Abstract
Here, we introduce a method for the fast and accurate analysis of DNA methylation based on bisulfite-treated DNA. The target region is PCR amplified using a T7 RNA polymerase promoter-tagged primer. A subsequent in vitro transcription leads to a transcript which contains guanosine residues only at sites that contained methylated cytosines before bisulfite treatment. In a single tube reaction using guanosine-specific cleavage by RNase T1, a specific pattern of RNA fragments is formed. This pattern directly represents the methylation state of the sample DNA and is analyzed using matrix-assisted laser desorption ionization time-of-flight technology. This method was successfully applied to the analysis of artificially methylated and unmethylated DNA, mixtures thereof and colon DNA samples. The applicability for the analysis of both PCR products and cloned PCR products is demonstrated. The observed methylation patterns were confirmed by bisulfite sequencing.
INTRODUCTION
DNA methylation in mammals is almost exclusively observed in position 5 of the cytosine base of the dinucleotide CpG. These dinucleotides are underrepresented in the genome and appear at only 20% of the expected frequency, as they are selected against during evolution due to their high mutability. Nevertheless, the genome contains CpG islands with a CpG dinucleotide frequency much higher than statistically expected. Promoter CpG methylation directly influences the transcriptional activity of the respective gene, e.g. by interfering with the binding of transcription factors (1) and is involved in fundamental processes like X-chromosome inactivation (2), imprinting (3), tumorigenesis (4), aging and cell differentiation (5).
Efficient methods are needed to analyze this epigenetic information. Most techniques for the analysis of methylation patterns depend on a preceding bisulfite treatment of the template DNA leading to a deamination of all unmethylated cytosines to uracil, leaving only methylated cytosines unaltered (6). Thus, the bisulfite reaction transforms the methylation information into sequence information which is easily analyzed using standard molecular biology techniques, most notably PCR. A powerful method for analyzing multiple CpG sites is bisulfite sequencing, which can be performed either directly on PCR products or on cloned PCR products generated on bisulfite-treated DNA. As direct bisulfite PCR sequencing reflects the mean of all subpopulations within a given sample bisulfite sequencing of cloned PCR products is the method of choice for accurate analysis of the methylation patterns present in a heterogeneous sample. It is also the method of choice for detecting stretches of co-methylated CpG positions which often carry biological information when occurring in promoter regions. Since many clones have to be sequenced in parallel and since only a fraction of the sequence trace information, namely the polymorphisms at CpG positions, are relevant for methylation analysis, a method which targets this information specifically and which can be run in high throughput at low cost, is highly desirable. The method presented in this paper is based on in vitro transcription of bisulfite PCR products, base-specific cleavage and final matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) analysis of the obtained RNA fragments. It represents a novel approach towards the efficient and low cost analysis of methylation pattern and is amenable to high throughput analysis. Fragmented RNA is a viable analyte for MALDI analysis, as it is more easily analyzed than DNA of the same size (7). The RNA fragment pattern can be correlated to the methylation pattern of the template DNA. In previous studies, the combination of the above-mentioned methods has already been successfully applied to comparative sequence analysis (8), discovery of single-nucleotide polymorphisms (9,10) and to the characterization of short tandem repeats (11).
MATERIALS AND METHODS
DNA preparation
Methylated DNA for the experiment described in Figure 2 was prepared by treating human genomic DNA (Promega) with SssI methyltransferase (New England Biolabs) in the presence of S-adenosyl-methionine according to the manufacturer's instructions. Unmethylated DNA was prepared by MDA (multiple displacement amplification), a genome-wide amplification method described by Dean et al. (12). For the preparation of mixtures with defined methylation states, a portion of this amplificate was treated with SssI as described above and mixed with the unmethylated amplificate to give mixtures with 0, 20, 40, 50, 60, 80 and 100% methylation in all CpG positions. DNA from colon tissue samples (Biocat) was extracted using the QIAamp DNA mini kit protocol (Qiagen). Bisulfite treatment was performed as described previously (13).
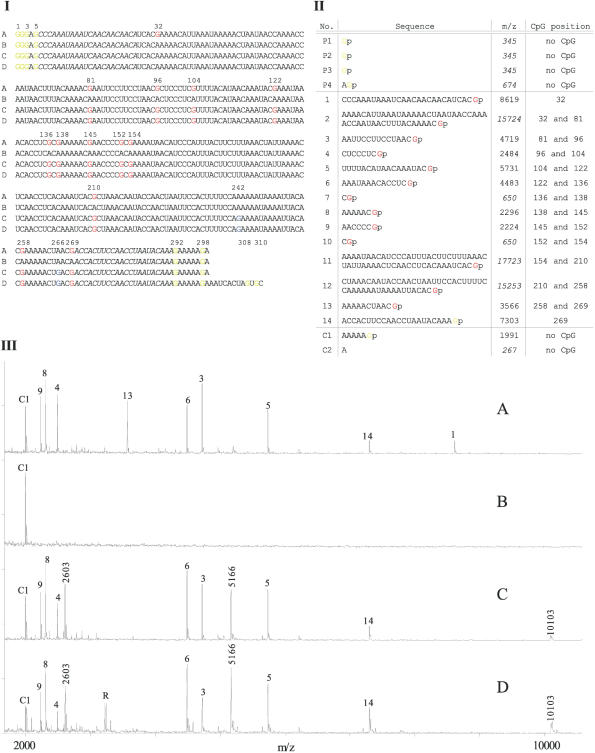
Figure 2.
(I) Multiple alignment of the virtual sequences of the investigated T7 transcripts derived from the bisulfite sequence traces of: PCR product from methylated, bisulfite-treated DNA (A), PCR product from unmethylated, bisulfite-treated DNA (B), PCR product from plasmid pEPI2383 DNA (C) and plasmid pEPI2383 without further PCR (D). Guanosines derived from originally methylated cytosines are highlighted in red, from non-converted cytosines in blue and from the residual vector, T7 Promoter and control tag in yellow. Gene-specific priming sites are marked in italic. (II) Fragmentation (sequences, their related m/z values and positions of the represented CpG) of the T7 transcript A (in I). Fragments from the T7 domain of the primer are tagged with P1–P4, gene-specific fragments are labeled with numbers (1–14) and fragments from the attached control tag are marked with C1–C2. Fragments size >11000 Da and <1000 Da could not be detected. These are highlighted in italic. Mass accuracy for the mass range 2000–10000 m/z is ± 3. (III) MALDI TOF spectra of the base-specific cleaved T7 transcripts A, B, C and D (in I).
PCR amplification, cloning and sequencing
A 289 bp fragment within the promoter region of the CDH13 gene, encoding H-cadherin was PCR amplified with the primers 5′-tctttttcTTTGTATTAGGTTGGAAGTGGT-3′ (forward) and 5′-gtaatacgactcactatagggagCCCAAATAAATCAACAACAACA-3′ (reverse). Gene-specific sequences are marked in capital letters and functional tags are given in small letters.
PCR was performed in a total volume of 25 μl containing 1 U Hotstar Taq polymerase (Qiagen), 12.5 pmol of forward and reverse primers, 1× PCR buffer (Qiagen), 0.2 mM of each dNTP (Fermentas) and 5 ng template. PCR of clones was performed using picked colony cells without DNA purification. Cycling was performed using a Mastercycler (Eppendorf) under the following conditions: 15 min at 95°C and 40 cycles at 95°C for 1 min, 55°C for 45 s and 72°C for 1 min.
The PCR product of the CDH13 fragment of SssI methylated and bisulfite-treated DNA was purified using the QIAquick kit (Qiagen) and subsequently cloned into the pGEM®-T Vector System (Promega) following the manufacturer's recommendations. Plasmid DNA from one clone (pEPI2383) was extracted using the Plasmid Mini Kit (Qiagen) protocol.
PCR products and plasmid pEPI2383 (restricted as described below) were sequenced using BigDye chemistry (Applied Biosystems) according to the manufacturer's recommendations.
RNA transcription and base-specific cleavage
Prior to T7 transcription, the plasmid DNA from clone pEPI2383 was hydrolyzed in 10 μl volume using 10 U Eco52I (Fermentas) in the recommended buffer. The reaction was performed for 1 h at 37°C. Inactivation was performed for 10 min at 65°C.
RNA transcription was performed in a 25 μl vol containing 10 μl PCR product or hydrolyzed plasmid DNA, respectively, containing 20 U T7 RNA polymerase (Fermentas), 1× transcription buffer (Fermentas), 0.5 mM of each NTP (Fermentas) and 4 U RNase inhibitor (Fermentas). After an incubation of 1.5 h at 37°C, 4 U of RNase T1 (Fermentas) was added immediately and incubated for another 45 min at 37°C.
MALDI-TOF analysis
Prior to the MALDI-TOF analysis, the RNA was desalted by adding 25 mg Clean Resin (Sequenom) to each preparation and incubating for 20 min at room temperature. The cleavage reaction mixture was diluted 5-fold, and 0.5 μl was mixed with 0.5 μl of organic matrix (saturated 3-hydroxypicolinic acid in 50% acetonitrile containing 0.05 M dibasic ammonium citrate) on a Scout™ 384 stainless steel target plate. Fragment analysis was carried out on a Biflex III mass spectrometer (Bruker Daltonics). Mass spectra were recorded in the negative ion reflector TOF mode. Potentials were 19 kV for the linear and 20 kV for the reflector acceleration. IS/2 potential was set at 17.75 kV. Ion extraction was delayed by 300 ns. The detector was gated to prevent saturation by molecules <700 Da. Usually 60 shots were accumulated per sample spot, smoothed using a Golay–Savitzky filter and baseline corrected. The interpretation of fragment masses was carried out using the software ‘Mongo Oligo Mass Calculator v2.05’ (http://medlib.med.utah.edu/masspec/mongo.htm).
RESULTS
Methodological background
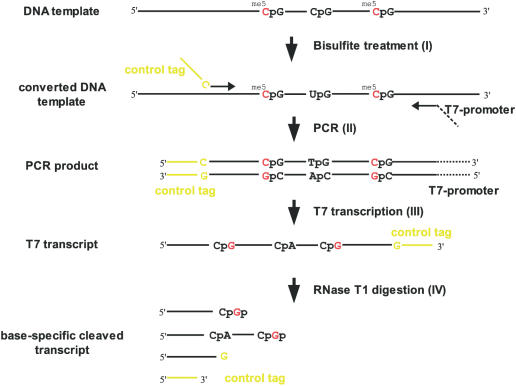
The presented novel approach for the detection of DNA methylation patterns requires bisulfite treatment of the template DNA. During the bisulfite treatment, only unmethylated cytosines are converted into uracils (Figure 1I). Thus, the methylation information is translated into sequence information. The double-stranded template DNA is converted into two single-stranded molecules, the cytosines of which represent the methylation information in the template DNA. In the subsequent PCR (Figure 1II) which is performed using a reverse primer containing a T7 RNA polymerase promoter tag (gtaatacgactcactatagggag), a complementary strand with low guanosine content is generated. The guanosines within this strand resemble the methylation information of the template DNA. After T7-mediated transcription (Figure 1III) these guanosine residues are subject to cleavage by RNase T1 from Aspergillus oryzae. Thus, the RNA fragmentation pattern corresponds to the methylation pattern of the original DNA (Figure 1IV). This methylation fingerprint is visualized by MALDI-TOF mass spectrometry. In addition, the T7 transcript is marked by a control tag at the 3′ end introduced by the forward primer. Using this control tag, the successful full-length transcription and a successful RNase T1 cleavage are easily monitored.
Figure 1.
CpG methylation pattern analysis using RNase T1 cleavage and MALDI-TOF: (I) deamination of unmethylated cytosines to uracils by bisulfite treatment, (II) PCR amplification with the reverse primer containing a T7 promoter site and the forward primer carrying a control tag (yellow), (III) transcription to RNA containing G-sites at originally methylated cytosines (me5C) and (IV) G-specific cleavage with RNase T1.
For validation of this method, a 289 bp fragment of the promoter region of the CDH13 gene was analyzed. This fragment contained 13 cytosines within a CpG context, all of which are methylated after SssI treatment.
Sequencing
PCR products derived from methylated and unmethylated bisulfite-treated DNA and the plasmid pEPI2383 were sequenced. In addition, the EcoR52I hydrolyzed plasmid pEPI2383 was sequenced. The expected sequences of the corresponding RNA in vitro transcripts are depicted in Figure 2I, A–D.
RNA fragmentation
Figure 2II shows the m/z values of all fragments expected after T1 cleavage of transcript A (Figure 2I, A) generated from completely methylated template DNA. In addition to the gene-specific fragments 1–14 the RNase T1 cleavage should result in the fragments C1, C2 and P1–P4. With the exception of those labeled italic, all fragments indeed were detected by MALDI-TOF analysis (Figure 2III, A). Fragments remaining undetected were either too small and thus not distinguishable from matrix background noise or too large and outside the detection range of the instrument.
In contrast to transcript A the T7 transcript derived from the originally unmethylated DNA contains no guanosines within the gene-specific sequence (Figure 2I, B). Thus, the subsequent G-specific cleavage generated only one gene-specific fragment (m/z = 90913) which is not detectable due to its large size. The control fragment C1 (m/z = 1991) was successfully detected with MALDI-TOF (Figure 2III, B), demonstrating successful transcription and fragmentation. Again, fragments C2 and P1–P4 were not detectable because of their sizes.
The MALDI-TOF spectrum of the fragmented transcript derived from a PCR product of the plasmid pEPI2383 is shown in Figure 2III, C. According to the virtual sequence of this transcript (Figure 2I, C), the spectrum differs in some additional and lacking fragments when compared with the spectrum derived from methylated DNA (Figure 2III, A). Fragment 13 caused by methylation of CpG sites at position 258 and 269 is lacking, which is easily explained by the additional guanosine at position 266 (Figure 2I, C). Additional guanosines are expected at low frequency due to incomplete bisulfite conversion. Thus, fragment 13 is cleaved into two fragments. Of these m/z = 2603 is detected, whereas m/z = 980 was not detectable due to its small size.
Fragment 12, generated by cleavage at positions 210 and 258 also possessed an internal cleaving site (position 242). Thus, two additional fragments of m/z = 5166 and m/z = 10103 appeared instead of fragment 12 (m/z = 15253).
The fragment pattern of the digested transcript derived from the PCR product of pEPI2383 is devoid of fragment 1 due to the unmethylated CpG site at position 32 (Figure 2I, C) which is explained by incomplete methylation of the template DNA. Hence, a fragment was generated which reflected the sum of fragments 1 and 2 (m/z = 24327). This fragment was too large to be detectable.
The MALDI-TOF spectra of the fragmented T7 transcript derived from pEPI2383 without prior PCR amplification is shown in Figure 2III, D. As expected, this spectrum corresponds well to the spectrum derived from the PCR product of this plasmid with the exception of an additional peak R which is observed due to the additional sequence (position 300–311 in Figure 2I) between the multiple cloning site of the vector and the recognition site of the applied restriction enzyme EcoR52I. This sequence attached to the end of the transcript resulted in three additional fragments, of which only fragment R (Figure 2III, D) was detected, since the other two (m/z = 651 and m/z = 243) are outside the detection range.
Analysis of colon DNA samples
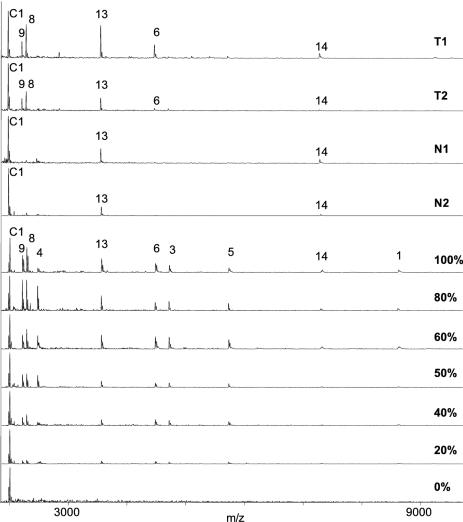
To demonstrate the viability of the novel method for the analysis of clinical sample material over a wide range of methylation levels, colon DNA samples were analyzed. Abberant methylation of the CDH13 promoter has been implicated with colorectal cancer (14). For the purpose of this study, two tumor DNA samples showing high methylation levels and two normal colon DNA samples showed low methylation levels were selected. Ten clones each of the amplified promoter region of the CDH13 gene were analyzed in direct comparison with sequencing (Figure 3).
Figure 3.
Analysis of CpG methylation in 10 clones (A–J) each derived from two bisulfite-converted colon tumor DNA samples (T1 and T2) and two normal colon DNA samples (N1 and N2), respectively, by RNA cleavage and MALDI-TOF (left) and sequencing (right). CpG methylated (black circle); CpG unmethylated (white circle); no fragment indicative of CpG methylation observed or ambiguous sequence (gray circle); CpG not accessible for analysis (cross).
There is excellent correlation of the methylation states of those CpG positions which are analyzed unambiguously by both methods for predominantly methylated (T1 and T2) as well as for predominantly unmethylated samples (N1 and N2). In some cases, sequencing failed to reveal the methylation state in position 32, 258 and 269 which are either close to the sequencing primer or to the end of the sequence. On the other hand, the mass range limitation of the MALDI instrument used in this study did not enable the unambiguous assignment of the methylation state of all CpG positions, since the absence of a fragment cannot be interpreted as the absence of methylation in a particular position unless this is justified by the detection of larger fragments. In clones A and J of sample T1, e.g. the presence of fragment 6+7 (m/z = 5117, Supplemental Material) is caused by methylation in positions 122 and 138 surrounding the unmethylated position 136. When several neighboring CpG positions are unmethylated, the resulting fragments become larger and are not easily detected. Positions 154 and 210 are considered to be non-analyzable, because the corresponding fragments are either too large to be detected reliably or too short to be distinguished from the background. However, this is not a general limitation. State of the art instrumentation has been described to detect RNAs as long as 2180 nt (15) and has been applied to sequence 50–100mer DNA and RNA fragments (16).
Finally, an aliquot of the same bisulfite-treated colon DNA samples was analyzed directly, i.e. without prior cloning. For comparison, mixtures of unmethylated and methylated DNA (0, 20, 40, 50, 60, 80 and 100% methylated) were prepared and analyzed in parallel (Figure 4). As expected, a decreased methylation rate results in a decrease of the intensities of the detected fragments, except the control fragment at m/z = 1991 which is formed independent of methylation and therefore has been utilized for signal normalization. In comparison with the methylation mixtures, the methylation signals of the clinical samples show a different intensity ratio which is due to some adjacent CpG sites showing stronger co-methylation than others as already indicated by the analysis of 10 clones from these samples. For example, the presence of strong signals for fragments 6, 8, 9, 13 and 14 in tumor sample T1 (for nomenclature see Figure 1) indicates a high relative degree of co-methylation around positions 122, 136, 138, 145, 152, 154, 258 and 269, respectively. These are exactly the CpG positions showing some degree of co-methylation in most of the clones analyzed for this sample (Figure 3). The normalized relative intensities of the signals indicate a minimum of 50% methylation at these positions. In contrast, the absence of or presence of weak signals for fragments 1, 3, 4 and 5 is explained by a low degree of co-methylation at positions 32, 81, 96 and 104. Both observations correspond well to the clone data. A similar methylation pattern is observed for tumor sample T2. The lower intensity of the observed signals corresponds well to the lower number of clones showing co-methylation for this sample (Figure 3). In the case of the normal colon samples N1 and N2, the absence of co-methylation is evidenced by both the direct method and clone analysis. In summary, the methylation information gained by direct analysis of clinical DNA samples compares well with the accumulated information obtained from clones of the same samples, albeit on a more qualitative level, i.e. the level of co-methylation.
Figure 4.
Direct analysis of two bisulfite-converted colon tumor DNA samples (T1 and T2) and two normal colon DNA samples (N1 and N2), respectively, by PCR, in vitro transcription, RNAse T1 cleavage and MALDI-TOF in comparison with DNA mixtures with defined methylation states. (top, mass spectrum of samples T1, T2, N1, N2, respectively; bottom, spectra of DNA mixtures with different, defined methylation states and signal intensity of control fragment C1 set as maximum, fragment numbers given for 100%).
DISCUSSION
DNA methylation markers have been discovered for a variety of clinical questions, most prominently cancer screening and monitoring. The validation of differential methylation patterns on large sample numbers represents a key step in the development of diagnostic test and requires both cost-efficient and high-throughput techniques. Here, we present a method for analyzing methylation patterns fulfilling these criteria. All PCR, in vitro transcription and guanine-specific cleavage of the generated transcript are performed in one pot. It has been demonstrated that cloned bisulfite DNA can be analyzed directly without prior PCR. When analyzing plasmid DNA directly, the T7 primer tag can be omitted because many cloning systems include a promoter sequence within the vector. Plasmid DNA was linearized by restriction to avoid transcription of the entire plasmid. Although the information generated by analyzing the RNAse T1 fragmentation pattern by MALDI-TOF is complementary to the methylation information contained in conventional bisulfite clone sequence traces, MALDI analysis does not require costly consumables like fluorescence labeled dNTPs.
In this study, the methylation pattern of unmethylated DNA and methylated DNA has been analyzed. The methylation information contained in the respective RNase T1 fragment patterns is in complete accordance with the sequencing data generated in parallel. In addition, it has been shown that occasional incomplete bisulfite conversion is detected position specifically by the appearance of specific shorter fragments appearing instead of an expected larger fragment. As the consequence, no methylation information is lost as long as these fragments are detected faithfully, which can be accomplished using sequence-based tracking algorithms.
Since there is an upper and lower m/z limit in the MALDI-TOF detection, there will always be fragments which cannot be detected. This also has been observed in the analysis of cloned bisulfite-treated colon DNA samples. However, the higher the mass range of the MALDI instrument, the higher will be the proportion of CpG positions, the methylation state of which can be unambiguously assigned. Even though, the presence of methylation over a stretch of neighboring CpG positions (co-methylation) is most reliably detected even within a limited mass range as demonstrated in Figure 3. It is this co-methylation of promoter regions which represents the most valuable information in the context of most clinical questions. The presence of co-methylation of two or more neighboring CpG positions in the same molecule within clinical samples is readily detected by the direct method as demonstrated in Figure 4. The relative abundance of co-methylation reveals itself in a semi-quantitative manner by comparison of methylation signal intensities with those of DNA mixtures with defined methylation states and normalization against the signal generated from a methylation-independent control tag. The selectivity of the technique for detecting co-methylation represents a significant advantage over the direct sequencing of bisulfite PCR products. Direct sequencing does not have the ability to distinguish specific methylation patterns from random methylation which usually does not contain clinically relevant information (17).
In summary, the combination of transcription, RNase T1 cleavage and fragment analysis by MALDI-TOF is suggested for methylation pattern analysis in bisulfite DNA either directly or after cloning.
Acknowledgments
ACKNOWLEDGEMENTS
We thank the German Ministry of Education and Research (BMBF) for financial support of this study by a grant no. KW0110. We also thank Daniela Friedrich, Institute for Zoo and Wildlife Research, for kindly performing the cloning experiments.
REFERENCES
- 1.Boyes J. and Bird,A. (1992) Repression of genes by DNA methylation depends on CpG density and promoter strength: evidence for involvement of a methyl-CpG binding protein. EMBO J., 11, 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riggs A.D. (2002) X chromosome inactivation, differentiation, and DNA methylation revisited, with a tribute to Susumu Ohno. Cytogenet. Genome Res., 99, 17–24. [DOI] [PubMed] [Google Scholar]
- 3.Recillas-Targa F. (2002) DNA methylation, chromatin boundaries, and mechanisms of genomic imprinting. Arch. Med. Res., 33, 428–38. [DOI] [PubMed] [Google Scholar]
- 4.Jones P.A. (2002) DNA methylation in cancer. Oncogene, 21, 5358–5360. [DOI] [PubMed] [Google Scholar]
- 5.Michalowsky L.A. and Jones,P.A. (1989) DNA methylation and differentiation. Environ. Health Perspect., 80, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frommer M., McDonald,L.E., Millar,D.S., Collis,C.M., Watt,F., Grigg,G.W., Molloy,P.L. and Paul,C.L. (1992) A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc. Natl Acad. Sci. USA, 89, 1827–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordhoff E., Cramer,R., Karas,M., Hillenkamp,F., Kirpekar,F., Kristiansen,K. and Roepstorff,P. (1993) Ion stability of nucleic acids in infrared matrix-assisted laser desorption/ionization mass spectrometry. Nucleic Acids Res., 21, 3347–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmer R., Storm,N., Boecker,S., Rodi,C.P., Hillenkamp,F., Jurinke,C. and van den Boom,D. (2003) RNase T1 mediated base-specific cleavage and MALDI-TOF MS for high-throughput comparative sequence analysis. Nucleic Acids Res., 31, e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bocker S. (2003) SNP and mutation discovery using base-specific cleavage and MALDI-TOF mass spectrometry. Bioinformatics, 19, i44–i53. [DOI] [PubMed] [Google Scholar]
- 10.Krebs S., Medugorac,I., Seichter,D. and Forster,M. (2003) RNaseCut: a MALDI mass spectrometry-based method for SNP discovery. Nucleic Acids Res., 31, e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seichter D., Krebs,S. and Forster M. (2004) Rapid and accurate characterisation of short tandem repeats by MALDI-TOF analysis of endonuclease cleaved RNA transcripts. Nucleic Acids Res., 32, e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dean F.B., Hosono,S., Fang,L., Wu,X., Faruqi,A.F., Bray-Ward,P., Sun,Z., Zong,Q., Du,Y., Du,J. (2002) Comprehensive human genome amplification using multiple displacement amplification. Proc. Natl Acad. Sci. USA, 99, 5261–5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olek A., Oswald,J. and Walter,J. (1996) A modified and improved method for bisulphite based cytosine methylation analysis. Nucleic Acids Res., 24, 5064–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toyooka S., Toyooka,K.O., Harada,K., Miyajima,K., Makarla,P., Sathyanarayana,U.G., Yin,J., Sato,F., Shivapurkar,N., Meltzer,S.J. and Gazdar,A.F. (2002) Aberrant methylation of the CDH13 (H-cadherin) promoter region in colorectal cancers and adenomas. Cancer Res., 62, 3382–3386. [PubMed] [Google Scholar]
- 15.Berkenkamp S., Kirpekar,F. and Hillenkamp,F. (1998) Infrared MALDI mass spectrometry of large nucleic acids. Science, 281, 260–262. [DOI] [PubMed] [Google Scholar]
- 16.Little D.P., Thannhauser,T.W. and McLafferty,F.W. (1995) Verification of 50- to 100-mer DNA and RNA sequences with high-resolution mass spectrometry. Proc. Natl Acad. Sci. USA, 92, 2318–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song J.Z., Stirzaker,C., Harrison,J., Melki,J.R. and Clark,S.J. (2002) Hypermethylation trigger of the glutathione-S-transferase gene (GSTP1) in prostate cancer cells. Oncogene, 21, 1048–1061. [DOI] [PubMed] [Google Scholar]