Abstract
Accumulating evidence has demonstrated that human cancers arise from various tissues of origin that initiate from cancer stem cells (CSCs) or cancer-initiating cells. The extrinsic and intrinsic apoptotic pathways are dysregulated in CSCs, and these cells play crucial roles in tumor initiation, progression, cell death resistance, chemo- and radiotherapy resistance, and tumor recurrence. Understanding CSC-specific signaling proteins and pathways is necessary to identify specific therapeutic targets that may lead to the development of more efficient therapies selectively targeting CSCs. Several signaling pathways—including the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR), maternal embryonic leucine zipper kinase (MELK), NOTCH1, and Wnt/β-catenin—and expression of the CSC markers CD133, CD24, CD44, Oct4, Sox2, Nanog, and ALDH1A1 maintain CSC properties. Studying such pathways may help to understand CSC biology and lead to the development of potential therapeutic interventions to render CSCs more sensitive to cell death triggered by chemotherapy and radiation therapy. Moreover, recent demonstrations of dedifferentiation of differentiated cancer cells into CSC-like cells have created significant complexity in the CSCs hypothesis. Therefore, any successful therapeutic agent or combination of drugs for cancer therapy must eliminate not only CSCs but differentiated cancer cells and the entire bulk of tumor cells. This review article expands on the CSC hypothesis and paradigm with respect to major signaling pathways and effectors that regulate CSC apoptosis resistance. Moreover, selective CSC apoptotic modulators and their therapeutic potential for making tumors more responsive to therapy are discussed. The use of novel therapies, including small-molecule inhibitors of specific proteins in signaling pathways that regulate stemness, proliferation and migration of CSCs, immunotherapy, and noncoding microRNAs may provide better means of treating CSCs.
Keywords: cancer stem cells, apoptosis resistance, death receptors, multidrug resistance, Bcl-2 family, c-FLIP
I. INTRODUCTION
The major limitation of cancer chemotherapy has proven to be apoptosis refractoriness and drug resistance, whether intrinsic to the malignancy due to resistance to cell death (colon cancer, pancreatic cancer, glioblastoma, and prostate cancer are typically refractory to cancer chemotherapy) or acquired by the malignancy after transient disease remission (e.g., after breast cancer chemotherapy). Numerous mechanisms have been found to cause chemotherapeutic resistance in cancer cells.1–5 Understanding the mechanisms of resistance will assist in the design of more effective strategies to overcome it in cancer cells and tumors. Defects in apoptotic signaling and redundant survival mechanisms in malignant cells contribute to drug resistance in various cancer types.5–9 Therefore, strategies to lower the thresholds for triggering apoptosis in CSCs and various cancers may lead to new and more effective therapeutic regimens.5–9
Drug resistance can emerge due to a host of environmental factors as well as genetic or epigenetic alterations in cancer cells.10–13 These factors are particularly important in the interconversion of CSCs to differentiated cells and vice versa,13 which make therapeutic approaches quite complicated. Evolutionary theory has contributed to our understanding of the dynamics of drug resistance mutations in a cancer cell population, the risk of resistance pre-existing the initiation of therapy, the drug cocktail composition necessary to prevent the emergence of resistance, and optimum drug administration schedules for patients at risk of evolving acquired resistance.14 Adding to the complexity of successful treatment of tumors is the recent demonstration of dedifferentiation of differentiated cancer cells into CSC-like cells due to epigenetic plasticity.13 This proves that any successful therapeutic agent or combination of drugs for cancer therapy must eradicate not only CSCs but the differentiated cancer cells and the entire bulk of tumor cells.
Several factors, including the tumor environment, pharmacodynamics, and intertumor and intratumor heterogeneities in individual patients, also contribute to unresponsiveness of cancer cells to therapies. Moreover, various genetic alterations that result in redundant and increased cytoprotective and survival pathways, as well as numerous defects in the apoptotic signaling machinery and epigenetic changes, contribute to highly aggressive tumors. The main cause of death in patients with incurable tumors is recurrence of the malignancy, which is attributed to treatment-resistant CSCs within the primary tumor and the tissues surrounding it. The concept of a subset of CSCs believed to play a significant role in tumor formation, metastasis, cancer recurrence, and resistance to anticancer therapies has gained significant traction. CSCs are a relatively rare cell population in a tumor and have been described by different molecular markers and cellular features in different types of cancers. Thorough and in-depth molecular characterization of the CSC population as well as delineation of the heterogeneity of these cells in many tumors will allow us to refine the targeted agents that force CSCs to apoptosis, thus enhancing the development of more effective treatments.
In this review article, signaling pathways that regulate the CSC survival, proliferation, and resistance to apoptosis and anticancer agents, and the potential of specific proteins in the apoptosis pathways for developing novel and effective inhibitors to eliminate CSCs, are discussed. There has been great interest in targeting CSCs for improved cancer treatment through triggering apoptosis, inhibiting metastasis, and overcoming drug resistance in the cells.13–16 Understanding and characterizing the molecular mechanisms that control unresponsiveness of CSCs to various apoptotic stimuli will help in the design of more effective therapies against a variety of cancers and prevent tumor recurrence.
II. APOPTOSIS PATHWAYS
A. Mitochondrial Pathway
Apoptosis is one of the most crucial and well-studied mechanisms regulating tissue development and homeostasis through a complex and orderly network of molecules that mediate death and survival signals. This orderly and tightly regulated programmed cell death involves signal transduction pathways that induce cells to self-destruct during embryonic development or in response to radiation-induced DNA damage and anticancer drugs. Apoptosis signaling pathways prevent cancer development, but specific mutations in normal stem cells transform these cells to CSCs (Fig. 1) and enable them to escape apoptosis and lead to tumor formation. Two major pathways, the extrinsic or cell surface death receptors pathway, and the intrinsic or mitochondria-initiated pathway, control apoptosis (Fig. 2).17–19 The extrinsic apoptotic program is initiated by the binding of membrane-bound death receptors (DRs) with their ligands—for example, Fas/Fas ligand interaction, tumor necrosis factor-α (TNF-α)/TNF receptor 1 (TNFR1), or TNF-related apoptosis-inducing ligand (TRAIL)/DR5 interaction and cell death (Fig. 2).
FIG. 1.
Cancer stem cells (CSCs) and their implications for the development and progression of tumors. CSCs are generated from normal stem cells (NSCs) through tumorigenic transformation of several potential pathways, including Hh: hedgehog (Hh); epithelial–mesenchymal transition (EMT); and mesenchymal–epithelial transition (MET). CSC and drug-induced CSCs (Di-CSCs) are enriched following conventional chemotherapy treatment.
FIG. 2.
Overview of the intrinsic and extrinsic apoptotic pathways, the TRADD/NF-κB survival pathway, and the growth factor (GF) receptors PI3K/AKT pro-survival signaling axis in CSCs. A logical strategy to target CSCs is to use inhibitors of the antiapoptotic and prosurvival proteins in these interconnected pathways as shown in this figure.
Ligand binding leads to aggregation of DRs and recruitment of the adaptor protein FADD, initiator pro-caspase-8, and pro-caspase-10 to form the DISC complex. Pro-caspase-8 or pro-caspase-10 is then converted to its active form, which further activates the effector caspases-3, -6, and -7. Active caspase-3 initiates the downstream caspase cascade leading to apoptosis. Active caspases-8 and -10 are known to cleave a proapoptotic Bcl-2 family member, Bid, and the truncated Bid induces mitochondrial cytochrome C release,19–22 thereby linking the two pathways. After activation, both caspases-8 and -9 activate caspase-3, which in turn cleaves other caspases and many cellular proteins.19
B. Death Receptor Pathway
The death receptor (DR)-initiated pathway is suppressed by c-FLIP, which inhibits death-inducing signaling (DISC) formation and activation of caspases- 8 and -10, and prevents downstream apoptotic signaling. The mitochondrial or intrinsic pathway is initiated by receptor-independent apoptotic stimuli (e.g., DNA damage, radiation, and chemotherapeutic agents), which trigger mitochondrial outer membrane permeabilization (MOMP) by activation of Bcl-2 homologous proapoptotic proteins (e.g., Bax, Bak, and Bad) and the Bcl-2 homology domain-3–(BH3-) only family of proteins including Bid, Bim, or PUMA.19–22 MOMP in turn causes inner membrane permeability, and the various anti- and pro-apoptotic members of the Bcl-2 family form an interactive network that finally regulates the release of apoptosis-triggering factors, including certain caspases, Smac/DIABLO, and others from the mitochondrial intramembrane space to the cytosol.19,23 Cytochrome C and dATP bind to apoptotic proteinase-activating factor-1 (Apaf-1); this complex, along with adenine nucleotides, promotes procaspase-9 autoactivation, which in turn activates caspases-2, -3, -6, -7, -8, and -10.
III. CANCER STEM CELLS AND APOPTOSIS
A. Cancer Stem Cell Phenotype
CSCs have the capacity for self-renewal, have multilineage differentiation potential capable of generating differentiated progenitor cells, are responsible for tumor cell heterogeneity, and can recapitulate the initial tumor. Escape from the complex apoptosis machinery is essential for CSCs. CSCs may arise from normal stem cells or progenitor cells (Fig. 1) following transforming mutations and as a result of epigenetic plasticity as well as interconversion and dedifferentiation of non-CSCs to CSCs.23,24 Three important criteria define CSCs: (a) only a low number of cancer cells within a tumor are usually endowed with the ability to recapitulate the original tumor; (b) they are identified and characterized by immunopheno-typing and express distinctive surface markers and can be differentially separated from non-CSCs by fluorescent activated cell sorting (FACS) analysis and flow cytometry; and (c) the tumors generated from CSCs express the phenotypic heterogeneity of the parent tumor containing mixed populations of CSCs and non-CSCs.13,25,26
CSCs are resistant to apoptosis, anticancer drug treatment, and radiation therapy; overexpress ABC-family efflux multidrug transporters to detoxify or mediate the release of cytotoxic agents from the cells27; display overexpression of Chk1/2 DNA damage repair proteins28,29; show rapid response to DNA damage29,30; express up-regulation of antiapoptotic proteins; and are resistant to oxidative stress. All of these characteristics contribute to the development of therapeutic resistance. Moreover, CSCs have been found to have crucial roles in various cancers, including breast, brain, ovarian, prostate, pancreatic, hepatocellular, head and neck, and hematological malignancies. While the identification and characterization of CSCs have been very useful in understanding their role in tumor initiation, oncogenesis, and distant metastasis, therapeutic targeting of CSCs has met significant difficulties due to their heterogeneous nature, which enables them to escape the cellular mechanisms that regulate apoptosis.
B. Stem Cell Signaling Pathways
Accumulating evidence has demonstrated that various cancers are initiated from CSCs that are responsible for the patient’s resistance to therapies. 13,23,30–33 Moreover, due to the heterogeneity, high diversity, and plasticity of CSCs, developing therapeutics to target these cells will be challenging. Furthermore, experimental evidence suggests a possibility of non-CSC reprogramming and conversion to CSCs13 (Fig. 1). Therefore, effective anticancer treatment must eliminate both CSCs and the entire bulk tumor population as well as prevent tumor dedifferentiation of non-CSCs to CSCs. Various signaling pathways, including activated PI3K/AKT/mTOR, maternal embryonic leucine zipper kinase (MELK), NOTCH1, and Wnt/β-catenin, regulate CSC survival and proliferation. 31–37 These pathways as well as expression of CSC markers including CD133, CD44, Oct4, Sox2, Nanog, and ALDH1A1 maintain CSC properties.
Olmez et al.38 recently demonstrated the difficulty of dealing with CSCs by inducing dedifferentiation of patient-derived GBM cell lines into GSC-like cells (induced GBM stem cells, iGSCs) through the expression of Oct4, Sox2, and Nanog transcription factors. The iGSCs formed neurospheres even in the absence of exogenous mitogens, were sensitive to the CSC inhibitor salinomycin, and exhibited resistance to temozolomide (TMZ) therapy. Moreover, NOTCH1 and Wnt/β-catenin signalings and expressions of CD133, CD44, and ALDH1A1 were induced in iGSCs. These results indicate that dedifferentiation of cancer cells to induced iCSCs causes complexity in treating malignant tumors and that any therapeutic intervention should be designed to eliminate GSCs as well as iGSCs that may result from treatment with a chemotherapeutic agent or radiation therapy.13
Recently, Iriondo et al.39 demonstrated that hypoxia-driven cancer stem–like cell enrichment results from a dedifferentiation process in breast cancer and that hypoxia-inducible factors (HIFs) are required for chemotherapy resistance of breast CSCs (BCSCs). The dedifferentiated cells upregulate multidrug resistance (MDR) genes via double-stranded RNA-dependent protein kinase– (PKR− like ER-resident kinase– (PERK-) nuclear factor erythroid 2-related factor 2 (Nrf2) (PERK-Nrf2) signaling, and suggest that targeting this pathway could sensitize drug-resistant cells to chemotherapy.40
We have demonstrated the indispensable role of DNA-dependent protein kinase (DNA-PK) in elevating the expression of P-glycoprotein (P-gp) and an siRNA or a small-molecule inhibitor of DNA-PK in preventing P-gp expression and drug resistance.41 Recently, the importance of the MDR1 gene and its product P-gp in GSC resistance to chemotherapy was reported by showing that, following prolonged chemotherapy, DNA-PK, P-gp, and CD133+ increase in recurrent GBM.42 Interestingly, doxorubicin markedly increased CD133, DNA-PK, and MDR1 expression in GBM cells.42 These results show that CD133 and DNA-PK may increase MDR1 expression via the phosphatidylinositol- 3-kinase– (PI3K-) Akt signaling pathway. The PI3K downstream targets Akt and nuclear factor NF-κB, which interact with the MDR1 promoter, are also increased in these cells. Downregulation of CD133 and DNA-PK by small interfering RNA or inhibition of PI3K or Akt decreased Akt, NF-κB, and MDR1 expression. These data collectively demonstrate that targeting CD133 and DNA-PK in combination with conventional chemotherapy may effectively eradicate GSCs and improve the prognosis for patients with GBM.
Another important protein, MELK, is a highly conserved serine/threonine kinase expressed in several human cancers and CSC populations.43 MELK plays critical roles in the formation and maintenance of CSCs, particularly in cell cycle control, cell proliferation, apoptosis, cell migration, cell renewal, embryogenesis, oncogenesis, and cancer treatment resistance and recurrence. MELK binds to and phosphorylates the oncogenic transcription factor FOXM1 in GSCs. Recent results show that the catalytic subunit of Polycomb repressive complex 2, EZH2, is targeted by the MELK-FOXM1 complex, which in turn promotes GSC resistance to radiation.44 The highly potent MELK selective inhibitor OTS167 exhibits strong in vitro activity, conferring an IC50 of 0.41 nM, and in vivo effects in various human cancer xenograft models.45
The characteristics of CSCs that may help in the development of anti-CSC therapies include specific cell surface markers and particular networks of transcription factor (TF) signaling, aberrant signaling pathways, epigenetic alterations, reprogramming and plasticity, interaction with the microenvironment and CSC niche, and use of particular metabolic pathways.
IV. MECHANISMS OF DEATH RESISTANCE IN CANCER STEM CELLS
A. Deficiencies in Apoptosis Pathways
Discovering distinct targets and delineating the molecular differences that prevent CSCs from proceeding to apoptosis hold enormous significance and promise for improving cancer therapy. Several mechanisms, including a deficiency in mitochondrial- mediated apoptosis,46 downregulation of death receptors,47,48 upregulation of c-FLIP, overexpression of antiapoptotic Bcl-2 family members and inhibitors of apoptosis proteins (IAPs), over-activation of ABC multidrug resistance transporters, and PI3K/AKT signaling contribute to enhanced resistance to cell death induction in CSCs in various cancers.31,32–35,48 These mechanisms are discussed in detail next.
B. Multidrug Resistance Transporters
Much evidence indicates that CSCs express ABCB1 (P-gp) and BRCP1/ABCB2. The ATP-binding cassette transporter (ABC) family induces resistance to chemotherapy in several malignancies. 42,49–53 Recently, based on the expression of the ABC family member ABCB5, melanoma cancer stem cells (MCSCs) were identified and it was shown that ABCB5 maintains melanoma-initiating cells through a proinflammatory cytokine signaling circuit.54 Wang et al.50 showed that Panc-1 pancreatic CSCs express high levels of CD133/CD44/Oct4/Nestin compared to Panc-1 cells, are resistant to gemcitabine, and express high levels of P-gp as well as high anti-apoptotic proteins, but exhibit weak proliferative potential. It was recently shown that epigallocatechin gallate treatment significantly downregulates expression of P-gp, but not that of ABCG2 or O6-methylguanine-DNA methyltransferase, and simultaneously enhances sensitivity to TMZ in GSCs.55
C. The Crucial Role of the PI3K/AKT/mTOR Signaling Pathway in CSCs
This signaling pathway is a master regulator for malignancies and plays important roles in many cellular processes, including proliferation, apoptosis, differentiation, metabolism, and migration. Aberrant upregulated PI3K/AKT/mTOR signaling is implicated in tumor resistance to conventional therapies in many cancers. Recent studies have revealed that this signaling pathway also plays a major role in the CSC phenotype in several tumors. 56–59 The combination of the antidiabetic drug metformin, an inhibitor of PI3K/AKT/mTOR signaling, and the RAF inhibitor sorafenib effectively impact reduced TMZ resistance, significantly reduce GSC oxidative stress and efflux pump activity, and synergistically kill these cells52. Moreover, a recent report showed that BFZ-235, an inhibitor of PI3K/AKT/mTOR signaling, effectively suppresses the stemness of colon CSCs.50
D. Dysregulated Antiapoptotoic Proteins in CSCs
1. Bcl-2 Family of Proteins
Prominent hallmarks of cancer are apoptosis evasion and the ability to self-govern growth and proliferation. While several steps within the extrinsic and intrinsic apoptotic pathways are dysregulated in various cancers, the aberrant expression levels and ratios of apoptotic and antiapoptotic proteins and their contribution to survival pathways in CSCs have not been characterized and delineated in detail. Bcl-2 family proteins consist of the antiapoptotic molecules Bcl-2, Bcl-xL, and Mcl-1, and the proapoptotic proteins Bax, Bak, Bid, Bim, Bik, Noxa, and Puma. High levels of antiapoptotic proteins, including Bcl-2 and Bcl-xL, have been described in GSCs.60 Moreover, high levels of Bcl-2, Bcl-xL, and Mcl-1 are associated with GSC resistance.61–63 The ratio of anti- to proapoptotic protein levels changes the balance of cell survival and controls the sensitivity of cancer cells to apoptotic stimuli.61 Furthermore, abnormal increases in the redux-sensing transcription factor Nrf2 promote CSC survival by elevating transcription of Bcl-2 and Bmi-1 genes (Bmi-1 is a member of Polycomb repressor complex 1).64 Since the expression of these antiapoptotic proteins is critical for the survival of CSCs, significant efforts have been directed toward therapeutic interventions to eliminate CSCs using inhibitors of the Bcl-2 family of proteins.
2. TRADD Expression and NF-κB Activity
As shown in Fig. 2, tumor necrosis factor receptor 1– (TNFR1-) associated death domain protein (TRADD) is a crucial adaptor protein in TNFR1 signaling and has an essential role in NF-κB activation and survival signaling in CSCs.65 Downstream of DR4 and DR5 and the death-inducing signaling complex (DISC), TRAIL also promotes the formation of the intracellular Complex II, which is composed of FADD, TRADD, caspase-8, caspase-10, RIP1, TRAF2, and IKK-γ.66 NF-κB is the transcription factor that promotes expression levels of various inflammatory cytokines and apoptosis inhibitory proteins. Cancer cells often contain constitutively activated NF-κB that provides them with increased survival and resistance to therapies. Increased expression of TRADD is sufficient to activate NF-κB in GSCs.67
In GBM, cytoplasmic TRADD expression is significantly associated with worse progression-free survival (PFS). Silencing TRADD in GSCs results in decreased NF-κB activity and decreased viability of these cells, suggesting that TRADD is required for maintenance of GBM stem cell populations. 67 Therefore, increased expression of cytoplasmic TRADD is both an important biomarker and a key driver of NF-κB activation in GBM, and supports an oncogenic role for TRADD in GBM. NF-κB activity supports the survival of CSCs in breast cancer, and inhibition of NF-κB by the small-molecule inhibitor parthenolide was shown to cause preferential induction of apoptosis in CSC and progenitor cells, but not in normal stem cells, in human prostate cancer populations.68 Similarly, NF-κB activity is important for the survival of breast cancer CSCs, and these cells are preferentially sensitive to inhibitors of the NF-κB pathway by parthenolide, pyrrolidinedithiocarbamate, and diethyldithiocarbamate, indicating that high activity of NF-κB plays a major role in the maintenance of CSCs.69
3. Inhibitor of Apoptosis Family Proteins in CSC
Increased expression of IAPs, a family of endogenous caspase inhibitors, helps cancer cells to evade apoptosis.70 The IAP family X-linked inhibitors of apoptosis include XIAP, cIAP1, cIAP2, survivin, ML-IAP, NAIP, and ILP-2.70–72 XIAP has the strongest antiapoptotic properties compared to other IAPs; it suppresses apoptosis signaling by binding to active caspase-3 and -7 and by preventing caspase- 9 activation.73 Interestingly, ZFP36, a mRNA binding protein that exerts antitumor activity in GBM by triggering cell death, promotes depletion of cIAP2 and XIAP and leads to the association of RIP1 to caspase-8 and FADD in GSCs.74
IAPs function through interactions of their BIR (baculoviral IAP repeat) protein domains; these interactions are antagonized by Smac/Diablo, an inverse regulator for IAP family membersthat are involved in apoptosis. The Smac mimetics in combination with TRAIL induce the degradation of cIAP1 and XIAP and thus induce apoptosis in vitro and in vivo.75 Therefore, they exert an antitumor effect on nasopharyngeal carcinoma CSCs. Combination treatment with TRAIL and other anticancer agents may be a promising strategy for the treatment of nasopharyngeal carcinoma. Survivin, another IAP family member, was shown to play a role in CD133+ cell chemoresistance to 5-fluorouracil (5-FU) through a mechanism related to survivin expression instead of MDR1, ABCG2, and AKT1 expression. Therefore, a survivin inhibitor may be a new targeted agent for effective treatment of CD133+ colon cancer.76
4. c-FLIP Overexpression in CSCs
Acutely induced chemosensitization of cancer cells occurs when a proapoptotic signaling program induced in neoplastic cells by a chemotherapy drug includes the disabling of a cytoprotective antiapoptotic response. This is shown by our discovery that acute exposure of human leukemia cells to Taxol triggers a proapoptotic program that entails coordinate caspase activation and downregulation of c-FLIP.77 c-FLIP is a major antiapoptotic protein and an important cytokine and chemotherapy resistance factor that suppresses cytokine- and chemotherapy-induced apoptosis. It is expressed as long (c-FLIPL), short (c-FLIPS), and c-FLIPR splice variants in human cells. c-FLIP binds to FADD and/or caspase-8 or -10 and TRAIL receptor 5 (DR5). This interaction in turn prevents deathDISC formation and subsequent activation of the caspase cascade.
c-FLIPL and c-FLIPS are also known to have multifunctional roles in various signaling pathways, and to activate and/or upregulate several cytoprotective and prosurvival signaling proteins that include Akt, ERK, and NF-κB. The possibility of developing novel modalities of cancer therapy that improve the efficacy and lessen the toxicity of cancer chemotherapy by targeting specific c-FLIP isoforms is very attractive.78
c-FLIP variants are involved in TRAIL and chemotherapeutic drug resistance in a wide range of human malignancies.78 Furthermore, a combined Taxol/c-FLIP targeted therapy may improve the therapeutic response to Taxol by enhancing downregulation of c-FLIP variants in concert with drug-induced apoptosis signaling.79 A similar observation was made when c-FLIP was silenced by its specific siRNA in breast CSCs, which rendered these cells sensitive to TRAIL-triggered apoptosis.
V. DRUGS KNOWN TO INCREASE APOPTOSIS IN CANCER STEM CELLS
A. Drugs Known to Downregulate c-FLIP and Increase DR5
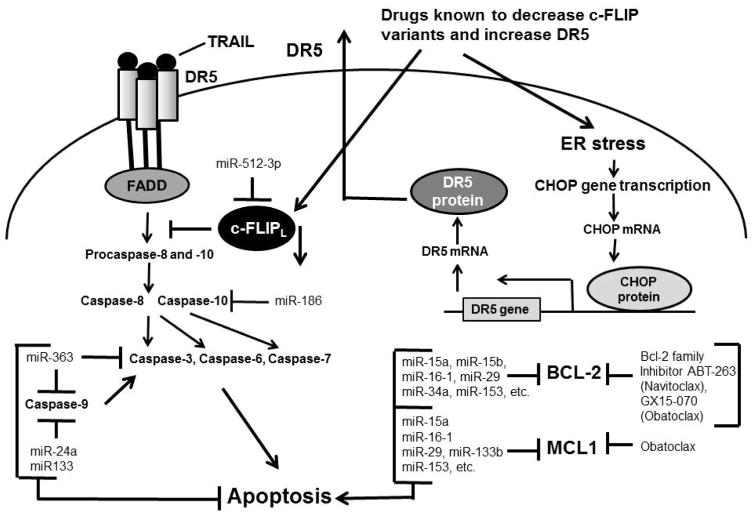
Activation of CSC signaling pathways is central to resistance to apoptosis and cancer therapy. At present, conventional chemotherapy and radio-therapy are largely ineffective in depleting the CSC pool because of CSCs’ apoptosis resistance phenotype, which suggests the need for novel therapies that specifically target the sustaining CSCs for tumor eradication and thus improve the poor prognosis of various cancers. Moreover, recent emerging reports have shown that some of the conventional chemotherapy agents trigger de-differentiation of cancer cells to CSCs by triggering epigenetic alterations as well as propagating these cells in the recurrent tumor.13, 38,80–83 Hence, recurrent tumors display even more resistance to these drugs than the initial tumors, which means that the design of better treatment strategies that can eliminate or otherwise control CSC populations in tumors is necessary for their eradication. Some of the antiapoptotic drugs for targeting CSCs are listed in Fig. 2 and Fig. 3.
FIG. 3.
Antiapoptotic drugs and miRNAs for targeting CSCs. Some of the drugs and miRNAs known to decrease c-FLIP variants, increase DR5, and target Bcl-2 family members are shown.
CSCs that contribute to cancer recurrence are frequently TRAIL resistant.79,84,85 Qi et al.84 showed that CD133-positive brain cancer stem cells are resistant to TRAIL and express lower levels of caspase-8 protein and higher levels of Bcl-2 protein when compared with CD133-negative cells (p < 0.05). One mechanism that causes resistance to TRAIL and chemotherapeutics in a wide range of human malignancies is upregulation of c-FLIP. Substantial levels of c-FLIP are expressed in deadly human cancers such as ovarian, colon, glioblastoma, breast, colorectal, and prostate cancers, as well as multiple myeloma. c-FLIP is also implicated in TRAIL resistance owing to its overexpression in a substantial proportion of these malignancies77,78 as well as in CSCs.79 Furthermore, interference with c-FLIP expression sensitizes these tumor cells to TRAIL and drugs like Taxol and gemcitabine.86,87 In addition to its function as an apoptosis modulator, c-FLIP has cellular functions including increased cell proliferation and tumorigenesis.78 Silencing c-FLIP in CSCs results in sensitization of these cells to TRAIL85 and cisplatin.48
Monensin (a polyether ionophore antibiotic) and salinomycin (a compound structurally related to monensin) cause effective cell death in CSCs and are currently recognized as anticancer drug candidates. Monensin as well as other polyether antibiotics, including salinomycin, nigericin, narasin, and lasalocid A, enhance TRAIL-mediated apoptosis in glioma cells via ER stress, CHOP-mediated DR5 upregulation, and c-FLIP downregulation (see Fig 3 for this signaling pathway). Salinomycin has been shown to be highly effective for eradicating CSCs both in vitro and in vivo.88–90 It induces apoptosis via death receptor-5 upregulation and decreased c-FLIP expression in cisplatin-resistant ovarian CSCs.91
Pancreatic ductal adenocarcinoma (PDA) is a very aggressive cancer that is lethal and has one of the worst outcomes among all cancers. PDA often recurs after initial treatment, resulting in patient death despite the use of any other treatment. Interestingly, in PDA patients DR5 is enriched in pancreatic CSCs compared with the bulk of tumor cells. Treating patient-derived PDA xenografts with gemcitabine, the first-line chemotherapeutic agent for PDA, initially reduces tumor size but does not affect CSC populations. 92 However, a combination of tigatuzumab, a fully humanized DR5 agonist monoclonal antibody, with gemcitabine is effective in killing CSCs and the bulk of tumor cells as well.92 This combination therapy has resulted in a remarkable reduction in pancreatic CSCs, later tumor remissions, and significant improvement in the time to tumor progression.92
It is believed that BCSCs mediate recurrence and drive the formation of metastatic tumors, the major cause of mortality in breast cancer patients. 93 Because of their heterogeneity, therapeutic targeting of BCSCs is hindered by their heterogeneous nature and resistance to conventional therapeutics. Piggott et al.79 identified a therapeutic approach to selectively killing BCSCs, irrespective of their clinical subtype and heterogeneity. They found that c-FLIP is upregulated in BCSCs from various breast cancer subtypes and that genetic suppression of c-FLIP by its siRNA partially sensitizes breast cancer cells to the anticancer agent TRAIL. Their results showed that in breast cancer cell lines, BCSCs are exquisitely sensitive to the derepression of this proapoptotic pathway due to loss of c-FLIP, which results in an 80% reduction in primary tumors, a 98% reduction in tumor metastases, and the loss of BCSC self-renewal. Taken together, the combination treatment of TRAIL or chemotherapy with agents that increase DR5, or inhibition of c-FLIP by pharmacological agents or genetic approaches, may offer an effective therapeutic strategy to eliminate these apoptosis-resistant CSCs.
B. Inhibitors of Bcl-2 Family Proteins
Activation of CSC signaling pathways is central to apoptosis and therapy resistance in various cancer types. ABT-263 (Navitoclax), a potent Bcl-2 family inhibitor, has been used against many tumor types.21,94 Pareja et al.95 recently reported a deregulated apoptotic pathway with elevated levels of Bcl-2 family proteins and increased activity of the PI3K signaling pathway in GBM and GSCs. These authors showed that ABT-263 and GDC-0941, a PI3K inhibitor, abrogate the ability of GSCs to form CSC neurospheres.95 Moreover, combining ABT-737 with the retinoid derivative fenretinide has been shown to target melanoma cancer stem cells by increasing caspase-dependent apoptosis.96 The antiapoptotic Bcl-2 family protein Mcl-1 is overexpressed in GBM and represents an important resistance factor to the BH-3 mimetic ABT-263. Additionally, GX15-070, a pan-Bcl-2 inhibitor that has shown promising antitumor activity in various malignancies, and combined treatment with ABT-263 and GX15-070 overcomes apoptotic resistance in established GBM cell lines, GBCs, primary cultures, and in vivo experiments97
ABT-263 has also been shown to inhibit cell proliferation and induce apoptosis in human esophageal cancer (EC) cells and their chemoresistant counterparts by targeting stemness pathways. 98 ABT-263 strongly suppresses CSCs, and the combination of ABT-263 and 5-Fu significantly reduces tumor growth in vivo and suppresses the expression of stemness genes. Therefore, the combination of ABT-263 with cytotoxic anticancer therapeutics may be a useful strategy for apoptosis- resistant cancer cells.
Obatoclax, a pan-Bcl-2 inhibitor with promising efficacy, was recently shown by Berghauser et al.99 to overcome apoptosis resistance to the histone deacetylase inhibitors SAHA and LBH589, and to act as a radiosensitizer in patient-derived GSCs. Obatoclax had a synergistic effect with SAHA and LBH589 and sensitizes cells to HDACi/radiation therapy (RTx). It enhances caspase-3/7 and inhibites the Bcl-2 family proteins Bcl-xL and Mcl-1 in these CSCs. The authors found that the gene predictive for treatment response is the F-box/WD repeat-containing protein-7, which is related to Bcl-2 inhibition and HDACi sensitivity.99
VI. ROLE OF MICRO RNA IN APOPTOSIS RESISTANCE AND ITS MODULATION
A decade ago, small non-protein-coding RNAs, a novel class of RNAs termed microRNAs (miRNAs), were found to be involved in carcinogenesis. These molecules function as endogenous suppressors of gene expression by binding to the 3’-untranslated region (UTR) of target mRNAs and trigger either cleavage of the mRNAs or induce translational repression in the cells. Functionally, two types of miRNAs have been described in cancer: tumor-suppressor and oncogenic.100–102 Ectopic expression of tumor-suppressor miRNAs and suppression of oncogenic miRNAs both generate antitumor effects by inhibiting cell proliferation and increasing cell death.103
miRNAs regulate the expression of various target genes and are dysregulated in CSCs, suggesting that they play a critical role in posttranscriptional gene regulation and function in various cellular processes in these cells.13,103 Various strategies, including miRNA mimics, miRNA antagonists, and nanodelivery, have been employed to suppress specific oncogenes or activate tumor suppressors.103 Accumulating evidence suggests that miRNAs play significant regulatory roles in CSC apoptotic and antiapoptotic pathways, proliferation, survival, differentiation, migration and invasion, drug resistance, and radiation resistance. 13,104–106 Moreover, they are innovative biomarkers and their expression patterns correlate with the developmental lineage and differentiation state of tumor cells.13,107–108 Several deregulated miRNAs have been strongly implicated in regulating GSC self-renewal capacity, maintenance of stemness and plasticity, resistance to drugs and radiation therapy, and unresponsiveness to various apoptotic stimuli.106–108 miRNAs regulate several groups of apoptosis-related proteins; those targeting caspases, Bcl-2, Mcl-1, and c-FLIP are shown in Fig. 3. Several studies have found that by antagonizing Bcl-2, miR-15a, miR16-1, and miR-34 trigger apoptosis in various CSCs.109–112
miRNA-34 is a tumor suppressor of great interest and is downregulated in several types of cancer. 113,114 As shown by Rathod et al.,115 it is downregulated by targeting many oncogenes related to proliferation, apoptosis, and invasion. miRNA-34a is a potential prognostic marker for glioma as its expression negatively correlates with patient survival in Grade III and IV glial tumors.115 Expression of miR-34a is decreased in a graded manner in glioma and glioma stem cell lines as compared to normal brain tissues. Ectopic expression of miR-34a in glioma stem cells decreases their proliferative and migratory potential, induces cell cycle arrest, and causes apoptosis. Rathod et al. showed that the tumor suppressive function of miR-34a in GBM is mediated via Rictor, a component of the mTORC2 complex that causes pronounced effects on glioma malignancy through its effects on the AKT/mTOR pathway and Wnt signaling.
We initially showed a 21-bp deletion in the p53 tumor suppressor protein in one of the four conserved regions within its conformational domain, spanning codons 126–133 at exon 5 in multidrug resistant MCF-7/ADR cells.116 Park et al. recently demonstrated that miR-34a expression is downregulated in these cells compared with MCF7 cells.117 The authors hypothesized that this reduction is due to the p53 mutation in MCF7/ADR cells. They found that miR-34a is suppressed by treatment with p53 RNAi or the dominant-negative p53 mutant in MCF7 cells. Ectopic miR-34a expression reduces breast cancer stem cell properties and increases sensitivity to doxorubicin treatment by directly targeting NOTCH1. Furthermore, tumors from nude mice treated with miR-34a were shown to be significantly smaller compared with those of mice treated with control lentivirus. Therefore, increased expression of miR-34a by systemic delivery of a miR-34a mimic118 represents a novel therapeutic approach in chemoresistant breast cancer.
Recently, Floyd et al.119 showed that two candidate oncogenic microRNAs, miR-582-5p and miR-363, target caspase-3, caspase-9, and Bim in glioblastoma by binding to the UTR of the genes for these apoptotic targets and suppressing their ex pression (Fig. 3). The authors performed a phenotypic rescue using siRNA against the targets in the context of miR inhibition and showed that miR-363 and miR-582-5p can increase GBM survival.
VII. CONCLUSIONS AND FUTURE DIRECTIONS
It is believed that many tumors contain CSCs that initiate tumors, metastasize, and cause tumor recurrence. CSCs have the capacity to self-renew and recapitulate the entire heterogeneous tumor population. Much evidence suggests that CSCs are more resistant to therapies that target cell proliferation and apoptosis. They escape from conventional therapy, which leads to their decreased sensitivity to prior treatment and CSC enrichment. Similarly, dedifferentiation of non-CSCs to CSCs after drug treatment can lead to cancer relapse. Therefore, effective and successful anticancer therapy must eradicate not only CSCs but also the bulk of tumor cells within the tumor.
CSCs exhibit enhanced resistance to apoptosis due to defects in the death receptor pathway, an impaired mitochondrial-mediated pathway, and upregulation of antiapoptotic proteins. Since the apoptotic pathways are deregulated in cancer cells and CSCs, restoration or activation of cell death machinery by the targeting of particular proteins in these pathways is a logical and attractive strategy for cancer therapy. Downregulation of death receptors, upregulation of c-FLIP, overexpression of antiapoptotic Bcl-2 family members and IAPs, and activation of PI3K/AKT signaling contribute to enhanced resistance to cell death induction in CSCs from various cancers. Increased expression of death receptors by small chemical inhibitors and their activation by recombinant ligands and agonistic antibodies, inhibition of c-FLIP expression by compounds that enhance its degradation and/or inhibit its transcription, suppression of antiapoptotic proteins by small-molecule antagonists or interfering RNAs, inhibitionof multidrug transporters by their chemical inhibitors, and inhibition of the PI3K/AKT pathway have all been shown to trigger cell death in CSCs that are resistant to conventional therapy. Moreover, while dysregulated miRNAs have been implicated in CSC stemness and tumorigenesis, proapoptotic miRNAs and miR-mimics have been discovered that potentially can be used to target CSCs. Activation of apoptosis programs in CSCs appears to make these cells more sensitive to current anticancer regimens and novel drugs designed or identified to target CSCs, and may open new strategies for effective cancer treatment.
While significant progress has been made toward identification, characterization, and understanding of the signaling pathways of CSCs, future research should be conducted to (a) determine the specific mechanisms of aberrant apoptotic programming that govern resistance to apoptosis in these cells; (b) explore whether the CSCs of each tumor type express various mechanisms of apoptosis resistance and whether different tumor types express common or different mechanisms of apoptotic resistance; (c) characterize what mechanisms of resistance to apoptosis evolve in CSCs treated with chemotherapy drugs and in therapy-induced dedifferentiation, resulting in the generation of CSCs that may behave differently than the initial CSCs within a tumor; (d) determine whether apoptosis-targeted therapies develop resistance in CSCs by mechanisms similar to those observed in conventional chemotherapy; and (e) develop strategies to combine conventional chemotherapy with CSC-targeted therapy to effectively trigger cell death in CSCs and eradicate them with acceptable toxicity to normal cells. Delineating these points may provide novel therapeutic approaches to triggering maximum cell death in the entire tumor cell population and better managing cancer therapy.
Acknowledgments
I would like to thank Dr. Mary D. Kraeszig for her excellent editorial assistance. The apoptosis and drug resistance research in the author’s laboratory was supported by research grants from the National Cancer Institute (CA 080734, CA 90878, CA-56078, and CA 101743.
ABBREVIATIONS
- AIC
apoptosis inhibitory complex
- Apaf-1
apoptotic proteinase-activating factor-1
- BH3
Bcl-2 homology domain-3
- c-FLIP
cellular FLICE (FADD-like IL-1β-converting enzyme inhibitory protein
- CSCs
cancer stem cells
- DED
death effector domain
- DISC
death-inducing signaling complex
- DNA-PK
DNA-dependent protein kinase
- DR5
death receptor 5
- ER
endoplasmic reticulum
- FADD
Fas-associated via death domain
- GBM
glioblastoma multiforme
- GSCs
GBM stem cells
- MELK
maternal embryonic leucine-zipper kinase
- Nrf2
nuclear factor erythroid 2-related factor 2
- PDA
pancreatic ductal adenocarcinoma
- PERK
protein kinase RNA-like ER kinase
- P-gp
P-glycoprotein
- PTP
permeability transition pore
- TNFR1
TNF receptor 1
- TNF-α
tumor necrosis factor-α
- TRADD
TNFR1-associated death domain protein
- TRAIL
TNF-related apoptosis- inducing ligand
References
- 1.Ween MP, Armstrong MA, Oehler MK, Ricciardelli C. The role of ABC transporters in ovarian cancer progression and chemoresistance. Crit Rev Oncol Hematol. 2015;96(2):220–56. doi: 10.1016/j.critrevonc.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Roberti A, La Sala D, Cinti C. Multiple genetic and epigenetic interacting mechanisms contribute to clonally selection of drug-resistant tumors: current views and new therapeutic prospective. J Cell Physiol. 2006;207(3):571–81. doi: 10.1002/jcp.20515. [DOI] [PubMed] [Google Scholar]
- 3.Delbridge AR, Strasser A. The Bcl-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015;22(7):1071–80. doi: 10.1038/cdd.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang F, Lin J, Xu R. The molecular mechanisms of TRAIL resistance in cancer cells: help in designing new drugs. Curr Pharm Des. 2014;20(42):6714–22. doi: 10.2174/1381612820666140929100735. [DOI] [PubMed] [Google Scholar]
- 5.Tang KD, Ling MT. Targeting drug-resistant prostate cancer with dual PI3K/mTOR inhibition. Curr Med Chem. 2014;21(26):3048–56. doi: 10.2174/0929867321666140414100127. [DOI] [PubMed] [Google Scholar]
- 6.Koff JL, Ramachandiran S, Bernal-Mizrachi L. A time to kill: targeting apoptosis in cancer. Int J Mol Sci. 2015;16(2):2942–55. doi: 10.3390/ijms16022942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fulda S. Targeting apoptosis for anticancer therapy. Semin Cancer Biol. 2015;31:84–8. doi: 10.1016/j.semcancer.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Zang F, Wei X, Leng X, Yu M, Sun B. c-FLIP(L) contributes to TRAIL resistance in HER2-positive breast cancer. Biochem Biophys Res Commun. 2014;450(1):267–73. doi: 10.1016/j.bbrc.2014.05.106. [DOI] [PubMed] [Google Scholar]
- 9.Safa AR, Pollok KE. Targeting the anti-apoptotic protein c-FLIP for cancer therapy. Cancers (Basel) 2011;3(2):1639–71. doi: 10.3390/cancers3021639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adorno-Cruz V, Kibria G, Liu X, Doherty M, Junk DJ, Guan D, Hubert C, Venere M, Mulkearns-Hubert E, Sinyuk M, Alvarado A, Caplan AI, Rich J, Gerson SL, Lathia J, Liu H. Cancer stem cells: targeting the roots of cancer, seeds of metastasis, and sources of therapy resistance. Cancer Res. 2015;75(6):924–9. doi: 10.1158/0008-5472.CAN-14-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan IN, Al-Karim S, Bora RS, Chaudhary AG, Saini KS. Cancer stem cells: a challenging paradigm for designing targeted drug therapies. Drug Discov Today. 2015;20(10):1205–16. doi: 10.1016/j.drudis.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Sørensen MD, Fosmark S, Hellwege S, Beier D, Kristensen BW, Beier CP. Chemoresistance and chemotherapy targeting stem-like cells in malignant glioma. Adv Exp Med Biol. 2015;853:111–38. doi: 10.1007/978-3-319-16537-0_7. [DOI] [PubMed] [Google Scholar]
- 13.Safa AR, Saadatzadeh MR, Cohen-Gadol AA, Pollok KE, Bijangi-Vishehsaraei K. Glioblastoma stem cells (GSCs) epigenetic plasticity and interconversion between differentiated non-GSCs and GSCs. Genes Dis. 2015;2(2):152–63. doi: 10.1016/j.gendis.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foo J, Michor F. Evolution of acquired resistance to anti-cancer therapy. J Theor Biol. 2014;355:10–20. doi: 10.1016/j.jtbi.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrido W, Rocha JD, Jaramillo C, Fernandez K, Oyarzun C, San Martin R, Quezada C. Chemoresistance in high-grade gliomas: relevance of adenosine signalling in stem-like cells of glioblastoma multiforme. Curr Drug Targets. 2014;15(10):931–42. [PubMed] [Google Scholar]
- 16.Liu H, Lv L, Yang K. Chemotherapy targeting cancer stem cells. Am J Cancer Res. 2015;5(3):880–93. [PMC free article] [PubMed] [Google Scholar]
- 17.Yadav N, Chandra D. Mitochondrial and postmitochondrial survival signaling in cancer. Mitochondrion. 2014;16:18–25. doi: 10.1016/j.mito.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkmann N, Marassi FM, Newmeyer DD, Hanein D. The rheostat in the membrane: Bcl-2 family proteins and apoptosis. Cell Death Differ. 2014;21(2):206–15. doi: 10.1038/cdd.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safa AR. Roles of c-FLIP in apoptosis, necroptosis, and autophagy. J Carcinog Mutagen. 2013;(Suppl 6) doi: 10.4172/2157-2518.S6-003. pii:003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opferman JT. Attacking cancer’s Achilles heel: antagonism of anti-apoptotic Bcl-2 family members. FEBS J. 2016;283(14):2661–75. doi: 10.1111/febs.13472. Epub 2015 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vela L, Marzo I. Bcl-2 family of proteins as drug targets for cancer chemotherapy: the long way of BH3 mimetics from bench to bedside. Curr Opin Pharmacol. 2015;23:74–81. doi: 10.1016/j.coph.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Correia C, Lee SH, Meng XW, Vincelette ND, Knorr KL, Ding H, Nowakowski GS, Dai H, Kaufmann SH. Emerging understanding of Bcl-2 biology: implications for neoplastic progression and treatment. Biochim Biophys Acta. 2015;1853(7):1658–71. doi: 10.1016/j.bbamcr.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Y-C, Zhou F-L, Shen Y, Liao D-F, Cao D. Apoptotic death of cancer stem cells for cancer therapy. Int J Mol Sci. 2014;15(5):8335–51. doi: 10.3390/ijms15058335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catalano V, Gaggianesi M, Spina V, Iovino F, Dieli F, Stassi G, Todaro M. Colorectal cancer stem cells and cell death. Cancers (Basel) 2011;3(2):1929–46. doi: 10.3390/cancers3021929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ajani JA, Song S, Hochster HS, Steinberg IB. Cancer stem cells: the promise and the potential. Semin Oncol. 2015;42(Suppl 1):S3–17. doi: 10.1053/j.seminoncol.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Kim JK, Jeon HY, Kim H. The molecular mechanisms underlying the therapeutic resistance of cancer stem cells. Arch Pharm Res. 2015;38(3):389–401. doi: 10.1007/s12272-014-0531-1. [DOI] [PubMed] [Google Scholar]
- 27.Dean M. ABC transporters, drug resistance, and cancer stem cells. J Mammary Gland Biol Neoplasia. 2009;14(1):3–9. doi: 10.1007/s10911-009-9109-9. [DOI] [PubMed] [Google Scholar]
- 28.Rassouli FB, Matin MM, Saeinasab M. Cancer stem cells in human digestive tract malignancies. Tumour Biol. 2016;37(1):7–21. doi: 10.1007/s13277-015-4155-y. Epub 2015 Oct 7. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed SU, Carruthers R, Gilmour L, Yildirim S, Watts C, Chalmers AJ. Selective inhibition of parallel DNA damage response pathways optimizes radiosensitization of glioblastoma stemlike cells. Cancer Res. 2015;75(20):4416–28. doi: 10.1158/0008-5472.CAN-14-3790. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida GJ, Saya H. Therapeutic strategies targeting cancer stem cells. Cancer Sci. 2016;107(1):5–11. doi: 10.1111/cas.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suresh R, Ali S, Ahmad A, Philip PA, Sarkar FH. The role of cancer stem cells in recurrent and drug-resistant lung cancer. Adv Exp Med Biol. 2016;890:57–74. doi: 10.1007/978-3-319-24932-2_4. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y-H, Scadden DT. Harnessing the apoptotic programs in cancer stem-like cells. EMBO Rep. 2015;16(9):1084–98. doi: 10.15252/embr.201439675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kise K, Kinugasa-Katayama Y, Takakura N. Tumor microenvironment for cancer stem cells. Adv Drug Deliv Rev. 2016;99(Pt B):197–205. doi: 10.1016/j.addr.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Marhold M, Tomasich E, El-Gazzar A, Heller G, Spittler A, Horvat R, Krainer M, Horak P. HIF1α regulates mTOR signaling and viability of prostate cancer stem cells. Mol Cancer Res. 2015;13(3):556–64. doi: 10.1158/1541-7786.MCR-14-0153-T. [DOI] [PubMed] [Google Scholar]
- 35.Xia P, Xu XY. PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. Am J Cancer Res. 2015;5(5):1602–9. [PMC free article] [PubMed] [Google Scholar]
- 36.Nakano I. Transcription factors as master regulator for cancer stemness: remove milk from fox? Expert Rev Anticancer Ther. 2014;14(8):873–5. doi: 10.1586/14737140.2014.940324. [DOI] [PubMed] [Google Scholar]
- 37.Hong IS, Jang GB, Lee HY, Nam JS. Targeting cancer stem cells by using the nanoparticles. Int J Nanomedicine. 2015;10(Spec Iss):251–60. doi: 10.2147/IJN.S88310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olmez I, Shen W, McDonald H, Ozpolat B. Dedifferentiation of patient-derived glioblastoma multiforme cell lines results in a cancer stem cell-like state with mitogen-independent growth. J Cell Mol Med. 2015;19(6):1262–72. doi: 10.1111/jcmm.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iriondo O, Rábano M, Domenici G, Carlevaris O, López-Ruiz JA, Zabalza I, Berra E, Vivanco MD. Distinct breast cancer stem/progenitor cell populations require either HIF1α or loss of PHD3 to expand under hypoxic conditions. Oncotarget. 2015;6(31):31721–39. doi: 10.18632/oncotarget.5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Vecchio CA, Feng Y, Sokol ES, Tillman EJ, Sanduja S, Reinhardt F, Gupta PB. Dedifferentiation confers multidrug resistance via noncanonical PERK-Nrf2 signaling. PLoS Biol. 2014;12(9):e1001945. doi: 10.1371/journal.pbio.1001945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong X, Safa AR. Phosphorylation of RNA helicase A by DNA-dependent protein kinase is indispensable for expression of the MDR1 gene product P-glycoprotein in multidrug-resistant human leukemia cells. Biochemistry. 2007;46(19):5766–75. doi: 10.1021/bi700063b. [DOI] [PubMed] [Google Scholar]
- 42.Xi G, Hayes E, Lewis R, Ichi S, Mania-Farnell B, Shim K, Takao T, Allender E, Mayanil CS, Tomita T. CD133 and DNA-PK regulate MDR1 via the PI3K- or Akt-NF-κB pathway in multidrugresistant glioblastoma cells in vitro. Oncogene. 2016;35(2):241–50. doi: 10.1038/onc.2015.78. [DOI] [PubMed] [Google Scholar]
- 43.Ganguly R, Mohyeldin A, Thiel J, Kornblum HI, Beullens M, Nakano I. MELK-a conserved kinase: functions, signaling, cancer, and controversy. Clin Transl Med. 2015;4:11. doi: 10.1186/s40169-014-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim SH, Joshi K, Ezhilarasan R, Myers TR, Siu J, Gu C, Nakano-Okuno M, Taylor D, Minata M, Sulman EP, Lee J, Bhat KP, Salcini AE, Nakano I. EZH2 protects glioma stem cells from radiation-induced cell death in a MELK/FOXM1-dependent manner. Stem Cell Reports. 2015;4(2):226–38. doi: 10.1016/j.stemcr.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho YS, Kang Y, Kim K, Cha YJ, Cho HS. The crystal structure of MPK38 in complex with OTSSP167, an orally administrative MELK selective inhibitor. Biochem Biophys Res Commun. 2014;447(1):7–11. doi: 10.1016/j.bbrc.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 46.Boland ML, Chourasia AH, Macleod KF. Mitochondrial dysfunction in cancer. Front Oncol. 2013;3:292. doi: 10.3389/fonc.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chandrasekaran S, Marshall JR, Messing JA, Hsu JW, King MR. TRAIL-mediated apoptosis in breast cancer cells cultured as 3D spheroids. PLoS One. 2014;9(10):e111487. doi: 10.1371/journal.pone.0111487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding L, Yuan C, Wei F, Wang G, Zhang J, Bellail AC, Zhang Z, Olson JJ, Hao C. Cisplatin restores TRAIL apoptotic pathway in glioblastoma-derived stem cells through up-regulation of DR5 and down-regulation of c-FLIP. Cancer Invest. 2011;29(8):511–20. doi: 10.3109/07357907.2011.605412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie ZY, Lv K, Xiong Y, Guo WH. ABCG2-meditated multidrug resistance and tumor-initiating capacity of side population cells from colon cancer. Oncol Res Treat. 2014;37(11):666–8. doi: 10.1159/000368842. [DOI] [PubMed] [Google Scholar]
- 50.Wang D, Zhu H, Zhu Y, Liu Y, Shen H, Yin R, Zhang Z, Su Z. CD133(+)/CD44(+)/Oct4(+)/Nestin(+) stem-like cells isolated from Panc-1 cell line may contribute to multi-resistance and metastasis of pancreatic cancer. Acta Histochem. 2013;115(4):349–56. doi: 10.1016/j.acthis.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Rentala S, Mangamoori LN. Isolation, characterization and mobilization of prostate cancer tissue derived CD133+ MDR1+ cells. J Stem Cells. 2010;5(2):75–81. [PubMed] [Google Scholar]
- 52.Nakai E, Park K, Yawata T, Chihara T, Kumazawa A, Nakabayashi H, Shimizu K. Enhanced MDR1 expression and chemoresistance of cancer stem cells derived from glioblastoma. Cancer Invest. 2009;27(9):901–8. doi: 10.3109/07357900801946679. [DOI] [PubMed] [Google Scholar]
- 53.Stacy AE, Jansson PJ, Richardson DR. Molecular pharmacology of ABCG2 and its role in chemoresistance. Mol Pharmacol. 2013;84(5):655–69. doi: 10.1124/mol.113.088609. [DOI] [PubMed] [Google Scholar]
- 54.Wilson BJ, Saab KR, Ma J, Schatton T, Pütz P, Zhan Q, Murphy GF, Gasser M, Waaga-Gasser AM, Frank NY, Frank MH. ABCB5 maintains melanoma-initiating cells through a proinflammatory cytokine signaling circuit. Cancer Res. 2014;74(15):4196–207. doi: 10.1158/0008-5472.CAN-14-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Wang SX, Ma JW, Li HY, Ye JC, Xie SM, Du B, Zhong XY. EGCG inhibits properties of glioma stem-like cells and synergizes with temozolomide through downregulation of P-glycoprotein inhibition. J Neurooncol. 2015;121(1):41–52. doi: 10.1007/s11060-014-1604-1. [DOI] [PubMed] [Google Scholar]
- 56.Sharma N, Nanta R, Sharma J, Gunewardena S, Singh KP, Shankar S, Srivastava RK. PI3K/AKT/mTOR and sonic hedgehog pathways cooperate together to inhibit human pancreatic cancer stem cell characteristics and tumor growth. Oncotarget. 2015;6(31):32039–60. doi: 10.18632/oncotarget.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Q, Rycaj K, Chen X, Tang DG. Cancer stem cells and cell size: a causal link? Semin Cancer Biol. 2015;35:191–9. doi: 10.1016/j.semcancer.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aldea MD, Petrushev B, Soritau O, Tomuleasa CI, Berindan-Neagoe I, Filip AG, Chereches G, Cenariu M, Craciun L, Tatomir C, Florian IS, Crivii CB, Kacso G. Metformin plus sorafenib highly impacts temozolomide resistant glioblastoma stem-like cells. J BUON. 2014;19(2):502–11. [PubMed] [Google Scholar]
- 59.Chen J, Shao R, Li F, Monteiro M, Liu JP, Xu ZP, Gu W. PI3K/Akt/mTOR pathway dual inhibitor BEZ235 suppresses the stemness of colon cancer stem cells. Clin Exp Pharmacol Physiol. 2015;42(12):1317–26. doi: 10.1111/1440-1681.12493. [DOI] [PubMed] [Google Scholar]
- 60.Tagscherer KE, Fassl A, Campos B, Farhadi M, Kraemer A, Böck BC, Macher-Goeppinger S, Radlwimmer B, Wiestler OD, Herold-Mende C, Roth W. Apoptosis-based treatment of glioblastomas with ABT-737, a novel small molecule inhibitor of Bcl-2 family proteins. Oncogene. 2008;27(52):6646–56. doi: 10.1038/onc.2008.259. [DOI] [PubMed] [Google Scholar]
- 61.Bhujade A, Gupta G, Talmale S, Das SK, Patil MB. Induction of apoptosis in A431 skin cancer cells by Cissus quadrangularis Linn stem extract by altering Bax-Bcl-2 ratio, release of cytochrome c from mitochondria and PARP cleavage. Food Funct. 2013;4(2):338–46. doi: 10.1039/c2fo30167a. [DOI] [PubMed] [Google Scholar]
- 62.Konopleva M, Zhao S, Hu W, Jiang S, Snell V, Weidner D, Jackson CE, Zhang X, Champlin R, Estey E, Reed JC, Andreeff M. The anti-apoptotic genes Bcl-X(L) and Bcl-2 are over-expressed and contribute to chemoresistance of non-proliferating leukaemic CD34+ cells. Br J Haematol. 2002;118(2):521–34. doi: 10.1046/j.1365-2141.2002.03637.x. [DOI] [PubMed] [Google Scholar]
- 63.Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM, Maecker H, O’Rourke K, Bazan F, Eastham-Anderson J, Yue P, Dornan D, Huang DC, Dixit VM. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature. 2010;463(7277):103–7. doi: 10.1038/nature08646. [DOI] [PubMed] [Google Scholar]
- 64.Jia Y, Chen J, Zhu H, Jia ZH, Cui MH. Aberrantly elevated redox sensing factor Nrf2 promotes cancer stem cell survival via enhanced transcriptional regulation of ABCG2 and Bcl-2/Bmi-1 genes. Oncol Rep. 2015;34(5):2296–304. doi: 10.3892/or.2015.4214. [DOI] [PubMed] [Google Scholar]
- 65.Wajant H, Scheurich P. TNFR1-induced activation of the classical NF-κB pathway. FEBS J. 2011;278(6):862–76. doi: 10.1111/j.1742-4658.2011.08015.x. [DOI] [PubMed] [Google Scholar]
- 66.Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010;29(34):4752–65. doi: 10.1038/onc.2010.221. [DOI] [PubMed] [Google Scholar]
- 67.Chakraborty S, Li L, Tang H, Xie Y, Puliyappadamba VT, Raisanen J, Chakraborty S, Li L, Tang H, Xie Y, Puliyappadamba VT, Raisanen J, Burma S, Boothman DA, Cochran B, Wu J, Habib AA. Cytoplasmic TRADD confers a worse prognosis in glioblastoma. Neoplasia. 2013;15(8):888–97. doi: 10.1593/neo.13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Birnie R, Bryce SD, Roome C, Dussupt V, Droop A, Lang SH, Berry PA, Hyde CF, Lewis JL, Stower MJ, Maitland NJ, Collins AT. Gene expression profiling of human prostate cancer stem cells reveals a proinflammatory phenotype and the importance of extracellular matrix interactions. Genome Biol. 2008;9(5):R83. doi: 10.1186/gb-2008-9-5-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou J, Zhang H, Gu P, Bai J, Margolick JB, Zhang Y. NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat. 2008;111(3):419–27. doi: 10.1007/s10549-007-9798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eckelman BP, Salvesen GS. The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J Biol Chem. 2006;281(6):3254–60. doi: 10.1074/jbc.M510863200. [DOI] [PubMed] [Google Scholar]
- 71.Budhidarmo R, Day CL. IAPs: modular regulators of cell signalling. Semin Cell Dev Biol. 2015;39:80–90. doi: 10.1016/j.semcdb.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 72.Fulda S. Smac mimetics as IAP antagonists. Semin Cell Dev Biol. 2015;39:132–8. doi: 10.1016/j.semcdb.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 73.Obexer P, Ausserlechner MJ. X-linked inhibitor of apoptosis protein—a critical death resistance regulator and therapeutic target for personalized cancer therapy. Front Oncol. 2014;4:197. doi: 10.3389/fonc.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Selmi T, Alecci C, dell’ Aquila M, Montorsi L, Martello A, Guizzetti F, Volpi N, Parenti S, Ferrari S, Salomoni P, Grande A, Zanocco-Marani T. ZFP36 stabilizes RIP1 via degradation of XIAP and cIAP2 thereby promoting ripoptosome assembly. BMC Cancer. 2015;15:357. doi: 10.1186/s12885-015-1388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu MS, Wang GF, Zhao ZQ, Liang Y, Wang HB, Wu MY, Min P, Chen LZ, Feng QS, Bei JX, Zeng YX, Yang D. Smac mimetics in combination with TRAIL selectively target cancer stem cells in nasopharyngeal carcinoma. Mol Cancer Ther. 2013;12(9):1728–37. doi: 10.1158/1535-7163.MCT-13-0017. [DOI] [PubMed] [Google Scholar]
- 76.Lee MR, Ji SY, Mia-Jan K, Cho MY. Chemoresistance of CD133(+) colon cancer may be related with increased survivin expression. Biochem Biophys Res Commun. 2015;463(3):229–34. doi: 10.1016/j.bbrc.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 77.Safa AR. c-FLIP, a master anti-apoptotic regulator. Exp Oncol. 2012;34(3):176–84. [PMC free article] [PubMed] [Google Scholar]
- 78.Safa AR, Day TW, Wu CH. Cellular FLICE-like inhibitory protein (c-FLIP): a novel target for cancer therapy. Curr Cancer Drug Targets. 2008;8(1):37–46. doi: 10.2174/156800908783497087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Piggott L, Omidvar N, Martí Pérez S, French R, Eberl M, Clarkson RW. Suppression of apoptosis inhibitor c-FLIP selectively eliminates breast cancer stem cell activity in response to the anti-cancer agent, TRAIL. Breast Cancer Res. 2011;13(5):R88. doi: 10.1186/bcr2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hashimoto N, Tsunedomi R, Yoshimura K, Watanabe Y, Hazama S, Oka M. Cancer stem-like sphere cells induced from de-differentiated hepatocellular carcinoma-derived cell lines possess the resistance to anti-cancer drugs. BMC Cancer. 2014;14:722. doi: 10.1186/1471-2407-14-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu H, Samanta D, Xiang L, Zhang H, Hu H, Chen I, Bullen JW, Semenza GL. Chemotherapy triggers HIF-1-dependent glutathione synthesis and copper chelation that induces the breast cancer stem cell phenotype. Proc Natl Acad Sci U S A. 2015;112(33):E4600–9. doi: 10.1073/pnas.1513433112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Samanta D, Gilkes DM, Chaturvedi P, Xiang L, Semenza GL. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc Natl Acad Sci U S A. 2014;111(50):E5429–38. doi: 10.1073/pnas.1421438111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Auffinger B, Tobias AL, Han Y, Lee G, Guo D, Dey M, Lesniak MS, Ahmed AU. Conversion of differentiated cancer cells into cancer stem-like cells in a glioblastoma model after primary chemotherapy. Cell Death Differ. 2014;21(7):1119–31. doi: 10.1038/cdd.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qi L, Ren K, Fang F, Zhao DH, Yang NJ, Li Y. Over expression of BCL2 and low expression of caspase 8 related to TRAIL resistance in brain cancer stem cells. Asian Pac J Cancer Prev. 2015;16(12):4849–52. doi: 10.7314/apjcp.2015.16.12.4849. [DOI] [PubMed] [Google Scholar]
- 85.Murphy ÁC, Weyhenmeyer B, Noonan J, Kilbride SM, Schimansky S, Loh KP, Kögel D, Letai AG, Prehn JH, Murphy BM. Modulation of Mcl-1 sensitizes glioblastoma to TRAIL-induced apoptosis. Apoptosis. 2014;19(4):629–42. doi: 10.1007/s10495-013-0935-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Day TW, Najafi F, Wu CH, Safa AR. Cellular FLICE-like inhibitory protein (c-FLIP): a novel target for Taxol-induced apoptosis. Biochem Pharmacol. 2006;71(11):1551–61. doi: 10.1016/j.bcp.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 87.Haag C, Stadel D, Zhou S, Bachem MG, Möller P, Debatin KM, Fulda S. Identification of c-FLIP(L) and c-FLIP(S) as critical regulators of death receptor-induced apoptosis in pancreatic cancer cells. Gut. 2011;60(2):225–37. doi: 10.1136/gut.2009.202325. [DOI] [PubMed] [Google Scholar]
- 88.Yoon MJ, Kang YJ, Kim IY, Kim EH, Lee JA, Lim JH, Kwon TK, Choi KS. Monensin, a polyether ionophore antibiotic, overcomes TRAIL resistance in glioma cells via endoplasmic reticulum stress, DR5 upregulation and c-FLIP downregulation. Carcinogenesis. 2013;34(8):1918–28. doi: 10.1093/carcin/bgt137. [DOI] [PubMed] [Google Scholar]
- 89.Zhang C, Tian Y, Song F, Fu C, Han B, Wang Y. Salinomycin inhibits the growth of colorectal carcinoma by targeting tumor stem cells. Oncol Rep. 2015;34(5):2469–76. doi: 10.3892/or.2015.4253. [DOI] [PubMed] [Google Scholar]
- 90.Ning X, Shu J, Du Y, Ben Q, Li Z. Therapeutic strategies targeting cancer stem cells. Cancer Biol Ther. 2013;14(4):295–303. doi: 10.4161/cbt.23622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parajuli B, Shin SJ, Kwon SH, Cha SD, Chung R, Park WJ, Lee HG, Cho CH. Salinomycin induces apoptosis via death receptor-5 up-regulation in cisplatin-resistant ovarian cancer cells. Anticancer Res. 2013;33(4):1457–62. [PubMed] [Google Scholar]
- 92.Rajeshkumar NV, Rasheed ZA, García-García E, López-Ríos F, Fujiwara K, Matsui WH, Hidalgo M. A combination of DR5 agonistic monoclonal antibody with gem-citabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model. Mol Cancer Ther. 2010;9(9):2582–92. doi: 10.1158/1535-7163.MCT-10-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Filipova A, Seifrtova M, Mokry J, Dvorak J, Rezacova M, Filip S, Diaz-Garcia D. Breast cancer and cancer stem cells: a mini-review. Tumori. 2014;100(4):363–9. doi: 10.1700/1636.17886. [DOI] [PubMed] [Google Scholar]
- 94.Zhou W, Xu J, Gelston E, Wu X, Zou Z, Wang B, Zeng Y, Wang H, Liu A, Xu L, Liu Q. Inhibition of Bcl-xL overcomes polyploidy resistance and leads to apoptotic cell death in acute myeloid leukemia cells. Oncotarget. 2015;6(25):21557–71. doi: 10.18632/oncotarget.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pareja F, Macleod D, Shu C, Crary JF, Canoll PD, Ross AH, Siegelin MD. PI3K and Bcl-2 inhibition primes glioblastoma cells to apoptosis through downregulation of Mcl-1 and phospho-BAD. Mol Cancer Res. 2014;12(7):987–1001. doi: 10.1158/1541-7786.MCR-13-0650. [DOI] [PubMed] [Google Scholar]
- 96.Norris DA, Shellman YG. Combining a BCL2 inhibitor with the retinoid derivative fenretinide targets melanoma cells including melanoma initiating cells. J Invest Dermatol. 2015;135(3):842–50. doi: 10.1038/jid.2014.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karpel-Massler G, Shu C, Chau L, Banu M, Halatsch ME, Westhoff MA, Ramirez Y, Ross AH, Bruce JN, Canoll P, Siegelin MD. Combined inhibition of Bcl-2/Bcl-xL and Usp9X/Bag3 overcomes apoptotic resistance in glioblastoma in vitro and in vivo. Oncotarget. 2015;6(16):14507–21. doi: 10.18632/oncotarget.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen Q, Song S, Wei S, Liu B, Honjo S, Scott A, Jin J, Ma L, Zhu H, Skinner HD, Johnson RL, Ajani JA. ABT-263 induces apoptosis and synergizes with chemotherapy by targeting stemness pathways in esophageal cancer. Oncotarget. 2015;6(28):25883–96. doi: 10.18632/oncotarget.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Berghauser Pont LM, Spoor JK, Venkatesan S, Swagemakers S, Kloezeman JJ, Dirven CM, van der Spek PJ, Lamfers ML, Leenstra S. The Bcl-2 inhibitor Obatoclax overcomes resistance to histone deacetylase inhibitors SAHA and LBH589 as radiosensitizers in patient-derived glioblastoma stem-like cells. Genes Cancer. 2014;5(11–12):445–59. doi: 10.18632/genesandcancer.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15(6):321–33. doi: 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oneyama C, Okada M. MicroRNAs as the fine-tuners of Src oncogenic signalling. J Biochem. 2015;157(6):431–8. doi: 10.1093/jb/mvv036. [DOI] [PubMed] [Google Scholar]
- 102.Takasaki S. Roles of microRNAs in cancers and development. Methods Mol Biol. 2015;1218:375–413. doi: 10.1007/978-1-4939-1538-5_24. [DOI] [PubMed] [Google Scholar]
- 103.Kaboli PJ, Rahmat A, Ismail P, Ling KH. MicroRNA-based therapy and breast cancer: a comprehensive review of novel therapeutic strategies from diagnosis to treatment. Pharmacol Res. 2015;97:104–21. doi: 10.1016/j.phrs.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 104.Garg N, Vijayakumar T, Bakhshinyan D, Venugopal C, Singh SK. MicroRNA regulation of brain tumor initiating cells in central nervous system tumours. Stem Cells Int. 2015;2015:141793. doi: 10.1155/2015/141793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jhanwar-Uniyal M, Labagnara M, Friedman M, Kwasnicki A, Murali R. Glioblastoma: molecular pathways, stem cells and therapeutic targets. Cancers (Basel) 2015;7(2):538–55. doi: 10.3390/cancers7020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Garofalo M, Croce CM. Role of microRNAs in maintaining cancer stem cells. Adv Drug Deliv Rev. 2015;81:53–61. doi: 10.1016/j.addr.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Swartling FJ, Čančer M, Frantz A, Weishaupt H, Persson AI. Deregulated proliferation and differentiation in brain tumors. Cell Tissue Res. 2015;359(1):225–54. doi: 10.1007/s00441-014-2046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takahashi RU, Miyazaki H, Ochiya T. The role of microRNAs in the regulation of cancer stem cells. Front Genet. 2014;4:295. doi: 10.3389/fgene.2013.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lima RT, Busacca S, Almeida GM, Gaudino G, Fennell DA, Vasconcelos MH. MicroRNA regulation of core apoptosis pathways in cancer. Eur J Cancer. 2011;47(2):163–74. doi: 10.1016/j.ejca.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 110.Liu J, Albrecht AM, Ni X, Yang J, Li M. Glioblastoma tumor initiating cells: therapeutic strategies targeting apoptosis and microRNA pathways. Curr Mol Med. 2013;13(3):352–7. [PubMed] [Google Scholar]
- 111.Willimott S, Wagner SD. Post-transcriptional and post-translational regulation of Bcl2. Biochem Soc Trans. 2010;38(6):1571–5. doi: 10.1042/BST0381571. [DOI] [PubMed] [Google Scholar]
- 112.Chen F, Zhu HH, Zhou LF, Wu SS, Wang J, Chen Z. Inhibition of c-FLIP expression by miR-512-3p contributes to taxol-induced apoptosis in hepatocellular carcinoma cells. Oncol Rep. 2010;23(5):1457–62. doi: 10.3892/or_00000784. [DOI] [PubMed] [Google Scholar]
- 113.Misso G, Di Martino MT, De Rosa G, Farooqi AA, Lombardi A, Campani V, Zarone MR, Gullà A, Tagliaferri P, Tassone P, Caraglia M. Mir-34: a new weapon against cancer? Mol Ther Nucleic Acids. 2014;3:e194. doi: 10.1038/mtna.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rokavec M, Li H, Jiang L, Hermeking H. The p53/miR-34 axis in development and disease. J Mol Cell Biol. 2014;6(3):214–30. doi: 10.1093/jmcb/mju003. [DOI] [PubMed] [Google Scholar]
- 115.Rathod SS, Rani SB, Khan M, Muzumdar D, Shiras A. Tumor suppressive miRNA-34a suppresses cell proliferation and tumor growth of glioma stem cells by targeting Akt and Wnt signaling pathways. FEBS Open Bio. 2014;4:485–95. doi: 10.1016/j.fob.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ogretmen B, Safa AR. Expression of the mutated p53 tumor suppressor protein and its molecular and biochemical characterization in multidrug resistant MCF-7/Adr human breast cancer cells. Oncogene. 1997;14(4):499–506. doi: 10.1038/sj.onc.1200855. [DOI] [PubMed] [Google Scholar]
- 117.Park EY, Chang E, Lee EJ, Lee HW, Kang HG, Chun KH, Woo YM, Kong HK, Ko JY, Suzuki H, Song E, Park JH. Targeting of miR34a-NOTCH1 axis reduced breast cancer stemness and chemoresistance. Cancer Res. 2014;74(24):7573–82. doi: 10.1158/0008-5472.CAN-14-1140. [DOI] [PubMed] [Google Scholar]
- 118.Daige CL, Wiggins JF, Priddy L, Nelligan-Davis T, Zhao J, Brown D. Systemic delivery of a miR34a mimic as a potential therapeutic for liver cancer. Mol Cancer Ther. 2014;13(10):2352–60. doi: 10.1158/1535-7163.MCT-14-0209. [DOI] [PubMed] [Google Scholar]
- 119.Floyd DH, Zhang Y, Dey BK, Kefas B, Breit H, Marks K, Dutta A, Herold-Mende C, Synowitz M, Glass R, Abounader R, Purow BW. Novel anti-apoptotic microRNAs 582-5p and 363 promote human glioblastoma stem cell survival via direct inhibition of caspase 3, caspase 9, and Bim. PLoS One. 2014;9(5):e96239. doi: 10.1371/journal.pone.0096239. [DOI] [PMC free article] [PubMed] [Google Scholar]