant Mortality | |||||
| Unadjusted RR (95% CI) | 1.66 (1.48, 1.87) | 1.27 (1.14, 1.41) | Referent | 0.94 (0.86, 1.03) | 1.21 (1.09, 1.33) |
| Adjusted RR* | 1.32 (1.17, 1.49) | 1.16 (1.04, 1.30) | Referent | 0.89 (0.81, 0.98) | 0.98 (0.88, 1.09) |
| Non-Hispanic White Infant Mortality | |||||
| Unadjusted RR (95% CI) | 1.59 (1.37, 1.85) | 1.28 (1.13, 1.46) | Referent | 0.99 (0.89, 1.10) | 1.20 (1.06, 1.36) |
| Adjusted RR** | 1.30 (1.11, 1.52) | 1.20 (1.06, 1.37) | Referent | 0.95 (0.85, 1.06) | 1.05 (0.92, 1.19) |
| Non-Hispanic Black Infant Mortality | |||||
| Unadjusted RR (95% CI) | 1.32 (1.08, 1.62) | 1.04 (0.86, 1.27) | Referent | 0.79 (0.67, 0.94) | 0.96 (0.81, 1.15) |
| Adjusted RR** | 1.29 (1.05, 1.59) | 1.05 (0.86, 1.28) | Referent | 0.79 (0.67, 0.94) | 0.93 (0.78, 1.12) |
Adjusted for marital status, socioeconomic status (Medicaid), smoking, maternal age, and race.
Adjusted for marital status, socioeconomic status (Medicaid), smoking, maternal age.
Acknowledgments
This study includes data provided by the Ohio Department of Health which should not be considered an endorsement of this study or its conclusions.
FUNDING:
This work was supported by the Perinatal Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA, and March of Dimes Prematurity Research Center Ohio Collaborative, USA, Grant 22-FY14-470
Footnotes
Disclosure Statement: The authors report no conflicts of interest.
Presentation information: The abstract for this study was a poster presentation at the Central Association of Obstetricians and Gynecologists annual meeting October 26–29, 2016 in Las Vegas, Nevada, USA.
References
- Atreya MR, Muglia LJ, Greenberg JM, DeFranco EA. Racial Differences in the Influence of Interpregnancy Interval on Fetal Growth. Matern Child Health J. 2016 Jul 30; doi: 10.1007/s10995-016-2140-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Blatt K, Moore E, Chen A, Van Hook J, DeFranco EA. Association of reported trimester-specific smoking cessation with fetal growth restriction. Obstet Gynecol. 2016 Jun;125(6):1452–9. doi: 10.1097/AOG.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody DJ, Bracken MB. Short interpregnancy interval: a risk factor for low birthweight. Am J Perinatol. 1987 Jan;4(1):50–4. doi: 10.1055/s-2007-999736. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention [Internet] Atlanta [updated 2016 Jan 12; cited 2016 July 28] Infant Mortality. Available from: http://www.cdc.gov/reproductivehealth/MaternalInfantHealth/InfantMortality.htm.
- Chen I, Jhangri GS, Chandra S. Relationship between interpregnancy interval and congenital anomalies. Am J Obstet Gynecol. 2014 Jun;210(6):564.e1–8. doi: 10.1016/j.ajog.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Conde-Agudelo A, Belizan JM. Maternal morbidity and mortality associated with interpregnancy interval: cross sectional study. BMJ. 2000 Nov 18;321(7271):1255–9. doi: 10.1136/bmj.321.7271.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Agudelo A, Belizan JM, Norton MH, Rosas-Bermudez A. Effect of the interpregnancy interval on perinatal outcomes in Latin America. Obstet Gynecol. 2005 Aug;106(2):359–66. doi: 10.1097/01.AOG.0000171118.79529.a3. [DOI] [PubMed] [Google Scholar]
- Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Birth Spacing and risk of adverse perinatal outcomes a meta-analysis. JAMA. 2006 Apr 19;295(15):1809–1823. doi: 10.1001/jama.295.15.1809. [DOI] [PubMed] [Google Scholar]
- Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Effects of birth spacing on maternal health: a systematic review. Am J Obstet Gynecol. 2007 Apr;196(4):297–308. doi: 10.1016/j.ajog.2006.05.055. [DOI] [PubMed] [Google Scholar]
- DeFranco EA, Seske LM, Greenberg JM, Muglia LJ. Influence of interpregnancy interval on neonatal morbidity. Am J Obstet Gynecol. 2015 Mar;212(3):386.e1–9. doi: 10.1016/j.ajog.2014.11.017. [DOI] [PubMed] [Google Scholar]
- Dietz PM, Bombard JM, Hutchings YL, Gauthier JP, Gambatese MA, Ko JY, Martin JA, Callaghan WM. Validation of obstetric estimate of gestational age on US birth certificates. Am J Obstet Gynecol. 2014 Apr;210(4):335.e1–5. doi: 10.1016/j.ajog.2013.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz PM, England LJ, Shapiro-Mendoze CK, Tong VT, Farr SL, Callaghan WM. Infant morbidity and mortality attributable to prenatal smoking in the U.S. Am J Prev Med. 2010 Jul;39(f1):45–52. doi: 10.1016/j.amepre.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Erickson JD, Bjerkedal T. Interpregnancy interval. Association with birth weight, stillbirth, and neonatal death. J Epidemiol Community Health. 1978 Jun;32(2):124–30. doi: 10.1136/jech.32.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eunice Kennedy Shriver National Institute of Child Health and Human Development [Internet] Bethesda [updated 2014 Aug 27; cited 2016 July 28] Infant Mortality: Topic Information. Available from: https://www.nichd.nih.gov/health/topics/infant-mortality/topicinfo/Pages/default.aspx.
- Fedrick J, Adelstein P. Influence of pregnancy spacing on outcome of pregnancy. Br Med J. 1973 Dec 29;4(5895):753–6. doi: 10.1136/bmj.4.5895.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussaini KS, Ritenour D, Coonrod DV. Interpregnancy intervals and the risk for infant mortality: a case control study of Arizona infants 2003–2007. Matern Child Health J. 2013 May;17(4):646–53. doi: 10.1007/s10995-012-1041-8. [DOI] [PubMed] [Google Scholar]
- Lengyel CS, Ehrlich S, Iams JD, Muglia LJ, DeFranco EA. Effect of Modifiable Risk Factors on Preterm Birth: A Population Based-Cohort. Matern Child Health J. 2016 Aug 3; doi: 10.1007/s10995-016-2169-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- MacDorman MF, Mathews T, Mohangoo AD, Zeitlin J. International comparisons of infant mortality and related factors: United States and Europe, 2010. National Vital Statistics Reports. 2014 Sep 24;63(5) [PubMed] [Google Scholar]
- Moore E, Blatt K, Chen A, Van Hook J, DeFranco EA. Relationship of trimester-specific smoking patterns and risk of preterm birth. Am J Obstet Gynecol. 2016 Jul;215(1):109.e1–6. doi: 10.1016/j.ajog.2016.01.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics [Internet] Atlanta [updated 2003; cited 2016 July 28] Guide to Completing the Facility Worksheets for the Certificate of Live Birth and Report of Fetal Death (2003 Revision) Available from: http://www.cdc.gov/nchs/data/dvs/GuidetoCompleteFacilityWks.pdf.
- Ohio Collaborative to Prevent Infant Mortality [Internet] Columbus [updated 2014; cited 2016 July 28] Ohio Infant Mortality Reduction Plan. 2015–2020 Available from: https://www.odh.ohio.gov/~/media/ODH/ASSETS/Files/cfhs/Infant%20Mortality/collaborative/2015/Infant%20Mortality%20Reduction%20Plan%202015-20.pdf.
- Ohio Department of Health [Internet] Columbus [updated 2014; cited 2016 July 28] Ohio Infant Mortality Data: General Findings. Available from: http://www.odh.ohio.gov/~/media/ODH/ASSETS/Files/cfhs/Infant%20Mortality/2014%20Ohio%20Infant%20Mortality%20Report%20Final.pdf.
- Reichman NE, Hade EM. Validation of birth certificate data. A study of women in New Jersey’s HealthStart program. Ann Epidemiol. 2001 Apr;11(3):186–193. doi: 10.1016/s1047-2797(00)00209-x. [DOI] [PubMed] [Google Scholar]
- Rothman K, Greenland S, Lash T. Modern Epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- Rowlings JS, Rawlings VB, Read JA. Prevalence of low birth weight and preterm delivery in relation to the interval between pregnancies among white and black women. N Engl J Med. 1995 Jan 12;332(2):69–74. doi: 10.1056/NEJM199501123320201. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Cole SR, Platt RW. Overadjustment Bias and Unnecessary Adjustment in Epidemiologic Studies. Epidemiology. 2009 Jul;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GC, Pell JP, Dobbie R. Interpregnancy interval and risk of preterm birth and neonatal death: retrospective cohort study. BMJ. 2003 Aug 9;327(7410):313. doi: 10.1136/bmj.327.7410.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephansson O, Dickman PW, Cnattingius S. The influence of interpregnancy interval on the subsequent risk of stillbirth and early neonatal death. Obstet Gynecol. 2003 Jul;102(1):101–8. doi: 10.1016/s0029-7844(03)00366-1. [DOI] [PubMed] [Google Scholar]
- Winkvist A, Rasmussen KM, Habicht JP. A new definition of materanl depletion syndrome. American Journal of Public Health. 1992 May;82(5):691–4. doi: 10.2105/ajph.82.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization [Internet] Geneva, Switzerland [updated 2005; cited 2016 July 28] Report of a WHO Technical Consultation on Birth Spacing. Department of Reproductive Health and Research; Available from: http://www.who.int/maternal_child_adolescent/documents/birth_spacing.pdf. [Google Scholar]


