Abstract
It is not clear whether alcohol consumption is associated with lung cancer risk. The relationship is likely confounded by smoking, complicating the interpretation of previous studies. We examined the association of alcohol consumption and lung cancer risk in a large pooled international sample, minimizing potential confounding of tobacco consumption by restricting analyses to never smokers. Our study included 22 case-control and cohort studies with a total of 2548 never-smoking lung cancer patients and 9362 never-smoking controls from North America, Europe and Asia within the International Lung Cancer Consortium (ILCCO) and SYNERGY Consortium. Alcohol consumption was categorized into amounts consumed (grams per day) and also modelled as a continuous variable using restricted cubic splines for potential non-linearity. Analyses by histologic sub-type were included. Associations by type of alcohol consumed (wine, beer and liquor) were also investigated. Alcohol consumption was inversely associated with lung cancer risk with evidence most strongly supporting lower risk for light and moderate drinkers relative to non-drinkers (>0–4.9g per day: OR=0.80, 95% CI=0.70–0.90; 5–9.9g per day: OR=0.82, 95% CI=0.69–0.99; 10–19.9g per day: OR=0.79, 95% CI=0.65–0.96). Inverse associations were found for consumption of wine and liquor, but not beer. The results indicate that alcohol consumption is inversely associated with lung cancer risk, particularly among subjects with low to moderate consumption levels, and among wine and liquor drinkers, but not beer drinkers. Although our results should have no relevant bias from the confounding effect of smoking we cannot preclude that confounding by other factors contributed to the observed associations. Confounding in relation to the non-drinker reference category may be of particular importance.
Keywords: Alcohol, lung cancer, wine, beer, liquor
Introduction
Lung cancer continues to be the most common cancer and the leading cause of cancer death worldwide, with 1.8 million new cases and 1.6 million deaths reported annually.1 Tobacco smoking is the primary cause of lung cancer accounting for more than 80% of all lung cancer diagnoses.2 Other known risk factors include exposure to occupational and environmental carcinogens such as asbestos and radon.3, 4 Although less common than lung cancer in smokers, lung cancer among never smokers still impacts a significant portion of the population, and is recognized as the 7th most common cause of cancer mortality worldwide.5
Alcohol is classified as a Group 1 carcinogen by the International Agency for Research on Cancer, and it has been hypothesized that alcohol consumption may modulate lung cancer risk. However, definite conclusions could not be drawn from previous epidemiologic investigations due to inconsistent results across studies.6–9 Since alcohol intake is strongly correlated with tobacco smoking,9 the confounding effect poses the main methodological challenge when investigating alcohol consumption and lung cancer risk. Although few previous studies have investigated the association between alcohol consumption and lung cancer risk in never smokers, they were limited in precision. Furthermore, associations by histologic subtype and beverage type (e.g., wine, beer and liquor) have not been thoroughly investigated among never smokers.8, 10, 11
In this study we investigated the association of alcohol consumption and lung cancer risk in never smokers in a large pooled dataset of 22 studies from the International Lung Cancer Consortium (ILCCO)12 and the SYNERGY project,13 in order to obtain sufficient sample size to thoroughly examine this association stratified by histologic subtype and beverage type while minimizing the effect of residual confounding by smoking.
Material and Methods
Study populations
Details regarding ILCCO and SYNERGY have been reported previously12, 13 and are available on web portals http://ilcco.iarc.fr and http://synergy.iarc.fr. Twenty-two studies from these consortia provided data for this analysis, including 10 studies in North America, 7 studies in Europe and 5 studies in Asia or other areas. All studies were either case-control or analyzed as nested case-control data sets, with 11 population-based, 7 hospital-based, 3 with mixed control groups and 1 cohort (see Supplementary Table 1 for further details). Control groups were at minimum matched on age and sex. Each study received approval from local ethics review boards.
Assessments of alcohol consumption
Consumption of alcohol and tobacco smoking was collected in each study by questionnaire. Never-smokers were defined as those who smoked less than 100 cigarettes in their lifetime whenever this information is available, or based on study questionnaire. Non-drinkers were defined as those who did not consume alcohol, or at least occasionally, in their lifetime (Supplementary Table 2). Most studies (n=18) included details regarding quantity and type of alcohol consumed (e.g., beer, wine and liquor) and duration of drinking. Some questionnaires included additional types of alcohol (e.g., Aperatif, Soku, Sachi), which were included in the estimation of average lifetime alcohol consumption (Supplementary Table 2). Duration of drinking data were generally available for multiple time periods (Supplementary Table 2).
Amount of alcohol consumption was converted to standardized drink units. These were then converted to grams per day using 12g of alcohol per drink unit based on on-line data from the International Agency for Research on Cancer (http://cancer-code-europe.iarc.fr/) and the Canadian Nutrient File by Health Canada. Lifetime average grams of alcohol consumed per day (overall and separately by beverage types) were estimated based on consumption frequency, changes in consumption patterns over the lifetime and beverage-specific alcohol content. For four studies where duration data were not available, we used current drinking as a proxy for average lifetime alcohol consumption. Non-drinkers were chosen as the reference category (instead of combining non-drinkers with low-level drinkers) to ensure that lung cancer risk related to low amounts of alcohol consumption could be assessed and that our results were comparable to previous large studies which also chose non-drinkers as the reference group. We also created detailed categories to capture the dose-response relationship for moderate and heavy alcohol consumption.
Statistical analysis
We applied unconditional logistic regression to estimate odds ratios and confidence limits for the association of average lifetime alcohol consumption with lung cancer risk based on the pooled dataset. To understand the association for different lung cancer histological subtypes we examined associations separately by histology. We also modelled average lifetime grams per day of wine consumption, beer consumption and liquor consumption separately for lung cancer risk, mutually adjusted by beverage type. The potential non-linear dose-response relationship was assessed using restricted cubic splines. All models were adjusted for sex, age, ethnicity, education and study centre/sub-centre. Race/ethnicity was collected according to investigator chosen categories (Table 1). We chose to adjust for race/ethnicity because alcohol consumption and lung cancer risk in non-smokers has been found to vary across race/ethnicity groups14, 15 indicating that race/ethnicity could confound the association between alcohol intake and lung cancer risk.
Table 1.
Demographic characteristics of study subjects.
| Characteristics | Case no. (%) | Control no. (%) |
|---|---|---|
| Total subjects | 2548 | 9362 |
| Sex | ||
| Female | 1978 (77.6) | 5351 (57.2) |
| Male | 570 (22.4) | 4011 (42.8) |
| Age (years) | ||
| Mean | 60.8 | 60.5 |
| Standard Deviation | 11.8 | 11.6 |
| Age groups | ||
| <50 | 453 (17.8) | 1710 (18.3) |
| 50<60 | 657 (25.8) | 2274 (24.3) |
| 60<70 | 810 (31.8) | 3155 (33.7) |
| 70+ | 628 (24.6) | 2223 (23.7) |
| Race/ethnicity | ||
| White, European | 1511 (59.3) | 6600 (70.5) |
| Black, African-American | 67 (2.6) | 463 (4.9) |
| Asian | 906 (35.6) | 2077 (22.2) |
| Latino | 41 (1.6) | 124 (1.3) |
| Other unknown | 23 (0.9) | 98 (1.0) |
| Education | ||
| Basic/elementary | 567 (22.3) | 2152 (23.0) |
| up to high school graduate | 572 (22.4) | 2791 (29.8) |
| some postsecondary and higher | 903 (35.4) | 2011 (21.5) |
| missing or unspecified | 506 (19.9) | 2408 (25.7) |
Because metabolism of alcohol varies between sexes, we conducted sex-specific analyses for overall alcohol consumption. To evaluate potential biases created by study design, stratified analysis (hospital- verses population-based/cohort) was conducted. For studies where data were available we examined whether potential confounders might at least in part influence the observed associations between overall alcohol intake and lung cancer risk. We examined confounding by occupational exposure (data available for 5 studies) by adjusting for study subjects’ job history (whether they held jobs known or suspected to be associated with excess risk of lung cancer such as mining, chemical industry, metal refining, and others).16, 17 We also adjusted for previous medical history of tuberculosis, chronic pulmonary disorder, emphysema or pneumonia (5 studies) and exposure to environmental tobacco smoke (10 studies) in regression models. Statistical analyses were performed with SAS (SAS Institute, Inc., Cary, NC) and R (Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 2548 never-smoking lung cancer patients and 9362 never-smoking controls from the 22 studies were included in this investigation (Table 1). Mean age of cases and controls were similar (60.8 for cases, 60.5 for controls). There were more females among cases (78%) than controls (57%), resulting from the original frequency matching by sex being performed in both ever and never smokers combined, with the tendency for females to be over-represented among cases in never smoking samples. Cases were slightly more educated than controls. The majority of the subjects were of European descent. Cases were less likely to be of European descent than controls due to the large number of controls from European-based EPIC study where frequency matching included 5 controls per case.
Overall alcohol consumption
The associations between average lifetime alcohol consumption and lung cancer risk by consumption categories are presented in Table 2. Low to moderate alcohol consumption was shown to be inversely associated with lung cancer risk when compared to non-drinkers with ORs of 0.80 (95%CI=0.70–0.90), 0.82 (95%CI=0.69–0.99) and 0.79 (95%CI=0.65–0.96) for the consumption of >0–4.9g per day, 5–9.9g per day, and 10–19.9g per day, respectively. Results from analyses stratified by histologic subtype showed inverse associations of low, moderate and heavier drinking with lung adenocarcinoma and squamous cell carcinoma. The inverse association with squamous cell carcinoma appeared to be more prominent. However, sample size for squamous cell carcinoma was limited, given the particularly strong association of this sub-type with tobacco. In contrast to these histologic sub-types, risk for small cell carcinoma of the lung was elevated ranging from 1.2 to 1.7 for all categories of alcohol consumption above 0–4.9g per day, although the confidence limits were wide given the small sample size (Table 2).
Table 2.
Risk estimates and 95% CI by histological type and average amount of alcohol consumed per day.
| Histological type | Average alcohol consumption (g/day) | Case no. (%) | Control no. (%) | OR | 95% CI |
|---|---|---|---|---|---|
| All Lung† | |||||
| Non-drinker | 1338 (52.5) | 3488 (37.3) | 1.00 | Reference | |
| >0–4.9 | 632 (24.8) | 2607 (27.8) | 0.80 | 0.70, 0.90 | |
| 5–9.9 | 217 (8.5) | 1111 (11.9) | 0.82 | 0.69, 0.99 | |
| 10–19.9 | 189 (7.4) | 1100 (11.7) | 0.79 | 0.65, 0.96 | |
| 20–29.9 | 78 (3.1) | 445 (4.8) | 0.82 | 0.62, 1.09 | |
| 30–44.9 | 36 (1.4) | 306 (3.3) | 0.68 | 0.47, 0.99 | |
| 45+ | 58 (2.3) | 305 (3.3) | 0.91 | 0.65, 1.29 | |
| Adenocarcinoma† | |||||
| Non-drinker | 702 (50.9) | 3488 (37.3) | 1.00 | Reference | |
| >0–4.9 | 376 (27.3) | 2607 (27.8) | 0.82 | 0.70, 0.96 | |
| 5–9.9 | 132 (9.6) | 1111 (11.9) | 0.91 | 0.72, 1.14 | |
| 10–19.9 | 98 (7.1) | 1100 (11.7) | 0.74 | 0.58, 0.96 | |
| 20–29.9 | 34 (2.5) | 445 (4.8) | 0.67 | 0.45, 0.99 | |
| 30–44.9 | 13 (0.9) | 306 (3.3) | 0.46 | 0.26, 0.83 | |
| 45+ | 24 (1.7) | 305 (3.3) | 0.72 | 0.44, 1.18 | |
| Squamous cell†† | |||||
| Non-drinker | 91 (52.9) | 3271 (37.7) | 1.00 | Reference | |
| >0–4.9 | 36 (20.9) | 2407 (27.7) | 0.51 | 0.33, 0.78 | |
| 5–9.9 | 15 (8.7) | 1066 (12.3) | 0.49 | 0.28, 0.89 | |
| 10–19.9 | 15 (8.7) | 1040 (12.0) | 0.51 | 0.28, 0.92 | |
| 20+ | 15 (8.7) | 903 (10.4) | 0.51 | 0.27, 0.95 | |
| Small cell‡ | |||||
| Non-drinker | 27 (43.5) | 2266 (32.6) | 1.00 | Reference | |
| >0–4.9 | 8 (12.9) | 2033 (29.3) | 0.47 | 0.21, 1.10 | |
| 5–9.9 | 9 (14.5) | 849 (12.2) | 1.45 | 0.64, 3.29 | |
| 10–19.9 | 7 (11.3) | 862 (12.4) | 1.23 | 0.49, 3.07 | |
| 20+ | 11 (17.7) | 933 (13.4) | 1.68 | 0.70, 4.06 |
Adjusted for age group, sex, ethnicity, education, and center/sub-centre. Includes all studies.
Adjusted for age group, sex, ethnicity, education, and centre (included: Aichi, CAPUA, CE, China, EAGLE, EPIC, ESTHER, FHS, HSPH, Hawaii, ICARE, Israel, Montreal, NCI-Maryland, Moffitt, Seoul, Toronto, UCLA).
Adjusted for age group, sex, ethnicity, education, and centre (included: Aichi, CAPUA, CE, China, EAGLE, EPIC, ESTHER, FHS, ICARE, Spain, Toronto, UCLA)
Figure 1 shows the dose-response relationship of average lifetime alcohol consumption in grams per day against the odds of being a case for all lung cancer, adenocarcinoma, squamous cell carcinoma and small cell lung cancer. A notable drop in the odds of being a case was seen for drinkers with low to moderate consumptions compared to non-drinkers for lung cancer overall, adenocarcinoma and squamous cell carcinoma. With increasing alcohol consumption, the confidence limits widened considerably which did not permit firm conclusions of the precise dose-response relationship for higher levels of alcohol intake.
Figure 1. Non-linear dose response relationship between alcohol consumption and lung cancer risk among never smokers based on restricted cubic splines.
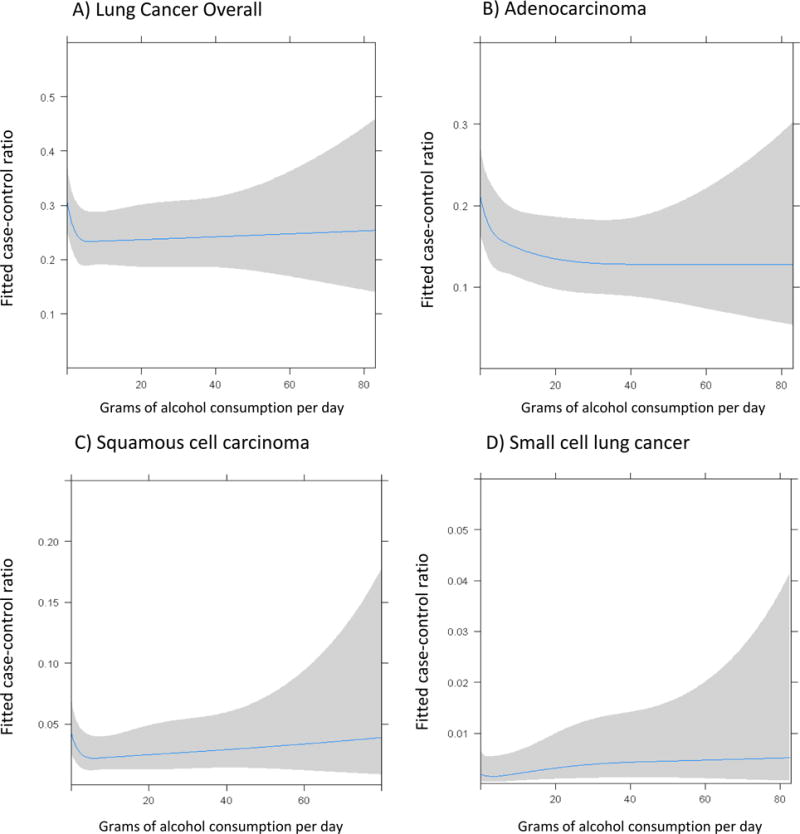
X-axis is grams of alcohol consumed per day and Y-axis is the fitted odds of being a case versus being a control, adjusted for sex, age, ethnicity, education and center.
Type of alcohol consumed
The associations between lung cancer risk and lifetime average consumption by different alcoholic beverage types (wine, beer and liquor) are reported in Table 3. Risk estimates for wine and liquor consumption were similar to those for overall consumption, whereas beer drinking showed statistically non-significant elevations in risk for alcohol consumption of 10g a day and higher. Low and moderate amounts of wine drinking were associated with reduced lung cancer risk (>0–4.9 g per day: OR=0.80, 95% CI=0.69–0.94; 20–29g per day: OR=0.62, 95% CI=0.43–0.89), while low amount of liquor drinking was associated with reduced lung cancer risk (0–4.9 g per day: OR=0.77, 95% CI=0.66–0.91). Trends for beer consumption differed from those for wine and liquor with point estimates being above 1 for moderate to high drinking categories (>10g per day) suggesting positive associations (Table 3).
Table 3.
Risk estimates and 95% CI by beverage type and average amount of alcohol consumed per day.
| Beverage type | Average Alcohol Consumption (g/day) | Case no. (%) | Control no. (%) | OR | 95% CI |
|---|---|---|---|---|---|
| Wine | |||||
| Non-drinker | 1138 (58.6) | 3377 (43.7) | 1.00 | Reference | |
| >0–4.9 | 480 (24.7) | 2387 (30.9) | 0.80 | (0.69,0.94) | |
| 5–9.9 | 133 (6.9) | 767 (9.9) | 0.87 | (0.69,1.10) | |
| 10–19.9 | 102 (5.3) | 552 (7.2) | 0.84 | (0.65,1.09) | |
| 20–29.9 | 41 (2.1) | 372 (4.8) | 0.62 | (0.43,0.89) | |
| 30+ | 47 (2.4) | 266 (3.5) | 0.94 | (0.64,1.38) | |
| Beer | |||||
| Non-drinker | 1427 (73.5) | 4647 (60.2) | 1.00 | Reference | |
| >0–4.9 | 378 (19.5) | 2221 (28.8) | 0.95 | 0.81, 1.11 | |
| 5–9.9 | 55 (2.8) | 453 (5.9) | 0.91 | 0.66, 1.26 | |
| 10–19.9 | 41 (2.1) | 229 (3.0) | 1.20 | 0.82, 1.75 | |
| 20–29.9 | 19 (1.0) | 79 (1.0) | 1.54 | 0.90, 2.65 | |
| 30+ | 21 (1.1) | 92 (1.2) | 1.35 | 0.78, 2.33 | |
| Liquor | |||||
| Non-drinker | 1459 (75.2) | 4806 (62.3) | 1.00 | Reference | |
| >0–4.9 | 383 (19.7) | 2382 (30.9) | 0.77 | (0.66, 0.91) | |
| 5–9.9 | 42 (2.2) | 233 (3.0) | 0.82 | (0.56, 1.19) | |
| 10–19.9 | 30 (1.6) | 137 (1.8) | 0.87 | (0.56, 1.36) | |
| 20–29.9 | 18 (0.9) | 73 (1.0) | 1.03 | (0.59, 1.81) | |
| 30+ | 9 (0.5) | 90 (1.2) | 0.41 | (0.19, 0.86) |
Adjusted for alcohol type (i.e. mutual adjustment for wine, beer, and liquor) age group, sex, ethnicity, education, and centre/sub-center. Includes: CAPUA, CE, China, EAGLE, EPIC, ESTHER, FHS, HSPH, HAWAII, ICARE, Israel, Montreal, NCI-Maryland, NELC, Moffitt, Spain, Toronto, UCLA, WELD.
Evaluation of effect modifiers and potential confounders
Associations between alcohol consumption and lung cancer risk were similar when stratified by gender. A significant inverse association with lower amounts of drinking was observed in females with OR of 0.80 (95% CI=0.69–0.93), while the estimate in males was comparable with OR of 0.89 (0.68–1.17) (Supplementary Table 3). The lack of significance in males may simply be due to the smaller sample size.
When we analyzed population-based and cohort studies separately from hospital-based studies, we found that significantly reduced odds ratios were restricted to the population-based/cohort studies. Risk estimates for the hospital-based study group did not provide strong evidence for an association with lung cancer risk (Supplementary Table 4).
Data for occupational exposure were available for 5 studies (CE, CAPUA, EAGLE, Montreal, Toronto: 494 cases, 2496 controls). No appreciable changes in odds ratios were found when variables representing lung cancer related occupational exposures were added to logistic regression models. Adjustment for medical history of tuberculosis, chronic pulmonary disorder, emphysema or pneumonia for 5 studies for which data were available (CE, FHS, NELCS, Toronto, UCLA, WELD: 516 cases, 2439 controls) also had negligible effects on odds ratios. We found no appreciable differences in odds ratios when controlling for exposure to environmental tobacco smoke (10 studies CE, EPIC, UCLA, FHS, Harvard, Hawaii, Moffitt, NELCS, Toronto, WELD: 851 cases, 3261 controls) (data not shown).
Discussion
In this study, the largest conducted on the association of alcohol consumption with lung cancer risk among never smokers, we found an inverse association between overall alcohol consumption and lung cancer risk with reduced risk estimates most consistently observed for low and moderate drinking. We also found alcohol consumption was associated with lower risk of both adenocarcinoma and squamous cell carcinoma. Analysis by alcoholic beverage type revealed that wine drinkers and liquor drinkers were at lower risk for lung cancer, with beer drinkers having modest non-significant increases in risk relative to non-drinkers for most drinking categories.
Consistent with our results, other large studies (including both ever and never smokers) found reduced lung cancer risk for lower levels of alcohol consumption. The NIH-AARP Diet and Health Study, a prospective cohort study, reported lower risk among drinkers who consumed less than 12g (1 drink) of alcohol per day18 while Freudenheim et al., using a pooled analysis of cohort studies, found lower risk for women who drank less than 15g of alcohol per day.8 A comprehensive meta-analysis by Bagnardi et al. (26,509 cases), also reported reduced risk for low levels of drinking (less than 12.5g per day).19 Specifically for never smokers, previous studies have not provided consistent evidence regarding alcohol consumption to lung cancer risk. A meta-analysis by Bagnardi et al., found no differences in risk between ever and never drinkers10. Among larger prospective cohort studies, one study found lung cancer risk increased with increased drinking in never smoking males but not females8, while two other studies reported null results15, 18.
We found differential associations by beverage type with inverse associations found for both wine and liquor consumption, but not beer consumption. Among larger studies that investigated association by beverage type (including both ever and never smokers), inverse associations for low levels of wine drinking (<12g or 1 drink per day), but positive associations with liquor drinking.7 However, similar to our results, the NIH-AARP Diet and Health Study (the largest cohort study investigating this association with 10,227 lung cancer cases) also found low to moderate consumption of wine or liquor was associated with reduced lung cancer risk.18 Consistent with our data, larger studies have reported positive associations between beer consumption and lung cancer risk.7, 18
Our results are compatible with the hypothesis that flavonoids found in wine may reduce the risk of some cancers. Support for a beneficial role of flavonoids is provided by several studies where higher dietary intake of flavonoids (including flavonols, flavanones and quercetin) was inversely associated with lung cancer risk.20–22 The inverse association between liquor consumption and lung cancer risk is more difficult to explain. It is possible that constituents of different beverage types have no direct effect on risk, but instead beverage type is correlated with lifestyle factors that are associated with lung cancer risk. For example, wine drinkers have been reported to have healthier diets than beer drinkers in several studies.23–26 A healthier diet for both wine and liquor drinkers relative to beer drinkers has also been reported, but not consistently.23, 24, 26
We observed differential association of lung cancer risk across different histologic sub-types, with inverse associations found between alcohol consumption and adenocarcinoma and squamous cell carcinoma but not for small cell lung carcinoma. Our finding of reduced risk for squamous cell carcinoma among alcohol consumers is in part supported by NIH-AARP Diet and Health Study where reduced risk of squamous cell carcinoma was found among low and moderate drinkers (<3 drinks per day) of alcohol.18 However, in general, results pertaining to the association of alcohol consumption with histologic sub-type of lung cancer in combined samples of ever and never smokers have been mixed.8, 18, 27 In addition to random variation it is possible that heterogeneity in results could be at least partially attributed to confounding by smoking, which can vary across populations and is differentially associated with different histologic subtypes. Even though our study is restricted to never smokers, we cannot preclude the possibility of residual confounding by tobacco smoking. However validation studies have shown that misclassification of never smokers with ever smokers is unlikely to have an important effect on results;28 therefore the potential residual confounding by tobacco smoking is not expected to be a driving factor of associations observed in our study.
In general, neither our categorical data analysis nor our analysis of non-linearity using restricted cubic splines indicated that heavy consumers of alcohol have higher lung cancer risk when comparing to non-drinkers, although we did observe a suggestive positive association with increased beer consumption. In most analyses, we found risk estimates were generally below the null for subjects who were categorized as heavier drinkers (30 or more grams per day) of total alcohol, wine or liquor. In contrast, results from several large cohort studies and a recent comprehensive meta-analysis indicated increased risk for heavier drinkers.8, 11, 18, 19 As these studies included smokers, residual confounding by smoking in heavier drinkers may explain the observed increased risk of lung cancer. Two recent cohort studies and a meta-analysis did not find heavier drinkers to be at higher risk for lung cancer among never smokers.10, 11, 18
The observed inverse associations we found between alcohol consumption and lung cancer risk may be explained by confounding related to differences between non-drinkers and drinkers. It has been postulated that non-drinkers may represent a unique subgroup of the population with either lower socio-economic status or medical conditions that could confound associations with lung cancer. Although we have controlled for confounding by socio-economic status by adjusting for education in logistic regression models, it is possible that this measure did not fully capture socio-economic status. To account for potential comorbidity, we also adjusted for medical history of tuberculosis, chronic pulmonary disorder, emphysema or pneumonia in a subset of our study where these data were available. We found no appreciable effects on odds ratios. Even though we did not observe any evidence of confounding based on socio-economic status or medical conditions, our results are compatible with the hypothesis that non-drinkers are a unique group of individuals which can drive the dose response relationship to show inverse associations with point estimates below the null throughout different categories of drinking.
To investigate whether study design could have introduced bias into our results, we compared results by study design (cohort, population-based and hospital-based case-control studies). Interestingly, inverse associations between alcohol consumption and lung cancer risk were found only for the population-based study. The most noticeable difference between the three sub-groups was that controls in hospital based studies were more likely to identify themselves as never drinkers than those in the population-based or cohort study (see Supplementary Table 4). A possible explanation for this is that controls recruited in the hospital-based studies may be more likely to abstain from alcohol due to other health conditions, and this resulted in associations remaining near the null in this sub-group. Given that most of the studies are based on case-control design, we cannot preclude the possibility of recall bias, which could further explain the lack of dose response, although it would not explain how recall bias would result in the observed association particularly in population-based case-control studies. Ideally, one would hope to address the recall issue in the prospective study, however we had limited number of non-smoking lung cancer cases from cohort study to be informative (Supplementary Table 4). We also stratified our subjects by sex since men and women metabolize alcohol differently. However, we did not find important differences in risk estimates between the sexes.
Although our results are consistent with wine and liquor consumption associated with a reduced risk of lung cancer, we cannot rule out residual confounding from known or unknown factors influencing observed associations with lung cancer risk. Recent results from Mendelian randomization studies conflict with the commonly cited view that light to moderate alcohol consumption is causally linked to lower risk for ischaemic heart disease, with results from genetic analyses clearly indicating that genetic variation that predisposes to less drinking is associated with lower risk in both light/moderate and heavier drinkers.29 This emphasizes the potential importance of confounding in studies that investigate associations between alcohol consumption and chronic diseases.
In summary, based on the largest study of alcohol consumption and lung cancer for never smokers to date, we investigated detailed dose-response relationships and potential effect modifiers by beverage type and histological subtype. We found an inverse association between wine and liquor consumption and lung cancer risk in never smokers. We cannot, however, rule out residual confounding from known or unknown risk factors influencing the observed associations with lung cancer risk, particularly those related to non-drinkers. Further research is needed to clarify associations between alcohol consumption and lung cancer risk with a focus on reducing or elucidating the role of confounding a priority for future studies.
Supplementary Material
Novelty and Impact.
It is not clear whether alcohol consumption is associated with lung cancer risk. Since the relationship is confounded by smoking, the authors conducted the largest study of lung cancer and alcohol consumption among never smokers to date, and found that alcohol consumption was inversely associated with lung cancer risk, particularly at low to moderate consumption levels, and among wine and liquor, but not beer drinkers. Confounding by factors other than smoking, particularly in relation to the non-drinkers reference group, cannot be ruled out.
Acknowledgments
The ILCCO data management is supported by Canadian Cancer Society (CCSRI no. 020214) and Cancer Care Ontario Research Chair Award. Individual ILCCO studies were funded or supported by various institutions and organizations. AICHI: MEXT Kakenhi (No. 170150181 and 26253041) from the Ministry of Education, Science, Sports, Culture and Technology of Japan, and by a Grant-in-Aid for the Third Term Comprehensive 10-Year Strategy for Cancer Control from Ministry Health, Labour and Welfare of Japan; CE: Supported by a grant from the European Commission’s INCO-COPERNICUS Program (contract IC15-CT96-0313; HSPH: National Institutes of Health and National Cancer Institute (Grants CA092824, CA074386, CA090578); EAGLE: Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics, Bethesda, Maryland; A. G. Schwartz (FHS, WELD) NIH R01CA060691, NIH R01CA87895, NIH P30CA022453, HHSN261201200011; C. C. Harris (NCI-Maryland) was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health; MSK: Steps for Breath, the Labrecque Foundation and the Society of Memorial Sloan-Kettering Cancer Center. Core Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center; The CAPUA STUDY was financed by FIS-FEDER/Spain grant numbers FIS-01/310, FIS-PI03-0365, and FIS-07-BI060604, FICYT/Asturias grant numbers FICYT PB02-67 and FICYT IB09-133 The University of Oviedo, Asturias, Spain, The Fundacion Caja de Ahorros de Asturias and the Ciber de Epidemiologia y Salud Publica, CIBERESP, Spain; ESTHER: Baden-Württemberg State Ministry of Research, Science and Arts; NELCS: Grant Number P20RR018787 from the National Center for Research Resources (NCRR), component of the National Institutes of Health (NIH). CE: research intention PRVOUK P28/1LF/6; The Danish Diet, Cancer and Health Study included in the EPIC-cohort was funded by the Danish Cancer Society.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. [Google Scholar]
- 2.Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32:605–44. doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Field RW, Withers BL. Occupational and environmental causes of lung cancer. Clin Chest Med. 2012;33:681–703. doi: 10.1016/j.ccm.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vineis P, Hoek G, Krzyzanowski M, Vigna-Taglianti F, Veglia F, Airoldi L, Overvad K, Raaschou-Nielsen O, Clavel-Chapelon F, Linseisen J, Boeing H, Trichopoulou A, et al. Lung cancers attributable to environmental tobacco smoke and air pollution in non-smokers in different European countries: a prospective study. Environ Health. 2007;6:7. doi: 10.1186/1476-069X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 6.Bagnardi V, Randi G, Lubin J, Consonni D, Lam TK, Subar AF, Goldstein AM, Wacholder S, Bergen AW, Tucker MA, Decarli A, Caporaso NE, et al. Alcohol consumption and lung cancer risk in the Environment and Genetics in Lung Cancer Etiology (EAGLE) study. Am J Epidemiol. 2010;171:36–44. doi: 10.1093/aje/kwp332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao C. Associations between beer, wine, and liquor consumption and lung cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2007;16:2436–47. doi: 10.1158/1055-9965.EPI-07-0386. [DOI] [PubMed] [Google Scholar]
- 8.Freudenheim JL, Ritz J, Smith-Warner SA, Albanes D, Bandera EV, van den Brandt PA, Colditz G, Feskanich D, Goldbohm RA, Harnack L, Miller AB, Rimm E, et al. Alcohol consumption and risk of lung cancer: a pooled analysis of cohort studies. Am J Clin Nutr. 2005;82:657–67. doi: 10.1093/ajcn.82.3.657. [DOI] [PubMed] [Google Scholar]
- 9.Korte JE, Brennan P, Henley SJ, Boffetta P. Dose-specific meta-analysis and sensitivity analysis of the relation between alcohol consumption and lung cancer risk. Am J Epidemiol. 2002;155:496–506. doi: 10.1093/aje/155.6.496. [DOI] [PubMed] [Google Scholar]
- 10.Bagnardi V, Rota M, Botteri E, Scotti L, Jenab M, Bellocco R, Tramacere I, Pelucchi C, Negri E, La Vecchia C, Corrao G, Boffetta P. Alcohol consumption and lung cancer risk in never smokers: a meta-analysis. Ann Oncol. 2011;22:2631–9. doi: 10.1093/annonc/mdr027. [DOI] [PubMed] [Google Scholar]
- 11.Klatsky AL, Li Y, Nicole Tran H, Baer D, Udaltsova N, Armstrong MA, Friedman GD. Alcohol intake, beverage choice, and cancer: a cohort study in a large kaiser permanente population. Perm J. 2015;19:28–34. doi: 10.7812/TPP/14-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung RJ, Christiani DC, Risch A, Popanda O, Haugen A, Zienolddiny S, Benhamou S, Bouchardy C, Lan Q, Spitz MR, Wichmann HE, LeMarchand L, et al. International Lung Cancer Consortium: pooled analysis of sequence variants in DNA repair and cell cycle pathways. Cancer Epidemiol Biomarkers Prev. 2008;17:3081–9. doi: 10.1158/1055-9965.EPI-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsson AC, Gustavsson P, Kromhout H, Peters S, Vermeulen R, Bruske I, Pesch B, Siemiatycki J, Pintos J, Bruning T, Cassidy A, Wichmann HE, et al. Exposure to diesel motor exhaust and lung cancer risk in a pooled analysis from case-control studies in Europe and Canada. Am J Respir Crit Care Med. 2011;183:941–8. doi: 10.1164/rccm.201006-0940OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS. Prevalence of alcohol dependence among US adult drinkers, 2009–2011. Prev Chronic Dis. 2014;11:E206. doi: 10.5888/pcd11.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thun MJ, Hannan LM, Adams-Campbell LL, Boffetta P, Buring JE, Feskanich D, Flanders WD, Jee SH, Katanoda K, Kolonel LN, Lee IM, Marugame T, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008;5:e185. doi: 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahrens W, Merletti F. A standard tool for the analysis of occupational lung cancer in epidemiologic studies. Int J Occup Environ Health. 1998;4:236–40. doi: 10.1179/oeh.1998.4.4.236. [DOI] [PubMed] [Google Scholar]
- 17.Mirabelli D, Chiusolo M, Calisti R, Massacesi S, Richiardi L, Nesti M, Merletti F. Database of occupations and industrial activities that involve the risk of pulmonary tumors. Epidemiol Prev. 2001;25:215–21. [PubMed] [Google Scholar]
- 18.Troche JR, Mayne ST, Freedman ND, Shebl FM, Abnet CC. The association between alcohol consumption and lung carcinoma by histological subtype. Am J Epidemiol. 2016;183:110–21. doi: 10.1093/aje/kwv170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, Scotti L, Jenab M, Turati F, Pasquali E, Pelucchi C, Galeone C, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. 2015;112:580–93. doi: 10.1038/bjc.2014.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam TK, Rotunno M, Lubin JH, Wacholder S, Consonni D, Pesatori AC, Bertazzi PA, Chanock SJ, Burdette L, Goldstein AM, Tucker MA, Caporaso NE, et al. Dietary quercetin, quercetin-gene interaction, metabolic gene expression in lung tissue and lung cancer risk. Carcinogenesis. 2010;31:634–42. doi: 10.1093/carcin/bgp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Marchand L, Murphy SP, Hankin JH, Wilkens LR, Kolonel LN. Intake of flavonoids and lung cancer. J Natl Cancer Inst. 2000;92:154–60. doi: 10.1093/jnci/92.2.154. [DOI] [PubMed] [Google Scholar]
- 22.Woo HD, Kim J. Dietary flavonoid intake and smoking-related cancer risk: a meta-analysis. PloS one. 2013;8:e75604. doi: 10.1371/journal.pone.0075604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adjemian MK, Volpe RJ, Adjemian J. Relationships between Diet, Alcohol Preference, and Heart Disease and Type 2 Diabetes among Americans. PloS one. 2015;10:e0124351. doi: 10.1371/journal.pone.0124351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barefoot JC, Gronbaek M, Feaganes JR, McPherson RS, Williams RB, Siegler IC. Alcoholic beverage preference, diet, and health habits in the UNC Alumni Heart Study. Am J Clin Nutr. 2002;76:466–72. doi: 10.1093/ajcn/76.2.466. [DOI] [PubMed] [Google Scholar]
- 25.Ruidavets JB, Bataille V, Dallongeville J, Simon C, Bingham A, Amouyel P, Arveiler D, Ducimetiere P, Ferrieres J. Alcohol intake and diet in France, the prominent role of lifestyle. Eur Heart J. 2004;25:1153–62. doi: 10.1016/j.ehj.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Sluik D, van Lee L, Geelen A, Feskens EJ. Alcoholic beverage preference and diet in a representative Dutch population: the Dutch national food consumption survey 2007–2010. Eur J Clin Nutr. 2014;68:287–94. doi: 10.1038/ejcn.2013.279. [DOI] [PubMed] [Google Scholar]
- 27.Chao C, Li Q, Zhang F, White E. Alcohol consumption and risk of lung cancer in the VITamins And Lifestyle Study. Nutr Cancer. 2011;63:880–8. doi: 10.1080/01635581.2011.582222. [DOI] [PubMed] [Google Scholar]
- 28.Nyberg F, Agudo A, Boffetta P, Fortes C, Gonzalez CA, Pershagen G. A European validation study of smoking and environmental tobacco smoke exposure in nonsmoking lung cancer cases and controls. Cancer Causes Control. 1998;9:173–82. doi: 10.1023/a:1008882227444. [DOI] [PubMed] [Google Scholar]
- 29.Holmes MV, Dale CE, Zuccolo L, Silverwood RJ, Guo Y, Ye Z, Prieto-Merino D, Dehghan A, Trompet S, Wong A, Cavadino A, Drogan D, et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ. 2014;349:g4164. doi: 10.1136/bmj.g4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


