Abstract
Background: The prevalence of Alzheimer's disease (AD) is approximately 4.5 million people in the United States, and it is projected that by 2050, the prevalence could be as high as 16 million. As one step to minimize this health care burden, we investigated the practices of primary care physicians (PCPs) to determine barriers to and incentives in diagnosing AD and assessed the feasibility of utilizing a caregiver-based phone interview for dementia in the primary care setting.
Method: This feasibility study was conducted in 2 phases. Phase I included PCP focus groups conducted to identify barriers and incentives to diagnosis of Alzheimer's disease, knowledge of available screening instruments, and current diagnostic procedures and patterns. A survey of a random sample of PCPs was also conducted to reassess the data obtained via the focus groups. Phase II assessed the feasibility of implementing a brief, caregiver-based dementia screening tool in the primary care setting. A participant's score on the screening tool was compared with the PCP's clinical impression using the κ statistic.
Results: A general willingness exists among PCPs to improve the level of care for patients with AD; however, time constraints are a sizeable barrier in the PCP setting. The incorporation of a telephone-based (or caregiver-based) assessment is feasible. The agreement between the caregiver ratings and the PCPs' clinical impressions was κ = 0.64 (95% CI = 0.19 to 1.0).
Conclusion: The caregiver-based telephone assessment has indications of good agreement with the PCP's general assessment of dementia or possible AD. The limited number of patients with positive screens hinders generalization of this phase of the study, but the results confirm the potential value of caregiver-based assessment and the desirability of a larger study to address the scientific properties of a telephone-based screening instrument.
In the United States, Alzheimer's disease (AD) afflicts approximately 4 million individuals and is considered to be the leading cause of dementia.1 There are substantial barriers to both the early recognition and treatment of AD, and there is growing literature on the recognition of symptoms of AD in the primary care setting.2,3 However, routine screening for dementia in the primary care setting is not yet recommended by the United States Preventive Services Task Force.4 In addition, according to our clinical experience and studies,5 primary care physicians (PCPs) do not feel comfortable providing pharmacologic treatment for AD.
Although there is currently no cure for AD, early diagnosis has particular advantages. For example, current treatments can help slow the progression of AD,6 and an early diagnosis may improve quality of life over the course of the disease and provide earlier opportunities for family education, planning, and assistance.7 Another benefit of early screening efforts is that in some cases AD can be ruled out in lieu of another, treatable condition.6 In the United States, most people aged 65 years and older have access to medical care through a PCP. Therefore, there is a need for involvement of PCPs in the early detection, diagnosis, and treatment of people who exhibit early symptoms of AD. Moreover, it may be reasoned that the most valuable benefit of diagnosis by a patient's PCP could be the continued care by the PCP whom the patient has come to trust and the possible reduction in the need for costly referrals to a specialist.7 However, problems exist with diagnosing AD in a primary care setting.
Most PCPs see a large volume of patients on a given day. A potential barrier to adding AD screening to the routine visit is whether an additional per-patient time commitment would be possible and cost-effective in a busy primary care setting. Other barriers include the failure of PCPs to recognize and respond to symptoms of dementia, the perceived lack of need to determine a specific diagnosis, and the negative attitude toward the importance of treatment.5 One possible solution to these barriers is for PCPs to utilize a screening tool administered by a third party.
A caregiver telephone interview that utilizes subsets of the Haycox Dementia Behavior Scale8 and the Blessed Dementia Functional Subscale9 was conducted in a random community sample. This approach specifically addressed the lack of time that PCPs have to screen for dementia. The results of a preliminary study indicated high reliability between the caregiver telephone interviews and psychiatrists' assessments of dementia.10 We hypothesized that if it were feasible to conduct this assessment in a primary care setting, it would facilitate the diagnosis of early-stage AD.
The Alzheimer Research and Clinical Studies Program at the Medical University of South Carolina (MUSC), Charleston, and The Clinical Innovation Group, Charleston, S.C., conducted this study. The purpose of the study was to assess the general beliefs of PCPs and caregivers in the diagnosis of AD and to determine the feasibility of utilizing the caregiver telephone interview developed by Mintzer et al.10 in order to assist with identification of elderly patients who exhibit symptoms of AD and receive treatment in a primary care setting.
METHOD
This feasibility study consisted of 2 phases. Phase I sought to preliminarily measure the general treatment practices and beliefs of PCPs in the treatment and diagnosis of AD. A combination of focus groups and a random sample survey was used to describe the screening, treatment, and referral patterns of PCPs in the greater metropolitan area of Charleston, S.C. (includes Charleston, Dorchester, and Berkeley counties). Physicians participating in phase I were asked if they wanted to take part in phase II of the project. In phase II, participating PCPs were asked to provide access to patients and caregivers to determine the feasibility of implementing the caregiver-based dementia screening instrument10 in the primary care setting. The project was reviewed and approved by the MUSC's Institutional Review Board.
Phase I: Focus Groups and Survey
Focus groups were conducted with general and family practice and internal medicine physicians from the greater Charleston area. Most of the physicians were affiliated with the MUSC's primary care network. The objectives of these focus groups were to (1) identify barriers and incentives to diagnosing AD, (2) identify knowledge of available screening instruments, and (3) identify current diagnostic procedures and patterns. Each focus group was professionally moderated and recorded and lasted approximately 1 hour.
A survey instrument was created based on feedback from the PCP focus groups to summarize the screening, treatment, and referral patterns of PCPs in the greater Charleston area. The PCPs indicated during the focus groups that a survey instrument would be useful and should meet the following criteria: (1) the survey instrument should not exceed one side of a piece of paper; (2) the number of open-ended questions should be minimal and responses to questions provided in a multi-response format; (3) the instrument must be anonymous, but a separate sheet for enrollment in phase II was acceptable; and (4) PCPs should be compensated monetarily for their cooperation.
To define the sampling frame, a list of licensed physicians practicing in the Charleston metropolitan area was obtained from the South Carolina Department of Health and Human Services. Each PCP on the list was assigned a unique identification number, and the list was randomly sorted using a sampling algorithm available in S-Plus11 to allow for the simple random sample. The selected PCPs received a survey instrument; a self-addressed, stamped envelope; an optional fax cover sheet; and $5.
Phase II: Caregiver-Based Dementia Assessment
Research staff contacted physicians who noted on their survey forms that they would like to be involved in the project. If the PCP agreed to participate, research staff provided necessary forms and instruction to PCP clinic staff. The inclusion criterion for participants in phase II was that the patient had to be aged 65 years or older or know a patient aged 65 years or older receiving care from the participating clinic. The PCP's office staff was instructed to distribute a research information sheet, study instructions, and a copy of the dementia screening tool to patients that met the inclusion criteria. After poor accrual and indication from PCP staff that time constraints hindered recruitment, the recruitment method was revised, and research staff were sent to the participating clinics to distribute study information. Once a patient agreed to participate in the research project, a time for the administration of the screening instrument was scheduled. The screening instrument could be conducted via telephone or face-to-face at the clinic. PCPs were requested to complete a follow-up form for all participants who completed the screening tool. The follow-up form provided an opportunity for the PCP to agree or disagree with the results of the screening tool and to indicate if any additional tests were performed as a result of the screening tool.
The screening tool combines selected items from the Haycox Dementia Behavior Scale8 and the Blessed Dementia Functional Subscale9 and was used previously in a random community sample.10 The Haycox Dementia Behavior Scale measures cognitive ability on a scale of 0 to 48 and consists of 8 items. The Blessed Dementia Functional Subscale measures functional abilities using 12 items with a total summated score ranging from 0 to 21. In both scales, a higher score is indicative of greater impairment, and the 2 measures are moderately correlated (r = 0.61, p < .001).8,10 If the total score of the Haycox Dementia Behavior Scale exceeded 8 or if the Blessed Dementia Functional Subscale total score exceeded 4, a patient was identified as possibly having dementia. This is the same scoring algorithm used by Mintzer et al.,10 reportedly selected based on clinical judgment of those investigators.
RESULTS
Phase I
The 2 PCP focus groups consisted of 7 and 6 physicians. Each group of physicians was diverse in years of practice, sex, and race. Most PCPs stated that they conducted “informal screening” for dementia through the evaluation of the patient's hygiene, behavior, and cognitive functions observed during a clinic visit. When PCPs did diagnose AD, they tended to identify the symptoms for the later stages of dementia, usually with help from family members. With the exception of the Mini-Mental State Examination,12 most PCPs were not familiar with formal screening tools, but they were interested in learning more about newly developed screening tools. The focus groups identified the benefit of early diagnosis of AD in allowing the patient and family time to prepare for the patient's progressing dementia and incapacitation, including addressing legal issues, such as choosing power of attorney, and plans for increasing support of activities of daily living. PCPs specified multiple disincentives to diagnosing and treating patients with dementia. The lack of a cure was the primary disincentive identified. PCPs also noted the large amount of time required for diagnosing and communicating the AD diagnosis to the patient and the lack of adequate monetary compensation. Regarding participation in the second phase of the project, the consensus was that the PCPs and their staff felt overstressed with little time to devote to outside projects. Most of the PCPs were in agreement that they would participate in the project if the protocol did not interfere with the office routine and was not too time consuming.
Physician survey.
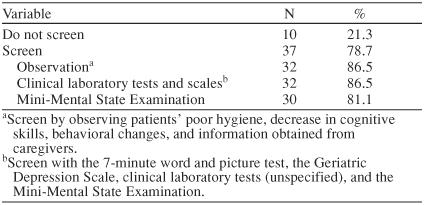
Table 1 illustrates physician specialty by county in the greater Charleston area. A total of 130 surveys were mailed. Thirty-nine mailings were returned due to an unknown address or no forwarding address. Of the remaining 91 mailings, 47 completed surveys were returned, yielding a response rate of 52%. Table 2 contains the demographics of the respondents. Table 3 presents the screening practices of the PCP respondents. The reported screening practices are consistent with the results from the focus groups. The Mini-Mental State Examination was used by 81% (N = 30) of the PCPs who screen for AD. Two PCPs used an assessment tool (laboratory test or scale) to screen but did not include the Mini-Mental State Examination.
Table 1.
Number of Physicians Practicing in Counties of the Greater Charleston, S.C., Area by Specialty
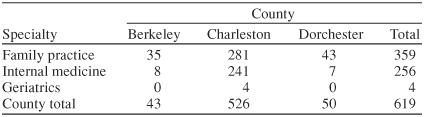
Table 2.
Demographics of 47 Primary Care Physician Survey Respondents
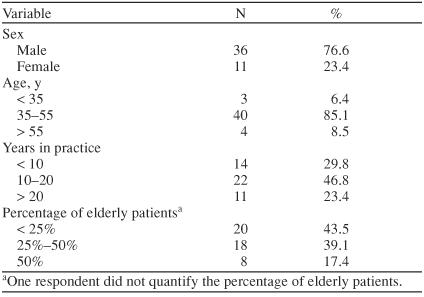
Table 3.
Alzheimer's Disease Screening Practices of 47 Primary Care Physician Survey Respondents
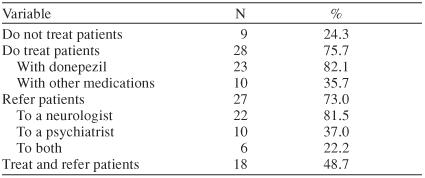
The types of treatment practices provided by the PCP respondents are shown in Table 4. Approximately 50% (N = 18) of responding PCPs indicated that if they suspected or diagnosed AD, they treated the condition with medications and referred the patient to a specialist. Done-pezil was the medication of choice among respondents. In addition, 7 (30%) of the 23 PCPs who prescribe done-pezil also prescribe vitamin and herbal supplements as an adjuvant therapy. The survey indicates that 82% (N = 22) of the PCPs who refer patients do so to a neurologist. About 22% (N = 6) of all referrals are made to both a neurologist and a psychiatrist.
Table 4.
Treatment Practices of 37 Primary Care Physician Respondents Who Screen for Alzheimer's Disease
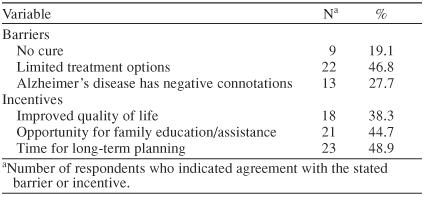
The barriers and incentives identified in the survey are presented in Table 5. Limited treatment options were the most frequent barrier. After the first 18 surveys were completed by respondents, an additional question was added to the survey to directly ask PCPs if the incentives to diagnosing AD outweighed the barriers. Eighty-six percent (25/29) of PCPs sampled after the question was added indicated that the incentives to diagnosing AD do outweigh the barriers. The survey also included an invitation for PCPs to learn about phase II of the study. Fourteen of 47 respondents (30%) stated that they would like to learn more, and 3 of 14 (21%) agreed to participate in phase II after learning what it would entail.
Table 5.
Barriers and Incentives to Diagnosing Alzheimer's Disease According to 47 Primary Care Physician Respondents
Phase II: Caregiver Assessment
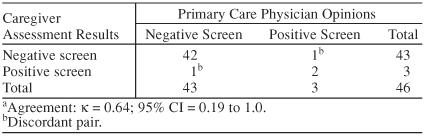
Forty-six caregivers completed assessments, and follow-up forms were obtained during phase II. The mean ± SD age of the respondents was 62.5 ± 13.1 years (28.9%, ≤ 55 years; 17.8%, 56–65 years; 53.3%, > 65 years). The relationships of the caregivers to the patients were spouse (59%), daughter (26%), paid helper (7%), sister (2%), and other (6%). The mean ± SD age of the screened patients was 74.4 ± 7.0 years. The caregiver assessment results and the PCP opinion were dichotomized into positive screen or negative screen categories, then cross-classified (Table 6) to obtain Cohen's κ, a measure of agreement. The observed value for κ was 0.64 (95% CI = 0.19 to 1.0. The agreement was classified, using the guidelines proposed by Landis and Koch,13 as “good” on a scale that includes marginal (κ < .4), good (.4 ≤ κ ≤ .75), and excellent (κ > .75). However, the CI for κ covered virtually all values for which κ can assume, and as such, indicated that the level of agreement could not be distinguished from either marginal or excellent. The internal consistencies measured by Cronbach's α for the Blessed Dementia Functional Subscale and the Haycox Dementia Behavior Scale subsets were 0.78 and 0.91, respectively. Internal consistency greater than 0.70 is considered indicative of good reliability.14 The discordant pairs provide additional interpretation of the screening feasibility. In the case of the negative caregiver assessment screen and the positive PCP screen, the physician responded that the patient “clearly has evidence of poor memory.” The scores for this patient were 3.5 and 5 for the Blessed Dementia Behavior Scale and Haycox Dementia Behavior Scale, respectively. The other discordant pair had a Blessed Dementia Behavior Scale score of 4.5 and a Haycox Dementia Behavior Scale score of 6. This patient was previously diagnosed with multi-infarct dementia. In both cases, the Blessed Dementia Behavior Scale scores were close to the cutoff value of 4 for a positive screen.
Table 6.
Cross-Classification of Agreement Between Caregiver Assessments and Primary Care Physician Opinions Regarding Alzheimer's Disease Diagnosisa
DISCUSSION
The primary objective of this study was to evaluate the feasibility of conducting a caregiver interview to identify patients suffering from symptoms of AD in the primary care setting. The time constraints in the primary care field are well documented,5 and the experiences of implementing and recruiting caregivers for this project reinforce this notion. However, the primary clinical finding of this study is that there is willingness from both caregivers and PCPs to engage in additional screening for dementia and AD. This research establishes preliminary feasibility in utilizing a caregiver screen in the primary care setting. The caregiver screening approach, once formally evaluated, will be one approach to improving PCP efficiency while responding to AD's projected health care burden.
The barriers illustrated during the focus groups and survey became evident during the caregiver assessment. The lack of time to perform additional screening measures other than those pertaining to the patient's presenting problem was understated during the focus groups. Previous research suggests that PCPs tend to participate in research studies that address issues important to their own practices that assist them in providing more efficient patient care as long as the studies do not interfere with their daily routine or overburden their staff.15 The study design for phase II addressed these concerns by selecting PCPs who expressed interest in conducting AD research and by utilizing a caregiver assessment that would screen patients independently of the PCP. The amount of effort required by the PCP and supporting staff was considered minimal at time of design. Once access was gained to the family practice clinics, assistance from office personnel was problematic due to stated time constraints. When researchers were sent to the clinics to enroll patients, participant accrual increased dramatically. This experience supports findings from focus groups regarding lack of time but also demonstrates a willingness to accept third-party involvement in the process as long as the involvement does not interrupt the normal flow of the PCP practice.
This study has several potential limitations. Although a combination of focus groups and a random sample survey was used to estimate the general perceptions of PCPs in the diagnosis and treatment of AD, the sampling frame only consisted of the Charleston, S.C., metropolitan area. While our results regarding time constraints and barriers to diagnosing AD may be common in other metropolitan areas not sampled, issues pertaining to referral patterns and treatment practices may have limited generalizability. With regard to phase II of this research, the caregiver assessment, consisting of the Blessed Dementia Functional Subscale and the Haycox Dementia Behavior Scale, has indications of agreement with the PCP's assessment; however, the interpretation of the agreement between the care-giver assessment and the PCP's clinical impression is limited due to the potential bias introduced by sharing the results of the caregiver assessment directly with the PCP before obtaining the PCP's judgment. Additional research is needed to refine the caregiver assessment and to pilot interventions utilizing the refined assessment in a manner that would enable the use to transition from a research-based setting to a practice-based setting.
Drug name: donepezil (Aricept).
Footnotes
This research was supported by Eisai Inc., Teaneck, N.J., and Pfizer Inc, New York, N.Y., as a grant for research on Alzheimer's disease in the family practice setting.
REFERENCES
- Hebert LE, Scherr PA, and Bienias JL. et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003 60:1119–1122. [DOI] [PubMed] [Google Scholar]
- Valcour VG, Masaki KH, and Curb JD. et al. The detection of dementia in the primary care setting. Arch Intern Med. 2000 160:2964–2968. [DOI] [PubMed] [Google Scholar]
- Mant A, Eyland EA, and Pond DC. et al. Recognition of dementia in general practice: comparison of general practitioners' opinions with assessments using the Mini-Mental State Examination and the Blessed Dementia Rating Scale. Fam Pract. 1988 5:184–188. [DOI] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force. Screening for dementia: recommendations and rationale. Available at: http://www.ahrq.gov/clinic/3rduspstf/dementrr.htm. Accessed July 30, 2004. [Google Scholar]
- Boise L, Camicioli R, and Morgan DL. et al. Diagnosing dementia: perspectives of primary care physicians. Gerontologist. 1999 39:457–464. [DOI] [PubMed] [Google Scholar]
- National Institute on Aging. Alzheimer's Disease: Unraveling the Mystery. Bethesda, Md: US Dept Health Human Services. 2002 [Google Scholar]
- Mace NL, Rabins PV. The 36-Hour Day: a Family Guide to Caring for Persons With Alzheimer's Disease, Related Dementing Illnesses, and Memory Loss in Later Life. Baltimore, Md: John Hopkins University Press. 1991 [Google Scholar]
- Haycox JA. A simple, reliable clinical behavioral scale for assessing demented patients. J Clin Psychiatry. 1984;45:23–24. [PubMed] [Google Scholar]
- Blessed G, Tomlison E, Martin R. The association between quantitative measures of dementia and of senile change in the cerebral gray matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Mintzer J, Nietert P, and Costa K. et al. Identifying persons with dementia by use of a caregiver telephone interview. Am J Geriatr Psychiatry. 1998 6:176–179. [PubMed] [Google Scholar]
- S-Plus [computer program]. Version 4.5. Seattle, Wash: Insightful Corporation. 1998 [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 2001;33:159–174. [PubMed] [Google Scholar]
- Nunnally JC, Bernstein IH. Psychometric Theory. 3rd ed. New York, NY: McGraw-Hill Companies. 1994 [Google Scholar]
- Plane M, Beasley J, and Wiesen P. et al. Physician attitudes toward research study participation: a focus group. Wis Med J. 2001 97:49–51. [PubMed] [Google Scholar]


