Abstract
Objectives:
n-Hexane, a common industrial organic solvent, causes multiple organ damage, especially neurotoxicity, which is proved to be caused by its metabolite 2,5-hexanedione (2,5-HD). We previously showed that 2,5-HD induced apoptosis of rat bone marrow mesenchymal stem cells (BMSCs). In the current study, we explored the mechanism of 2,5-HD-induced apoptosis, especially the role played by reactive oxygen species (ROS).
Methods:
Intracellular ROS levels after 2,5-HD treatment were measured by the dichloro-dihydro-fluorescein diacetate (DCFH-DA) method, and the antioxidant N-acetyl cysteine (NAC) was used to scavenge ROS. Apoptosis, mitochondrial membrane potential (MMP), and caspase-3 activity were measured after 2,5-HD exposure with or without NAC pretreatment.
Results:
In rat BMSCs, 20 mM 2,5-HD significantly increased ROS levels and apoptosis. In addition, MMP activity was decreased and caspase-3 activity was increased. With NAC pretreatment, ROS increases were prevented, cells were rescued from apoptosis, and both MMP and caspase-3 activity returned to normal levels. Western blotting analysis of malondialdehyde-modified proteins and superoxide dismutase (SOD) 1 showed that after 2,5-HD exposure, BMSCs had oxidative damage and abnormal SOD1 expression. These returned to normal when cells were pretreated with NAC in addition to 20 mM 2,5-HD. Furthermore, the expressions of NF-κB p65/RelA and phospho-NF-κB p65/RelA (Ser536) were suppressed after 2,5-HD exposure and restored by NAC pretreatment.
Conclusions:
2,5-HD-induced apoptosis in rat BMSCs is potentially mediated by excessive ROS production.
Keywords: Apoptosis; 2,5-hexanedione; Rat bone marrow mesenchymal stem cells; Reactive oxygen species
n-Hexane is a common organic solvent widely used in the fields of industrial cleaning, oil extraction, and leather adhesion. It can be absorbed by inhalation and is then distributed to lipid-rich tissues and organs such as the brain, peripheral nerves, liver, spleen, and kidneys1). Chronic exposure leads to severe neuropathy in humans and experimental animals2,3). 2,5-Hexanedione (2,5-HD), a metabolite of n-hexane, is proved to be the causative agent in neurotoxicity2,4). It can result in axonal atrophy of the peripheral and central nervous systems5,6). The mechanisms of 2,5-HD-induced neurotoxicity are unclear but likely involve its interaction with lysine residues of neurofilaments to form pyrrole adducts, followed by the cross-linked proteins produced by the adducts5,7). n-Hexane can also lead to damage to the reproductive systems8).
2,5-HD has been demonstrated to induce apoptosis in mouse dorsal root ganglia neurons9) and the human neuroblastoma cell line SH-SY5Y10). It can also induce apoptosis in ovarian granulosa cells through the Bcl-2, Bax, and caspase-3 signaling pathways11,12) and apoptosis in testicular germ cells through the Fas and caspase-3 pathways13). Therefore, apoptosis is one of the likely mechanisms for 2,5-HD-induced multiple organ toxicity.
Reactive oxygen species (ROS) increase has been detected in the spermatogenic cells after 2,5-HD exposure and can be scavenged by an antioxidant14). In addition, ROS are involved in the 2,5-HD-induced cytotoxicity of neural progenitor cells4). 2,5-HD-induced oxidative damage has been demonstrated recently in rat tissues and organs, including nerves, ovary, uterus, liver, and kidney15-17). Our previous data have shown that 2,5-HD can induce apoptosis in the bone marrow mesenchymal stem cells (BMSCs) through a mitochondrial pathway18). Because excessive ROS are usually produced by uncoupling of the mitochondrial respiratory chain by the toxins19), we hypothesized that ROS play a role in the 2,5-HD-induced apoptosis in BMSCs.
BMSCs are multipotential stem cells and can be easily acquired from bone marrow aspirate18,20,21). BMSCs have unique properties, such as self-renewal and differentiation into bone, fat, and cartilage. Many studies have demonstrated that BMSCs can directly differentiate into neurons and glial cells21-23). Therefore, they have therapeutic potential for regenerative medicine. In the current study, we evaluated 2,5-HD-induced apoptosis in BMSCs, detected the ROS production and subsequently investigated the role of ROS in apoptosis. NF-κB proteins are a family of transcription factors that are crucial to inflammation and immunity and are also involved in many pathological conditions24), including oxidative stress. Thus, we investigated the expression of NF-κB p65/RelA and phospho-NF-κB p65/RelA (Ser536) to explore the signaling pathways involved in 2,5-HD-induced BMSC apoptosis.
Materials and Methods
Animal care
Experiments were performed using male Sprague Dawley rats (120-140 g). All procedures were conducted in accordance with the Animal Guide of Dalian Medical University and approved by the Animal Care and Use Committee of Dalian Medical University.
BMSC culture and treatment
Isolation and expansion of BMSCs were performed as described previously18,25). Briefly, after rat euthanasia by cervical dislocation, BMSCs were harvested by repeated flushing of the femoral and tibial cavities and were cultured in low-glucose Dulbecco's Modified Eagle's Medium (L-DMEM; HyClone, Beijing, China) with 10% fetal bovine serum (NQBB, Hong Kong). Cells were passaged at a ratio of 1:3 when they reached 90% confluence. The BMSCs surface markers CD29, CD45, and CD90 were analyzed by flow cytometry to ensure that the purity of the cell preparation was greater than 95%. 2,5-HD (Sigma, Switzerland) solutions were prepared in cell culture medium. At about 60% confluence, cells were treated with 2,5-HD media (10, 20, 40 mM) at 37°C for 24 h. Where indicated, they were instead pretreated with 10 mM N-acetyl cysteine (NAC; Sigma, China) in culture medium for 1 h before adding 2,5-HD to the final concentrations of 20 or 40 mM and incubating for 24 h. Cell culture media with no additions or with only NAC were used as vehicles.
Analysis of apoptosis by the TUNEL assay
The TUNEL assay was performed using the In Situ Cell Death Detection Kit and Fluorescein (Roche, Mannheim, Germany). All procedures were based on the manufacturer's protocol with slight modifications. Briefly, cells with various treatments on 35-mm cell culture dishes were rinsed with PBS, fixed in 4% paraformaldehyde, then treated with permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate) for 2 min on ice. Samples were incubated with a terminal deoxynucleotidyl transferase (TdT) reaction mixture for 1 h at 37°C in a humidified chamber, then stained with Hoechst 33342 (2 μg/ml in PBS) for 5 min. Finally, samples were mounted with fluorescence mounting medium and visualized under a confocal microscope (TCS SP5; Leica, Mannheim, Germany).
ROS measurements
Intracellular ROS were measured by the dichloro-dihydro-fluorescein diacetate (DCFH-DA) method26). After various treatments, cells were rinsed twice with cold PBS, loaded with DCFH-DA (10 μM) for 40 min at 37°C and then washed. The cell number was adjusted to 5×105/ml before fluorescent intensity was detected in a fluorescence spectrophotometer (F-2700; Hitachi, Tokyo, Japan). Excitation and emission wavelengths were 488 and 525 nm, respectively. Results were expressed as fluorescent intensity per 5×105 cells.
MMP evaluation using JC-1
Mitochondrial membrane potential (MMP) in BMSCs was measured using the mitochondrion-specific lipophilic cationic dye, JC-127). JC-1 forms J-aggregates and emits red fluorescence (excitation/emission wavelength=525/590 nm) in the mitochondria with higher membrane potentials, but remains monomers and emits green fluorescence (excitation/emission wavelength=490/530 nm) in apoptotic or damaged cells with low MMP. The ratio of green to red fluorescence therefore provides a reliable estimate of the impairment of MMP. For this assay, cells were incubated with JC-1 (5 μM) in L-DMEM at 37°C for 30 min and analyzed with a confocal microscope. The mean optical density (OD) value of each sample from at least six random fields was obtained using Image-Pro Plus 6.0 (Media Cybernetics, MD), and the OD ratio of green to red fluorescence was calculated.
Caspase-3 activity assay
Caspase-3 activity was determined using a caspase-3 activity kit by measuring levels of p-nitroanilide (p-NA) cleaved from the substrate N-Ac-DEVD-pNA (Beyotime Institute of Biotechnology, Haimen, China). Supernatants of cell lysates were prepared after their respective treatments. Protein concentrations of supernatants were measured by the Bradford assay (Bio-Rad, CA). Caspase-3 activity assays were performed on the 96-well plates by incubating 40-μl supernatant with 50-μl reaction buffer (1% NP-40, 20 mM Tris-HCl, 137 mM NaCl, and 10% glycerol) and 10-μl caspase-3 substrate (2 mM Ac-DEVD pNA). Reactions were incubated at 37°C for 4 h. The OD values were measured with a microplate reader at 405 nm, and the caspase-3 activities were expressed as μM pNA/mg protein.
Western blotting analysis
After various treatments, the BMSCs were collected in PBS with 1% Halt™ Protease and Phosphatase Inhibitor (Thermo, IL) and stored at -80°C until use. Total protein concentrations in the lysates were determined using the Pierce™ 660nm protein assay kit (Thermo, IL). Then, 10-μg/lane proteins were loaded on SDS-PAGE gels. After blocking, the PVDF membrane (Bio-Rad) was incubated overnight at 4°C with one of the following antibodies: rabbit anti-superoxide dismutase (SOD) 1 antibody (1:2000; LifeSpan, WA), goat anti-malondialdehyde (MDA) antibody (1:2500; LifeSpan), rabbit anti-NF-κB p65, and anti-phospho-NF-κB p65 (Ser536) antibodies (1:1000; Cell Signaling, MA). The HRP-conjugated secondary antibodies were from Jackson ImmunoResearch (PA). Signals were detected using ECL Prime Western Blotting Detection Reagent (GE Healthcare, Buckinghamshire, UK) and ChemiDocXRS+ (Bio-Rad), then quantified densitometrically by the software Image Lab 4.1 (Bio-Rad).
Statistical analysis
Data are presented as means±SEM from at least three independent experiments. All data were analyzed with SPSS 11.5 for Windows. Differences in mean values were assessed with the Student's unpaired t test or one-way ANOVA followed by a post hoc LSD test. p values less than 0.05 were considered significant.
Results
ROS levels increased after 2,5-HD exposure
ROS levels were measured with ROS-sensitive probe DCFH-DA. Exposure of BMSCs to 2,5-HD at 20 mM and 40 mM significantly increased ROS levels from control levels of 2.5±0.09 to 4.5±0.19 and 10.3±0.35, respectively (p<0.01, Fig. 1). With the antioxidant NAC (10 mM), the increase in the ROS levels in response to 20 mM 2,5-HD was suppressed. Because of the limited ROS scavenging ability of 10 mM NAC, the ROS levels were significantly decreased (p<0.01, Fig. 1), but not restored to the normal levels in the NAC+40 mM 2,5-HD group (3.6±0.14, p<0.01, Fig. 1). Higher NAC concentrations were not tested because of possible toxic effects to the BMSCs.
Fig. 1. Effects of 2,5-HD and N-acetyl cysteine (NAC) on ROS levels. Intracellular ROS were measured by the DCFH-DA method. Results are expressed as fluorescent intensity per 5×105cells. Means ± SEM. n=3, Student's unpaired t test or one-way ANOVA with post hoc LSD test. **p<0.01.
2,5-HD induced BMSC apoptosis through ROS
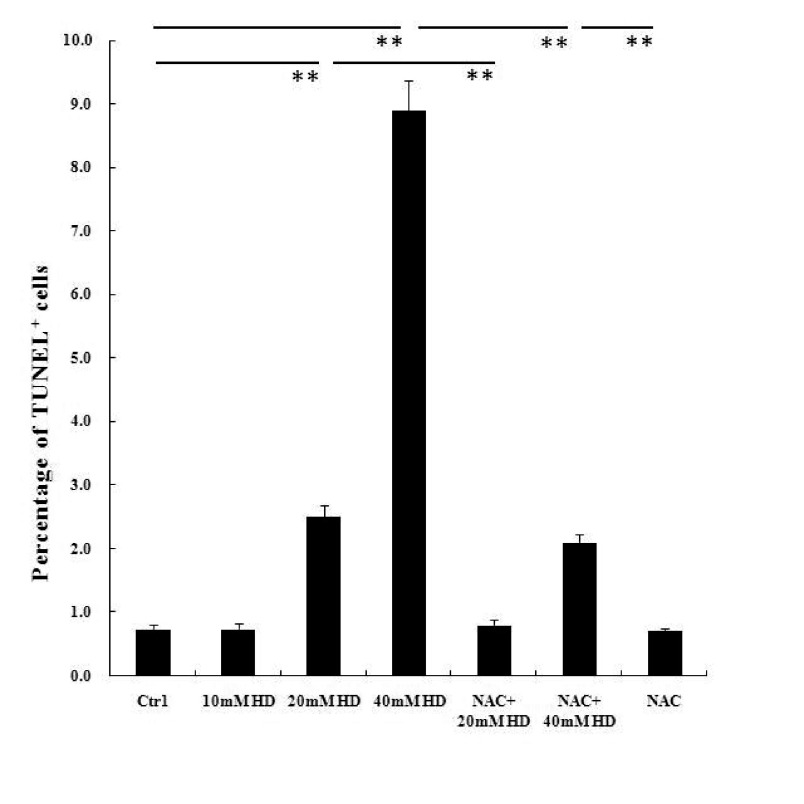
Apoptosis of BMSCs was detected by the TUNEL assay. After exposure for 24 h, 2,5-HD at 20 mM and 40 mM induced 2.5±0.18% and 8.9±0.46% apoptosis, respectively, both significantly higher than that of control (0.74±0.06%, p<0.01, Fig. 2) and corresponding to the higher ROS productions. NAC pretreatment returned apoptosis to normal in the NAC+20 mM 2,5-HD group (0.80±0.07%, p>0.05, Fig. 2), but only partially reduced apoptosis in the NAC+40 mM 2,5-HD group (2.1±0.12%, p<0.01, Fig. 2) with higher ROS level than that in the NAC group (0.71±0.07%); this indicates that ROS played important role in it.
Fig. 2. Effects of 2,5-HD and N-acetyl cysteine (NAC) on TUNEL-positive cells. Apoptosis was detected by the TUNEL assay, and samples were mounted with fluorescence mounting medium and visualized under the confocal microscope. To determine percentage of apoptotic cells, 300-500 cells were counted for each sample. Means ± SEM. n=3, Student's unpaired t test or one-way ANOVA with post hoc LSD test. **p<0.01.
Apoptosis was mediated by mitochondria and the caspase-3 pathway
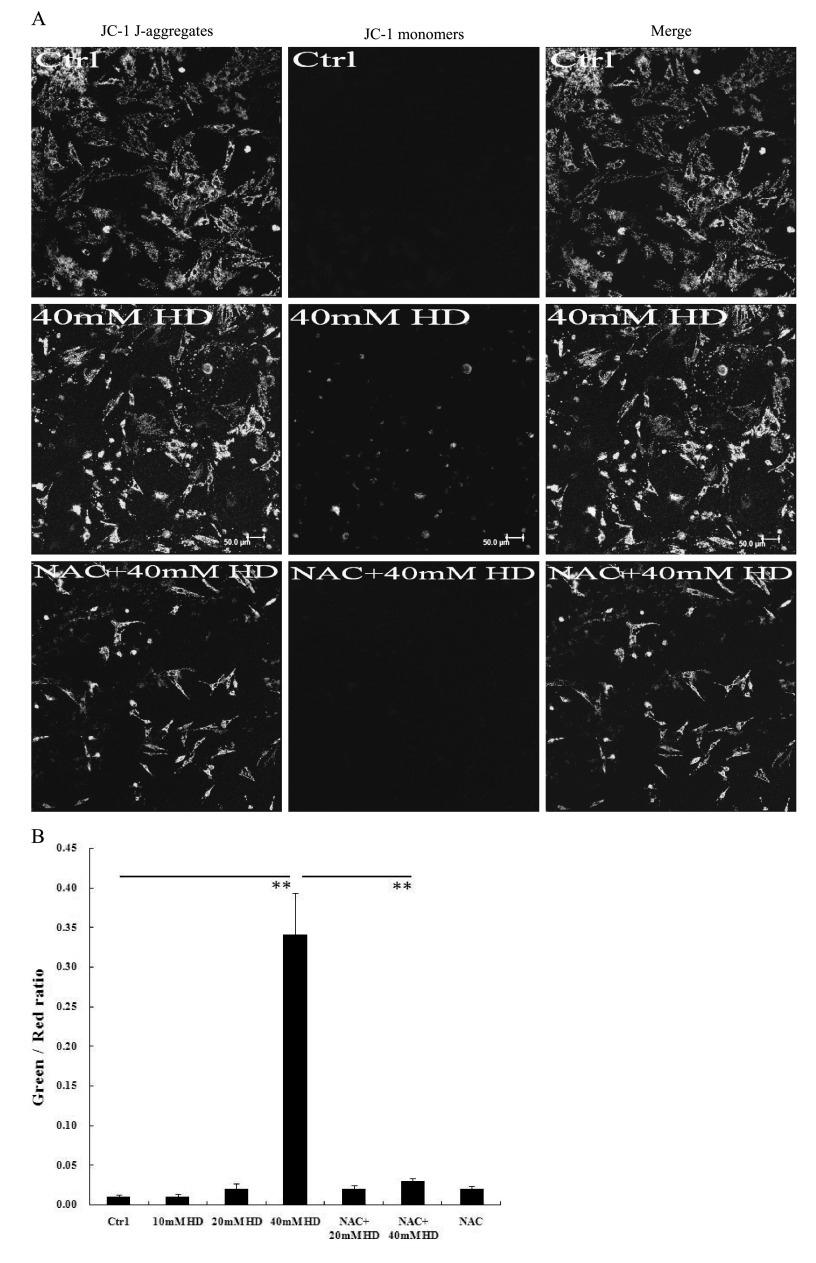
MMP was detected by staining of mitochondria with the JC-1 probe. Control cells were primarily stained red, indicating normal MMP (Fig. 3), whereas with 40 mM 2,5-HD treatment, more cells were stained green, indicating a loss of MMP (Fig. 3). MMP returned to almost normal in the NAC pretreatment group, providing a link between ROS overproduction and MMP loss (Fig. 3).
Fig. 3. Effects of 2,5-HD and N-acetyl cysteine (NAC) on mitochondrial membrane potential (MMP). Cells were incubated with JC-1 in L-DMEM at 37°C for 30 min and analyzed with a confocal microscope. The mean optical density (OD) value of each sample from at least six random fields was obtained using Image-Pro Plus 6.0, and the OD ratio of green to red fluorescence was calculated. (A) Representative JC-1 staining results. Control cells with normal MMP are primarily stained red (indicating JC-1 aggregates). Apoptotic cells with low MMP are stained green (indicating JC-1 monomers). Scale bar=50µm. (B) The OD ratio of green-to-red fluorescence with JC-1 staining. Means ± SEM. n=6-12, Student’s unpaired t test or one-way ANOVA with post hoc LSD test. **p<0.01.
There was a significant increase in the caspase-3 activity in cells after 20 mM and 40 mM 2,5-HD exposure, as compared with that of control cells (30.16 and 36.56 vs. 11.27 μM pNA/mg protein, p<0.01, Fig. 4). NAC pretreatment reduced ROS levels and returned the caspase-3 activity to normal in the NAC+20 mM 2,5-HD group (10.98±0.50 μM pNA/mg protein, p>0.05, Fig. 4), but not in the NAC+40 mM 2,5-HD group (18.17±0.85 pNA/mg protein, p<0.01, Fig. 4), indicating that more ROS were produced and higher apoptosis rate was observed. The changes in caspase-3 activity after 2,5-HD exposure with or without NAC pretreatment further supported a connection between ROS productions and apoptosis.
Fig. 4. Effects of 2,5-HD and N-acetyl cysteine (NAC) on caspase-3 activity. Caspase-3 activity was determined by measuring levels of p-NA cleaved from the substrate N-Ac-DEVD-pNA. Reactions were incubated at 37°C for 4 h. The optical density (OD) values were measured, and the caspase-3 activity expressed as μM pNA/mg protein. Means ± SEM. n=3, Student's unpaired t test or one-way ANOVA with post hoc LSD test. **p<0.01.
Western blotting
MDA levels are widely used as an indicator of lipid peroxidation28). The antibody against MDA is used to detect MDA-modified proteins. Levels of MDA-modified proteins were significantly increased in cells treated with 20 mM and 40 mM 2,5-HD (1.52±0.11 and 1.66±0.06, respectively, p<0.01, Fig. 5) as compared with those in control cells. The levels of MDA-modified proteins were reduced to normal in the NAC+20 mM 2,5-HD group (0.98±0.06, Fig. 5), but expressed high in the NAC+40 mM 2,5-HD group (1.38±0.13, p<0.05, Fig. 5) as compared with that of the NAC group (0.88±0.11). These differences corresponded well to the relative ROS levels in each group. SOD1 is the main form of three superoxide dismutases to clear free superoxide radicals in the cells29). The SOD1 expression level was significantly higher in the 20 mM 2,5-HD group (1.72±0.16, p<0.01, Fig. 5), returned to normal in the NAC+20 mM 2,5-HD group (0.94±0.01), and remained elevated in the NAC+40 mM 2,5-HD group (1.40±0.09, p<0.05, Fig. 5), consistent with the relatively high ROS levels in this group.
Fig. 5. Effects of 2,5-HD and N-acetyl cysteine (NAC) on levels of MDA and SOD1. (A) Representative Western blotting images showing MDA and SOD1. (B) Quantitative densitometric analysis of MDA and SOD1 using Image Lab 4.1. All densitometry values were normalized to that of actin, and the ratio of the control group was defined as 1.00. Means ± SEM. n=3, Student's unpaired t test or one-way ANOVA with post hoc LSD test. *p<0.05, **p<0.01.
Activation of transcription factor NF-κB can translocate p65/RelA to the nucleus to regulate gene expressions24). Expression levels of p65/RelA were significantly lower in the 20 and 40 mM 2,5-HD groups (0.67±0.07 and 0.54±0.03, respectively, p<0.01, Fig. 6) as compared with that of control, and increased significantly in the NAC+20 mM and NAC+40 mM 2,5-HD groups (1.1±0.06 and 0.98±0.06, p<0.05 and p<0.01, respectively, Fig. 6). Phosphorylation of p65/RelA at serine 536 is important for its enhanced transcription activity30). Expression levels of phospho-NF-κB p65/RelA (Ser536) were decreased significantly in all the 2,5-HD-treated groups (0.08-0.52, p<0.01, Fig. 6). With NAC, the expression of phospho-NF-κB p65/RelA (Ser536) in the NAC+20 mM (0.42±0.06) and NAC+40 mM (0.35±0.08) 2,5-HD groups increased significantly compared to that without NAC pretreatment (0.08-0.16) and returned to the levels in the NAC group (0.46±0.07).
Fig. 6. Effects of 2,5-HD and N-acetyl cysteine (NAC) on levels of NF-κB p65/RelA and phospho-NF-κB p65/RelA (Ser536). (A) Representative Western blotting images showing NF-κB p65/RelA and phospho-NF-κB p65/RelA (Ser536). (B) Quantitative densitometric analysis of NF-κB p65/RelA and phospho-NF-κB p65/RelA (Ser536) using Image Lab 4.1. All densitometry values were normalized to that of actin, and the ratio of the control group was defined as 1.00. Means ± SEM. n=3, Student’s unpaired t test or one-way ANOVA with post hoc LSD test. *p<0.05, **p<0.01.
Discussion
In this study, 20 mM 2,5-HD induced significant BMSC apoptosis. These apoptosis levels returned to normal by the antioxidant NAC, indicating that the apoptosis was caused by excessive ROS production. When BMSCs were treated with 40 mM 2,5-HD, higher ROS and apoptosis levels were detected, and the same concentration of NAC could not clear the ROS to the normal levels as shown by the ROS value and the Western blotting results using the antibody against MDA; consequently, apoptosis was apparent in the NAC+40 mM 2,5-HD group. MMP was reduced significantly in the 20 mM and 40 mM 2,5-HD groups with concomitant increases in caspase-3 activities; moreover, both MMP and caspase-3 activity returned to normal in the NAC+20 mM 2,5-HD group but remained abnormal in the NAC+40 mM 2,5-HD group. These data indicated an important role for ROS in 2,5-HD-induced apoptosis. ROS-induced cell death has been reported in 2,5-HD-treated neural precursor and spermatogenic cells4,14) with apoptosis demonstrated in the spermatogenic cells14). In the current study, BMSCs were used, and the role ROS played in the apoptosis was shown.
2,5-HD concentration of 8.8 mM (0.1%) and 20 mM has been shown to induce apoptosis in the mouse neurons and rat ovarian granulosa cells, respectively9,12). In a rat toxicity model, the serum 2,5-HD level is 2.8 mM31), which is lower than the 10-40 mM used in our study. However, because of the accumulation of toxic effects of 2,5-HD during long-term exposure in vivo, the higher concentrations we used were appropriate for our short-term exposure experiments.
SOD1 participates in the clearance of ROS29); the high expression of SOD1 in the 20 mM 2,5-HD group indicated that more antioxidant enzyme was induced as more ROS formed29). In the 40 mM 2,5-HD group, although more ROS were produced and more SOD1 was needed to clear them, the SOD1 level was lower than that in the 20 mM group. This was probably because of the inhibition effect of high concentration of 2,5-HD on SOD1 synthesis. Excessive ROS are usually produced by uncoupling of the mitochondrial respiratory chain by the toxins19). In the current study, excessive ROS are probably produced by uncoupling of the mitochondria respiratory chain, that is, inhibition of complexes I to IV by the toxin 2,5-HD acting in concert with the limited clearance ability of antioxidant enzymes such as SOD1. The high ROS levels and relative low SOD1 expression in the 40 mM 2,5-HD group were consistent with this explanation.
Low MMP has been used as the indicator of apoptosis32). MMP was significantly lower in the 2,5-HD-treated groups, which was followed by the release of cytochrome c through the membrane pore. The cytochrome c switched the signal to activate the executioner caspase-333) and the apoptosis, as shown in our study. Related mechanisms of mitochondria- and caspase-3-mediated apoptosis have been reported in 2,5-HD-exposed ovarian granulosa, sperm cells, and BMSCs11-13),18). Similarly, decreased Bcl-2/Bax ratio is observed in ovarian granulosa cells11,12) and in the BMSCs that we used for the present study18).
NF-κB activation can translocate p65/RelA to the nucleus where it regulates gene expression, including inhibiting apoptosis34). Liver apoptosis is demonstrated in the p65/RelA-deficient mice35), and combined inhibition of NF-κB and Bcl-2 induces apoptosis in the melanoma cells36). Our results showed decreased expressions of p65/RelA in 20 and 40 mM 2,5-HD groups, which would reduce p65/RelA-mediated transactivation. The inhibition of Bcl-2 is reported in our previous work18). Whether NF-κB and Bcl-2 act in BMSCs as they are reported to act in the melanoma cells needs to be studied further. The phosphorylation of p65 at serine 536 has been reported to be important for the enhanced transcriptional activity30). NAC is found to induce the phosphorylation of p65 in the cytoplasm independent of its antioxidant function, indicating that it may modulate cellular functions either independent or dependent of ROS37). In our study, NAC pretreatment improved total and phospho-NF-κB p65/RelA expression, indicating that excessive ROS inhibited p65/RelA production directly. However, the role that low NF-κB expression played in the 2,5-HD-mediated apoptosis needs further investigation, including examining the specific activation and inhibition of this factor.
In summary, in BMSCs, ROS overproduction induced by exposure to the toxin 2,5-HD decreased MMP, which resulted in activation of the downstream executioner caspase-3 and apoptosis. The ROS scavenger NAC normalized MMP and caspase-3 activity and rescued apoptosis. These data support our hypothesis that ROS are involved in 2,5-HD-mediated toxicity of BMSCs. However, the detailed molecular mechanism of this apoptosis must be further explored.
Conflicts of interest: None.
Acknowledgments: This research was supported by National Natural Science Foundation of China (No. 81273038)
References
- 1). Huang CC. Polyneuropathy induced by n-hexane intoxication in Taiwan. Acta Neurol Taiwan 2008; 17: 3-10. [PubMed] [Google Scholar]
- 2). Neghab M, Soleimani E, Khamoushian K. Electrophysiological studies of shoemakers exposed to sub-TLV levels of n-hexane. J Occup Health 2012; 54: 376-382. [DOI] [PubMed] [Google Scholar]
- 3). Rao DB, Jortner BS, Sills RC. Animal models of peripheral neuropathy due to environmental toxicants. ILAR J 2014; 54: 315-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Kim MS, Park HR, Park M, et al. Neurotoxic effect of 2,5-hexanedione on neural progenitor cells and hippocampal neurogenesis. Toxicology 2009; 260: 97-103. [DOI] [PubMed] [Google Scholar]
- 5). LoPachin RM, DeCaprio AP. gamma-Diketone neuropathy: axon atrophy and the role of cytoskeletal protein adduction. Toxicol Appl Pharmacol 2004; 199: 20-34. [DOI] [PubMed] [Google Scholar]
- 6). Song F, Zhang C, Yu S, Zhao X, Yu L, Xie K. Time-dependent alteration of cytoskeletal proteins in cerebral cortex of rat during 2,5-hexanedione-induced neuropathy. Neurochem Res 2007; 32: 1407-1414. [DOI] [PubMed] [Google Scholar]
- 7). Graham DG, Amarnath V, Valentine WM, Pyle SJ, Anthony DC. Pathogenetic studies of hexane and carbon disulfide neurotoxicity. Crit Rev Toxicol 1995; 25: 91-112. [DOI] [PubMed] [Google Scholar]
- 8). Sallmen M, Neto M, Mayan ON. Reduced fertility among shoe manufacturing workers. Occup Environ Med 2008; 65: 518-524. [DOI] [PubMed] [Google Scholar]
- 9). Ogawa Y, Shimizu H, Kim SU. 2,5-Hexanedione induced apoptosis in cultured mouse DRG neurons. Int Arch Occup Environ Health 1996; 68: 495-497. [DOI] [PubMed] [Google Scholar]
- 10). Zilz TR, Griffiths HR, Coleman MD. Apoptotic and necrotic effects of hexanedione derivatives on the human neuroblastoma line SK-N-SH. Toxicology 2007; 231: 210-214. [DOI] [PubMed] [Google Scholar]
- 11). Sun Y, Lin Y, Li H, Liu J, Sheng X, Zhang W. 2,5-Hexanedione induces human ovarian granulosa cell apoptosis through BCL-2, BAX, and CASPASE-3 signaling pathways. Arch Toxicol 2012; 86: 205-215. [DOI] [PubMed] [Google Scholar]
- 12). Zhang W, Huang L, Kong C, Liu J, Luo L, Huang H. Apoptosis of rat ovarian granulosa cells by 2,5-hexanedione in vitro and its relevant gene expression. J Appl Toxicol 2013; 33: 661-669. [DOI] [PubMed] [Google Scholar]
- 13). Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology 1997; 138: 2081-2088. [DOI] [PubMed] [Google Scholar]
- 14). Mishra DP, Pal R, Shaha C. Changes in cytosolic Ca2+levels regulate Bcl-xS and Bcl-xL expression in spermatogenic cells during apoptotic death. J Biol Chem 2006; 281: 2133-2143. [DOI] [PubMed] [Google Scholar]
- 15). Abolaji AO, Adedara IA, Soladogun A, Salau V, Oguaka M, Farombi EO. Exposure to 2,5-hexanedione is accompanied by ovarian and uterine oxidative stress and disruption of endocrine balance in rats. Drug Chem Toxicol [published online ahead of print November 13, 2014]. (doi: 10.3109/01480545.2014.974265). [DOI] [PubMed] [Google Scholar]
- 16). Adedara IA, Abolaji AO, Odion BE, Okwudi IJ, Omoloja AA, Farombi EO. Impairment of hepatic and renal functions by 2,5-hexanedione is accompanied by oxidative stress in rats. J Toxicol [published online ahead of print November 8, 2014]. (doi: 10.1155/2014/239240). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Torres ME, Dos Santos AP, Goncalves LL, Andrade V, Batoreu MC, Mateus ML. Role of N-acetylcysteine in protecting against 2,5-hexanedione neurotoxicity in a rat model: Changes in urinary pyrroles levels and motor activity performance. Environ Toxicol Pharmacol 2014; 38: 807-813. [DOI] [PubMed] [Google Scholar]
- 18). Chen R, Liu S, Piao F, et al. 2,5-Hexanedione induced apoptosis in mesenchymal stem cells from rat bone marrow via mitochondria-dependent caspase-3 pathway. Ind Health 2015; 53: 222-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Sinha K, Das J, Pal PB, Sil PC. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol 2013; 87: 1157-1180. [DOI] [PubMed] [Google Scholar]
- 20). Scanu M, Mancuso L, Cao G. Evaluation of the use of human mesenchymal stem cells for acute toxicity tests. Toxicol In Vitro 2011; 25: 1989-1995. [DOI] [PubMed] [Google Scholar]
- 21). Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 2000; 61: 364-370. [DOI] [PubMed] [Google Scholar]
- 22). Bossolasco P, Cova L, Calzarossa C, et al. Neuro-glial differentiation of human bone marrow stem cells in vitro. Exp Neurol 2005; 193: 312-325. [DOI] [PubMed] [Google Scholar]
- 23). Kim BJ, Seo JH, Bubien JK, Oh YS. Differentiation of adult bone marrow stem cells into neuroprogenitor cells in vitro. Neuroreport 2002; 13: 1185-1188. [DOI] [PubMed] [Google Scholar]
- 24). Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res 2011; 21: 103-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Wang Y, Sun Z, Qiu X, Li Y, Qin J, Han X. Roles of Wnt/beta-catenin signaling in epithelial differentiation of mesenchymal stem cells. Biochem Biophys Res Commun 2009; 390: 1309-1314. [DOI] [PubMed] [Google Scholar]
- 26). Sohn JH, Han KL, Lee SH, Hwang JK. Protective effects of panduratin A against oxidative damage of tert-butylhydroperoxide in human HepG2 cells. Biol Pharm Bull 2005; 28: 1083-1086. [DOI] [PubMed] [Google Scholar]
- 27). Lai HC, Yeh YC, Wang LC, et al. Propofol ameliorates doxorubicin-induced oxidative stress and cellular apoptosis in rat cardiomyocytes. Toxicol Appl Pharmacol 2011; 257: 437-448. [DOI] [PubMed] [Google Scholar]
- 28). Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011; 283: 65-87. [DOI] [PubMed] [Google Scholar]
- 29). Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med 2002; 33: 337-349. [DOI] [PubMed] [Google Scholar]
- 30). Yang F, Tang E, Guan K, Wang CY. IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J Immunol 2003; 170: 5630-5635. [DOI] [PubMed] [Google Scholar]
- 31). Yin HY, Guo Y, Song FY, Zeng T, Xie KQ. Toxicokinetic study of pyrrole adducts and its potential application for biological monitoring of 2,5-hexanedione subacute exposure. Int Arch Occup Environ Health 2014; 87: 655-662. [DOI] [PubMed] [Google Scholar]
- 32). Green DR, Reed JC. Mitochondria and apoptosis. Science 1998; 281: 1309-1312. [DOI] [PubMed] [Google Scholar]
- 33). Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 2007; 35: 495-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 2006; 72: 1493-1505. [DOI] [PubMed] [Google Scholar]
- 35). Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature 1995; 376: 167-170. [DOI] [PubMed] [Google Scholar]
- 36). Watanabe M, Umezawa K, Higashihara M, Horie R. Combined inhibition of NF-kappaB and Bcl-2 triggers synergistic reduction of viability and induces apoptosis in melanoma cells. Oncol Res 2013; 21: 173-180. [DOI] [PubMed] [Google Scholar]
- 37). Liu J, Yoshida Y, Yamashita U. DNA-binding activity of NF-kappaB and phosphorylation of p65 are induced by N-acetylcysteine through phosphatidylinositol (PI) 3-kinase. Mol Immunol 2008; 45: 3984-3989. [DOI] [PubMed] [Google Scholar]