Psoriasis is a chronic inflammatory disease affecting up to 3% of the world’s population.1 Between 10% and 30% of those with psoriasis also develop psoriatic arthritis.2 Histologically, psoriasis is characterized by hyperproliferative keratinocytes and infiltration of prominent T cells, dendritic cells and neutrophils in the dermis. There is growing evidence suggesting that the interleukin (IL)-23/T-helper (Th)17-γδT17 axis and Th17-related cytokines play critical roles in disease development and pathogenesis.3–7 Indeed, the U.S. Food and Drug Administration has recently approved anti-IL-17A (secukinumab) for the treatment of moderate-to-severe plaque psoriasis. Despite these studies, the aetiology and immunopathogenesis of psoriasis have not been fully understood. In addition, identifying biomarkers for psoriasis diagnosis and treatment response is still a daunting task in the field due to the heterogeneity of disease. In this issue of the BJD, Kang et al. provide candidate biomarkers using a systems metabolomics approach.8 Specifically, they used a combined full-scan mode untargeted metabolomics approach and selected ion monitoring (SIM)-targeted metabolomics approach to extensively profile metabolites from serum samples of patients with psoriasis compared with those from healthy individuals. They found that patients with psoriasis had enhanced amino acid metabolic activity and increased glycolysis while the fatty acid biosynthesis pathway was significantly decreased in patients with psoriasis. Taken together, this study provides an array of biomarkers that may be related to psoriasis pathogenesis and diagnosis.
Metabolomics is an emerging systems biology approach to measure the abundance of various metabolites thus profiling different metabolic pathways. Previous studies have examined and compared metabolic patterns between unaffected skin and psoriatic lesional skin tissues using one-dimensional 1H nuclear magnetic resonance spectroscopy.9 They found that psoriatic skin contained lower metabolite levels of myo-inositol and glucose but higher levels of choline and taurine compared with unaffected skin tissues. It appears that these metabolite levels are correlated with the therapeutic effect of corticosteroid treatment although the study cohort is relatively small, with 10 samples from each group. The more comprehensive metabolomics analysis using 96 sex-balanced individuals (32 healthy donors, 32 mild and 32 severe psoriasis) with nontargeted liquid chromatography mass spectrometry (LC-MS) metabolomics approach revealed three altered psoriasis-related metabolic pathways: (i) arginine and proline; (ii) glycine, threonine and serine; and (iii) alanine, aspartate and glutamate.10 These circulating metabolite levels before and after anti-tumour necrosis factor (TNF)-α (etanercept) treatment correlated well with psoriasis area and severity index clinical score, suggesting that these metabolites might be used as biomarkers for treatment response. Although the LC-MS metabolomics approach provides rich data with a wide range of different metabolites, gas chromatography (GC)-MS offers a cost-effective way to analyse a variety of endogenous metabolites, particularly fatty acids. Kang et al. used the GC-MS approach with full-scan mode and SIM mode to profile circulating metabolites from serum samples of patients with psoriasis.8 The full-scan mode can monitor a range of masses and is useful for identifying unknown compounds while the SIM mode detects a particular metabolite of interest. They found four metabolic perturbations that are associated with psoriasis: (i) increased amino acid levels including ornithine, proline, glutamine and branched-chain amino acids; (ii) elevated glycolytic activity such as increased lactic acid; (iii) increased urea cycle activity; and (iv) decreased fatty acid syntheses including decreased levels of crotonic acid and azelaic acid. These findings are largely consistent with previous studies,10 particularly increased amino acid levels and urine cycle activity. The increased metabolites in these two pathways may well explain the observed keratinocyte hyperproliferation and elevated proteolysis activity in patients with psoriasis. Elevated amino acid levels and an enhanced influx of amino acids may lead to the production of psoriasis-enriched protein synthesis such as small proline-rich proteins, hornirine and late cornified envelope 3A (LCE3A), which are found in psoriatic skin at levels up to 500 times greater than in healthy skin.11 Increased urea cycle activity further supports this notion as the urea cycle is an entry pathway for polyamine synthesis, which is essential in cell proliferation, and a hallmark of keratinocytosis in psoriasis. Interestingly, this study also identified the levels of crotonic acid and azelaic acid associated with psoriasis pathogenesis. Both were significantly lower in patients with psoriasis compared with healthy individuals. Indeed, azelaic acid topical treatment has been shown to have therapeutic efficacy in psoriasis vulgaris.12
In contrast to metabolites associated with psoriasis pathogenesis, this study reported for the first time that glycolytic activity was significantly increased as revealed by high lactic acid levels in patients with psoriasis. Although it is unknown how lactic acid production is related to psoriasis pathogenesis, it is tempting to speculate that lactic acid production may be a result of host response to inflammation elicited in psoriasis, resembling the phenotype found in wound healing. Lactic acid can tip macrophages into an anti-inflammatory phenotype.13 In addition, moisturizers with therapeutic concentrations of lactic acid may be helpful in psoriasis. As summarized in Figure 1, metabolites revealed in psoriasis are involved in a number of different metabolic pathways. High levels of most of these metabolites provide energy fuels for keratinocyte hyperproliferation, psoriasis-enriched protein synthesis and collagen synthesis, ultimately contributing to psoriasis pathogenesis. Despite these studies, more work needs to be done to further validate these metabolic biomarkers, particularly in the setting of psoriasis treatment such as anti-IL-17 or anti-TNF-α biologics therapy. In addition, the exact mechanism by which these metabolites are involved in psoriasis pathogenesis needs to be determined. Nevertheless, this study provides a list of candidate metabolic biomarkers that may be relevant to psoriasis diagnosis and treatment.
Fig 1. Metabolites revealed from differential metabolic pathways by systems metabolomics approaches are involved in psoriasis pathogenesis.
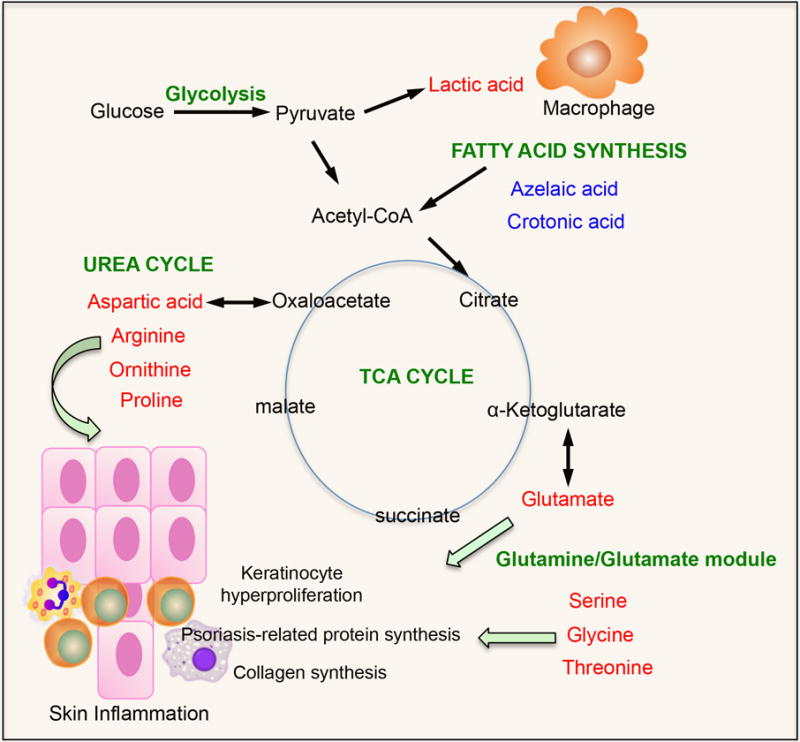
font denotes upregulated metabolites while font represents downregulated metabolites in psoriasis. Acetyl-CoA, acetyl coenzyme A; TCA, tricarboxylic acid.
Acknowledgments
Funding sources
J.Y. is supported by NIH P01CA150947, R21AI124235, AI124081 and the Kentucky Research Challenge Trust Fund.
Footnotes
Conflicts of interest
None declared.
References
- 1.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–73. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 2.Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl. 2):ii14–17. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–50. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 4.Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009;129:79–88. doi: 10.1038/jid.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J Invest Dermatol. 2010;130:362–70. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blauvelt A. T-helper 17 cells in psoriatic plaques and additional genetic links between IL-23 and psoriasis. J Invest Dermatol. 2008;128:1064–7. doi: 10.1038/jid.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Y, Shen X, Ding C, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang H, Li X, Zhou Q, et al. Exploration of candidate biomarkers for human psoriasis based on gas chromatography-mass spectrometry serum metabolomics. Br J Dermatol. 2016 doi: 10.1111/bjd.15008. (in press) [add details at production] [DOI] [PubMed] [Google Scholar]
- 9.Sitter B, Johnsson MK, Halgunset J, et al. Metabolic changes in psoriatic skin under topical corticosteroid treatment. BMC Dermatol. 2013;13:8. doi: 10.1186/1471-5945-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamleh MA, Snowden SG, Grapov D, et al. LC-MS metabolomics of psoriasis patients reveals disease severity-dependent increases in circulating amino acids that are ameliorated by anti-TNFalpha treatment. J Proteome Res. 2015;14:557–66. doi: 10.1021/pr500782g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Koning HD, van den Bogaard EH, Bergboer JG, et al. Expression profile of cornified envelope structural proteins and keratinocyte differentiation-regulating proteins during skin barrier repair. Br J Dermatol. 2012;166:1245–54. doi: 10.1111/j.1365-2133.2012.10885.x. [DOI] [PubMed] [Google Scholar]
- 12.Iraji F, Faghihi G, Siadat AH, et al. Efficacy of 15% azelaic acid in psoriasis vulgaris: a randomized, controlled clinical trial. J Drugs Dermatol. 2010;9:964–8. [PubMed] [Google Scholar]
- 13.Colegio OR, Chu NQ, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]


