Abstract
Objective:
The purpose of this study was to investigate the effects of Nickel (Ni) -smelting fumes on oncogenic proteins in vivo and in vitro.
Methods:
Ni fallout beside a Ni smelting furnace in a factory was sampled to study its toxic effect. The effects of Ni-smelting fumes on the regulation of PI3K and ERK signaling pathways and the important downstream hypoxia inducible factor, HIF-1α, were studied both in NIH/3T3 cells and in the lung tissue of rats. NIH/3T3 cell transformation induced by Ni-smelting fumes was also observed.
Results:
Ni-smelting fumes activated PI3K, p-AKT, p70S6K1, and ERK proteins and increased HIF-1α expression in a time- and dose-dependent manner. However, activation was suppressed when NIH/3T3 cells were pretreated with PI3K/AKT or ERK inhibitors. Ni-smelting fumes caused malignant transformation of NIH/3T3 cells.
Conclusions:
Ni-smelting fumes increased the expression of HIF-1α through the PI3K/ERK pathway in NIH/3T3 cells and induced malignant transformation in these cells indicating that Ni-smelting fumes may be a potential carcinogen in mammalian cells.
Keywords: Animal toxicity, Cytotoxicity, HIF-1α, Kinase, Nickel-smelting fumes
Introduction
In this industrial society, the demand for metal products has significantly increased, and environmental pollution due to heavy metals is inevitable. During the use and cleaning of metal products, occupational exposure is the main source of exposure to heavy metals via inhalation into the respiratory tract in humans, followed by the intake of food with a high metal content, particularly in metal welders, miners and smelters. Metal accumulation in the human body can lead to a variety of diseases, including cancer.
Nickel (Ni) is one of the essential trace elements in the human body, and animal experiments have showed that Ni is beneficial for the skeleton, reproductive function, sensory function, and energy metabolism1). Ni is widely used in a variety of industrial products and consumer goods such as batteries, stainless steel, coins, etc2). Ni compounds can be released into the environment during each production process3). Occupational exposure to Ni compounds occurs mainly in Ni ore mining, ore dressing, smelting of Ni and Ni alloy production plating and welding processes4). Ni compounds are absorbed into the respiratory tract, the digestive system and through skin contact5-7). Of the different levels of damage to human health following exposure to Ni compounds, the most serious is carcinogenesis8,9).
There are two forms of Ni compounds, namely, soluble and insoluble; the toxicity of the latter is greater than the former and epidemiological studies have shown that both forms are related to lung cancer and carcinoma of the nasal cavity10). An enormous amount of effort has been devoted to the carcinogenic mechanism of Ni compounds, and Ni carcinogenicity was first reported in 1943. A number of epidemiological studies have shown that Ni-smelter workers have a high risk of lung cancer. It is recognized that the process of carcinogenesis is complex, as Ni compounds cause cancer through multiple mechanisms, and the carcinogenic effect is likely to be activated by specific transcription factors through gene expression changes11).
A study found that type IA PI3K and its downstream protein kinase B (PKB or Akt) were closely related to the occurrence and progression of human tumors. P70S6K1, an important downstream member of the PI3K/AKT pathway, is the direct substrate of mTOR, which can integrate amino acid and signaling pathways stimulated by growth factors12). PI3K/AKT plays an important role in the occurrence and development of many types of tumors13-17). Mitogen-activated protein kinase (MAPK) plays an important role in controlling a variety of physiological processes in the signal network, such as cell growth, development, division, and death. As a member of the MAPK family, ERK (extracellular signal-regulated kinase) was the first to be identified and is the best known. Research has shown that Ni compounds can activate the MAPK signaling pathway, including ERK1/218). More recently, the activation of ERK was closely related to tumor formation and was found to be highly expressed in many cancers, including lung cancer19).
Hypoxia inducible factor-1 (HIF-1) is a transcription factor consisting of two subunits, HIF-1α and HIF-1β. HIF-1α is the main activity unit, and its expression is controlled by the partial pressure of oxygen. HIF-1α is highly expressed under the condition of hypoxia; thus, a series of reactions activate tumor cells, which respond to hypoxia. Ni is a strong hypoxia inducing agent, which can activate HIF-1α and induce many hypoxia signals to transfer related target gene transcription. Previous research indicated that ERK1/2 and the PI3K/AKT pathway can be activated under hypoxia in a variety of cell lines20-22). Minet et al. pointed out that bovine pulmonary artery adventitial fibroblast proliferation can be stimulated only by hypoxia through the ERK1/2 and PI3K/AKT1 pathways23).
There have been numerous studies on the toxicology of Ni compounds; however, these studies focused on the Ni toxicology mechanism using pure Ni compounds such as Ni sulfate, Ni acetate, and Ni oxide, which do not represent the compounds found in occupationally exposed populations24). In a previous study by our research group, an analysis of the components of Ni refining dust was carried out using inductively coupled plasma mass spectrometry (ICP-MS)25). The results showed that the dust contained 48.3% Ni and levels of copper, chromium, manganese and other metal elements which indicated that Ni-smelting fumes consisted of various metals. In this study, we sampled Ni-smelting fumes from a Ni smelting furnace to simulate the environment in an occupationally exposed population. Our research group has certain base already on nickel smelting fumes26). The results showed that nickel-smelting fumes had strong cytotoxicity which laid the foundation for our research. Our laboratory has a certain research base for the nickel smelting fume collected by different processes. This study is the first to research the possible carcinogenic mechanism of nickel-smelting fumes involving key oncogene signaling proteins in humans. This has not been published in other journals. Our study not only provides new experimental basis for the mechanism of Nickel-smelting fumes carcinogenicity but also brings out great practical significance to protect the workers health.
Materials and Methods
Reagents and Antibodies
Antibodies against PI3K (#4249), phospho-AKT (#4060), and p70S6K1 (#2708), ERK (#4370) and HIF-1α (#14179) were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against β-actin (YT0099) and HIF-1α (YT2133) for immunohistochemistry were obtained from Immunoway (DE, USA). Rapamycin (#9904), U0126 (#9903), LY294002 (#9901) were purchased from Cell Signaling Technology (Beverly, MA, USA). Stocks of U0126, LY294002, and rapamycin were prepared in DMSO and stored at -20°C. Normal melting-point agarose and low melting-point agarose were purchased from Gibco (CA, USA).
Cell Culture
NIH/3T3 cells were purchased from the Shanghai Institutes for Biological Sciences (Shanghai, China) and routinely cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL) supplemented with 10% fetal bovine serum (FBS; Sijiqing, China), 1% penicillin G, and 100 mg/mL streptomycin at 37°C in a humidified chamber with a 5% carbon dioxide atmosphere. The cells were exposed to different concentrations of Ni-smelting fumes for different periods of time (0h,6h,12h,24h,48h) which was determined by the specific individual experiment.
Animals
This study was performed in accordance with the "Guide for the Care and Use of Laboratory Animals" of National Institutes of Health guidelines (NIH) and was authorized by the Pharmacia Animal Care and Use Committee of Harbin medical university.
Male Wistar rats aged 6-7 weeks were obtained from Vitalriver (Beijing, China). The animals were housed in specific pathogen-free conditions at the temperature of 23°C± 2°C with free access to food and water. Forty rats were randomly divided into 5 different groups (n=8 per group) and exposed to different concentrations of Ni-smelting fumes (0.00 mg/kg per rat, 0.50 mg/kg per rat, 1.00 mg/kg per rat, 2.00 mg/kg per rat, 3.00 mg/kg per rat) by endotracheal instillation every fifteen days (a total of two exposures). After treatment for 4 weeks, the rats were sacrificed, and their lung tissues were sampled (integral dose: 0.00 mg/kg per rat, 1.00 mg/kg per rat, 2.00 mg/kg per rat, 4.00 mg/kg per rat and 6.00 mg/kg per rat).
Preparation of Test Materials
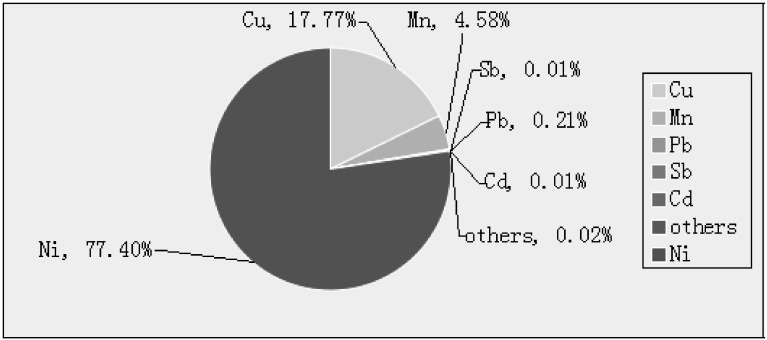
Ni-smelting fumes were sampled from the process of nickel purify smelting furnace in a Chinese refining plant. Particle diameter in the fumes was less than 5 μm in 99%, and the particles were insoluble. The components of the Ni-smelting fumes were analyzed by ICP-MS (Fig. S.1). The fumes were diluted in phosphate-buffered saline (PBS; Maxim) prior to use.
Fig. S.1.
Testing results of six metals in Ni-smelting fumes.
Determination of metal content in Ni-smelting fumes by ICP-MS
The mixing standards of Ni, Mn, Cu, etc (Agilent, USA) were diluted in a standard series with 1% HNO3, and the concentrations were 0.0, 2.5, 10.0, 20.0, 50.0 mg/mL. Ni-smelting fumes (0.2 g) were dispelled in accordance with the microware digestion procedure. After digestion, the liquid was placed in a volumetric flask and ultrapure water was added to 50 mL. In addition, a blank control group was prepared using the same method with 5 mL HNO3, 1 mL H2O2 and 0.1 mL HF. The blank control group, standard series and sample liquid were introduced into the inductively coupled plasma mass spectrometer (Agilent, 7500 series, USA), which determined the concentration of each metal.
Western Blot
Cells were treated with different concentrations of Ni-smelting fumes for different times and then harvested at 70%-80% confluence. Cellular protein was extracted by lysis buffer and centrifuged at 12,000 rpm for 10 min. Concentrations of the protein extracts were measured using BCA protein assay reagent (Beyotime, China) and a microplate reader (Molecular Devices, CA, USA). Cellular protein was subjected to 8%-15% sodium dodecyl sulfate polyacrylamide gel, and the resolved proteins were electroblotted onto PVDF (polyvinylidene fluoride) membranes. The membranes were blocked with nonfat milk in Tris-buffered saline containing 0.1% Tween-20 for 10 min at 37°C and incubated with appropriate primary antibodies at 4°C overnight and horseradish peroxidase conjugated secondary antibody for 30 min. The protein blots were visualized by incubation with Western Blue (Promega, USA) to detect positive protein bands, and protein blots were analyzed using ChemiAnalysis image analysis software (Clinx Science Instruments, China). Equal loading of the samples was determined by quantitation of proteins and by reprobing membranes for β-actin.
Cell Transformation Activity
Using the method of cell focus, cell suspension inoculation fluid was prepared in 25 mL culture bottles, and each bottle was inoculated with 5.0×105 cells. After 24 h, five concentration groups (6.25, 12.50, 25.00, 50.00, 100.00 μg/mL) were prepared with three culture bottles in each group. Negative controls without cells were also prepared. After 24 h, the cells were washed with PBS three times and placed in 10% serum DMEM growth medium, once every 3 days for growth medium. The medium was changed to 5% serum DMEM growth medium when 80% of the cells covered the bottom of the culture bottle until termination of the experiment at 21 days.
Observation of cell transformation was carried out as follows: Transformed cells were taken from 2 culture bottles per concentration group, fixed with methanol, stained with Giemsa, and transformed cells were identified (a cell aggregation of more than 50 cells was regarded as a cell focus).
Soft Agar Culture
Transformation cell suspension was prepared in 24 pore plate, and each hole was inoculated with 0.8mL underlying soft agar (1.2% agarose and 2× DMEM mixed by 1:1). After solidification, top agar (1.2% agarose and 2× DMEM mixed by 1:1 ratio) were added in each hole. After 14 d, the colony was observed and the colony numbers were counted when more than 20.
Hematoxylin-eosin Staining
The fixed lung tissue samples were embedded in paraffin, sectioned at 5 μm, dewaxed and dehydrated with gradient alcohol from 70% to 100% then stained with hematoxylin and eosin to evaluate alveolar integrity.
Immunohistochemistry
Paraffin-embedded sections (5 mm/4 μm in thickness) of rat lung were used to evaluate the expression of PI3K, p-AKT, p70S6K1, ERK and HIF-1α. These sections were deparaffinized and rehydrated. The slides were subjected to antigen retrieval by microwave heating (800 W for 30 min) in citrate buffer for 20 min. The sections were exposed to primary antibody: anti-PI3K, anti-p-AKT, anti-P70S6K1, anti-ERK, and anti-HIF-1α monoclonal antibodies at dilutions of 1:50-1:250, and were incubated overnight at 4°C. This was followed by incubation with the secondary biotinylated antibody for 20 min at room temperature.
Statistical Analysis
Data were analyzed by SPSS18.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as mean ± standard error of the mean (SEM). Statistical comparisons between groups were estimated using one-way analysis of variance (ANOVA). Further pairwise comparisons were carried out using the Least Significant Difference t test (LSD) and Fisher's Exact Test. Dose-response relationship was assessed using the linear independent method. A p value of ≤0.05 indicated statistical significance.
Results
Nickel-smelting fumes were composed primarily of six metal elements
The composition of Ni-smelting fumes was determined by ICP-MS, which showed that the fumes were composed of six metals (Ni, Mn, Cu, Cd, Pb, Sb). As shown in Fig. S1, the main component of occupational dusts in the Ni smelting workshop was Ni, which accounted for 77.39% of all components. Small amounts of the other metals were found. These results indicated that the main source of effect was Ni. Furthermore, a synergistic effect between these metals may occur during the pathogenic process.
Time-Dependent Alterations in PI3K, p-AKT, P70S6K1, ERK, and HIF-1α Expression after Exposure to Ni-Smelting Fumes
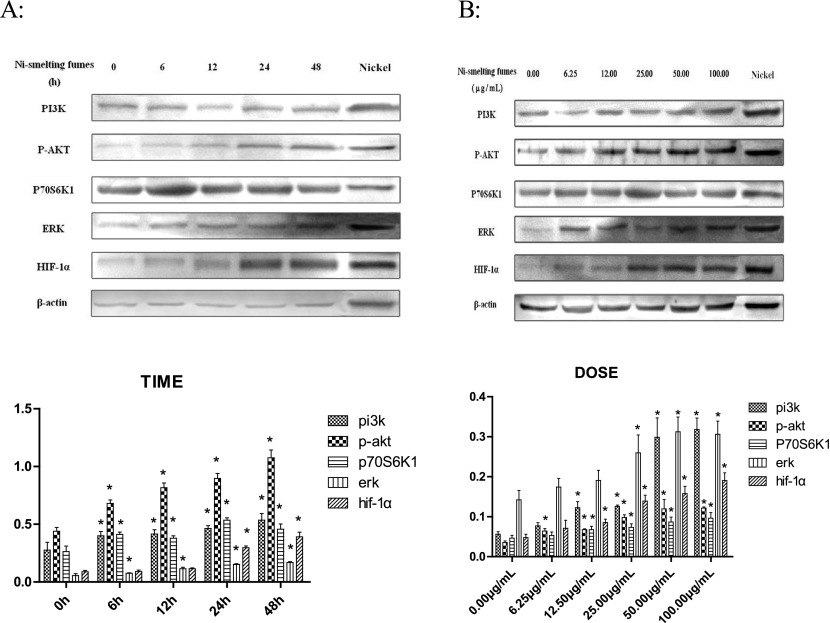
To determine the effects of Ni-smelting fumes on signal transduction in NIH/3T3 cells, we assessed whether Ni-smelting fumes changed the expression of the PI3K and ERK signaling pathways and their downstream signaling molecule HIF-1α. NIH/3T3 cells were exposed to 50.00 μg/mL Ni-smelting fumes for different periods of time (6, 12, 24, and 48 h). A negative control without cells and a positive control (Ni powder at 50.00 μg/mL for 24 h) were also included. The effects of Ni-smelting fumes on PI3K, p-AKT, p70S6K1, and ERK signaling were analyzed by immunoblot (Fig. 1A). The experiment was repeated three times. The results showed that Ni-smelting fumes induced the activation of PI3K, p-AKT, p70S6K1 from 6 h to 24 h (PI3K from 0.400±0.036 to 0.535±0.056; p-AKT from 0.680±0.029 to 1.075±0.067; p70S6K1 from 0.412±0.019 to 0.456±0.045), compared with negative control, *p<0.05. ERK was activated from 6 h to 24 h (from 0.114±0.013 to 0.168±0.008), compared with negative control, *p<0.05. Ni-smelting fumes increased the level of HIF-1α at 24 h (0.296±0.015, *p<0.05). Activation was time-dependent (R2=0.830 for PI3K, p<0.05; R2=0.929 for p-AKT, p<0.05; R2=0.811 for p70S6K1, p<0.05; R2=0.971 for ERK, p<0.05; R2=0.912 for HIF-1α, p<0.05). There was marked activation of HIF-1α following exposure to Ni-smelting fumes for 24 h and 48 h (*p<0.05).
Fig. 1.
A: Ni-smelting fumes activated PI3K and ERK pathways in a time-dependent manner. NIH/3T3 cells were exposed to 50.00 μg/mL Ni-smelting fumes for 6, 12, 24, and 48 hours and then analyzed by Western blot with antibodies against PI3K, p-AKT, P70S6K1, ERK, HIF-1α, and β-actin. The experiment was repeated 3 times, compared with the negative control, *P<0.05. B: Ni-smelting fumes activated PI3K and ERK pathways and HIF-1α in a dose-dependent manner. NIH/3T3 cells were exposed to Ni-smelting fumes for 24 hours with concentrations 0.00 μg/mL, 6.25 μg/mL, 12.50 μg/mL, 25.00 μg/mL, 50.00 μg/mL and 100.00 μg/mL then analyzed by Western blot with antibodies against PI3K, p-AKT, P0S6K1, ERK, HIF-1α, and β-actin. The experiment was repeated 3 times, compared with the negative control, *P<0.05.
Dose-Dependent Alterations in PI3K, P-AKT, P70S6K1, ERK, and HIF-1α Expression after Exposure to Ni-Smelting Fumes
NIH/3T3 cells were exposed to Ni-smelting fumes at concentrations of 0.00, 6.25, 12.50, 25.00, 50.00, and 100.00 μg/mL for 24 h (Fig. 1B). A negative control without cells and a positive control (Ni powder at 100.00 μg/mL for 24 h) were also included. The experiment was repeated three times. The results showed that exposure to Ni-smelting fumes activated PI3K, p-AKT, p70S6K1, and HIF-1α expression from 12.50 μg/mL to 100.00 μg/mL (PI3K from 0.122±0.015 to 0.318±0.0285; p-AKT from 0.670±0.002 to 0.121±0.003; p70S6K1 from 0.067±0.008 to 0.096±0.013; HIF-1α from 0.085±0.008 to 0.190±0.0195). Compared with negative control, *p<0.05. Ni-smelting fumes markedly increased the expression of HIF-1α at 25.00 μg/mL (compared with negative control, *p<0.05). When the concentration of Ni-smelting fumes increased, the protein expression of ERK was activated at 25.00 μg/mL (0.259±0.044, compared with negative control,*p<0.05). The activation of oncogene expression was dose-dependent (R2=0.908 for PI3K, p<0.05; R2=0.906 for p-AKT, p<0.05; R2=0.793 for p70S6K1, p<0.05; R2=0.856 for ERK, p<0.05; R2=0.913 for HIF-1α, p<0.05).
The expression of HIF-1α induced by Ni-smelting fumes was suppressed by PI3K/AKT or ERK inhibitors
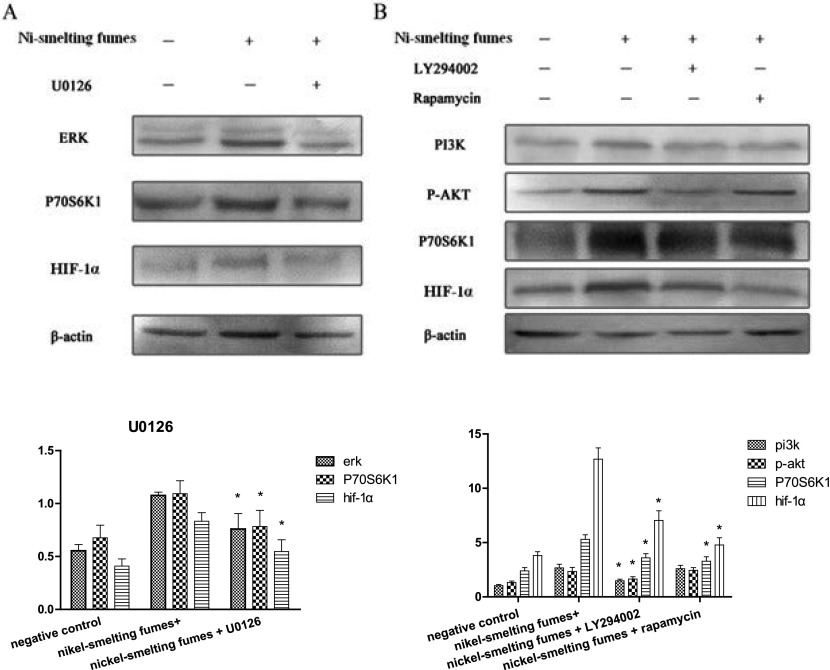
NIH/3T3 cells were pretreated with the PI3K/AKT inhibitor, U0126, the p70S6K1 inhibitor, LY294002, or the ERK inhibitor, Rapamycin, for 0.5-1 h before exposure to 50.00 μg/mL Ni-smelting fumes (Fig. 2). The experiment was repeated three times. This was carried out to determine whether the ERK and PI3K pathways are necessary for the Ni-smelting fumes-induced increase in HIF-1α expression to identify the relationship between the two signaling pathways and HIF-1α. The results showed that U0126 inhibited the expression of ERK (decreased from 1.076±0.030 to 0.759±0.146, *p<0.05) and P70S6K1 (decreased from 1.092±0.123 to 0.783±0.152, *p<0.05) and significantly reduced the level of HIF-1α (from 0.832±0.022 to 0.546±0.110, *p<0.05). Both LY294002 and Rapamycin inhibited the expression of PI3K, p-AKT and p70S6K1 (LY294002 and Rapamycin inhibited PI3K from 2.665±0.193 to 1.480±0.142 and 1.450±0.181, respectively, *p<0.05; LY294002 and Rapamycin inhibited p-AKT from 2.332±0.247 to 1.645±0.095 and 1.820±0.179, respectively, *p<0.05; LY294002 and Rapamycin inhibited P70S6K1 from 5.290±0.078 to 3.594±0.473 and 3.284±0.435, respectively, *p<0.05). The level of HIF-1α was also suppressed by these agents (LY294002 and Rapamycin inhibited HIF-1α from 1.092±0.208 to 0.684±0.115 and 0.652±0.257, respectively, *p<0.05). These results indicated that Ni-smelting fumes increase HIF-1α expression through PI3K/ERK-dependent signaling pathways in NIH/3T3 cells.
Fig. 2.
NIH/3T3 cells were pretreated with 25IM U0126, 20uM LY294002 or 10nM rapamycin for 1 h and then exposed to 50.00 μg/mL Ni-smelting fumes for 24 h. The experiment was repeated 3 times. The activation of ERK signaling induced by Ni-smelting fumes was suppressed by the specific inhibitors U0126 (A). The activation of PI3K/AKT/P70S6K1 signaling induced by Ni-smelting fumes was suppressed by the specific inhibitors LY294002 and Rapamycin (B). The protein band with antibodies against PI3K, p-AKT, p70S6K1, ERK, HIF-1α, and β-actin were analyzed by western blotting system. The difference between the treatment groups and the inhibitor treatment groups were statistically significant, *P<0.05.
Cell Transformation in the Cell Line
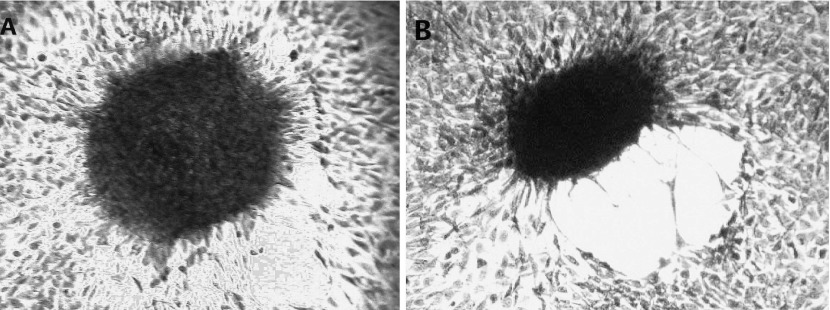
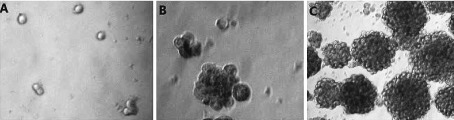
With regard to cell transformation, contact inhibition in the negative control group was observed during the cultivation process. After 21 d of culture, the cells did not transform into a focus spontaneously and were arranged regularly in the focus. Fasciculation of cells in the focus in the peripheral zone showed monolayer growth (Fig. 3A). Cells exposed to each concentration of Ni-smelting fumes showed a transformed focus formation, cells lost growth direction, were disordered; focal cells changed from normal long fusiform to short fusiform, lost contact inhibition, showed stratified growth and disordered arrangement, and formed whirlpool (Fig. 3B).
Fig. 3.
Morphological transformation of NIH/3T3 cells induced by Ni-smelting fumes. Cells transformantion. The experiment was repeated 3 times. A: Normal cells focus (100×). Cells were neat arrangement and growed in a monolayer which around the focus. The shape of cells were short fusiformis were long fusiformis. B: Transformed cells focus. Cells were growed in a multilayer and irregular arrangement. The shape of cells were short fusiformis.
Three repeated tests showed similar results. Cells exposed to each concentration of Ni-smelting fumes showed growth when cultured on soft agar (Fig. S2). Negative control cells on soft agar did not grow and slowly died, without colony formation. Following cultivation for 14 d, the colony formation rate in each concentration group was counted, and as the concentration of Ni-smelting fumes increased the colony formation rate increased (Table 1).
Fig. S.2.
Growth of transformation cells in soft agarose. The experiment was repeated three times. A: Cells were cultured 1 day (100×). B: Cells were cultured 7 day (100×). C: Cells were cultured 21 day (100×).
Table 1.
The affects of smelting furnace dust on the colony of NIH/3T3 cells.
| Ni-smelting fumes (μg/mL) | Inoculated cell number (×103/hole) | Holes | Colony number | colony forming efficiency (10–3) |
|---|---|---|---|---|
| 0.00 | 2.5 | 4 | 0 | 0.0 |
| 12.50 | 2.5 | 4 | 28 | 2.8 |
| 25.00 | 2.5 | 4 | 32 | 3.2 |
| 50.00 | 2.5 | 4 | 37 | 3.7 |
| 100.00 | 2.5 | 4 | 49 | 4.9 |
Hematoxylin-eosin Staining
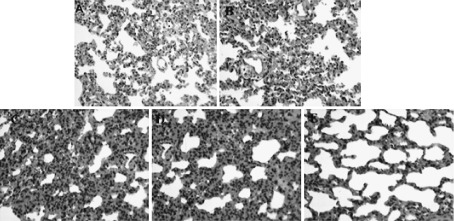
Paraffin-embedded sections of rat lung were assessed to determine the morphological changes in vivo after exposure to Ni-smelting fumes using hematoxylin-eosin staining (Fig. S3). The 0 mg/kg group was used as the negative control and Ni powder (3 mg/kg) as the positive control (Fig. S3A). With increased concentrations of Ni-smelting fumes, the alveolar septum widened slightly and edema occurred around the blood vessels in the 2 mg/kg group (Fig. S3D). In the 3 mg/kg group, the alveolar septum widened obviously, and angiotelectasis and congestion were observed (Fig. S3E). The positive control group was found to have similiar characteristics to the 3 mg/kg Ni-smelting fumes group. These results indicated that increased concentrations of Ni-smelting fumes exacerbated pathological changes.
Fig. S.3.
Ni-smelting fumes effect on morphological by hematoxylin-eosin staining. Embedded sections were exposed to different concentration of Ni-smelting fumes. The experiment was repeated three times. A: Negative control. B: Exposed to 0.5 mg/kg Ni-smelting fumes per rat every fifteen days for a total of twice. Alveolar wall capillaries were expanded and congested. C: Exposed to 1.0 mg/kg Ni-smelting fumes per rat every fifteen days for a total of twice. Alveolar wall capillaries were more expanded and congested. D: Exposed to 2.0 mg/kg Ni-smelting fumes per rat every fifteen days for a total of twice. There were a small number of red blood cells in the alveolar cavity, and the alveolar septum was widened. E: Exposed to 3.0 mg/kg Ni-smelting fumes per rat every fifteen days for a total of twice. Alveolar wall capillaries were expanded and congested. Infiltration by neutrophils granulocytes and lymphocytes around the blood vessels was observed.
Immunohistochemistry
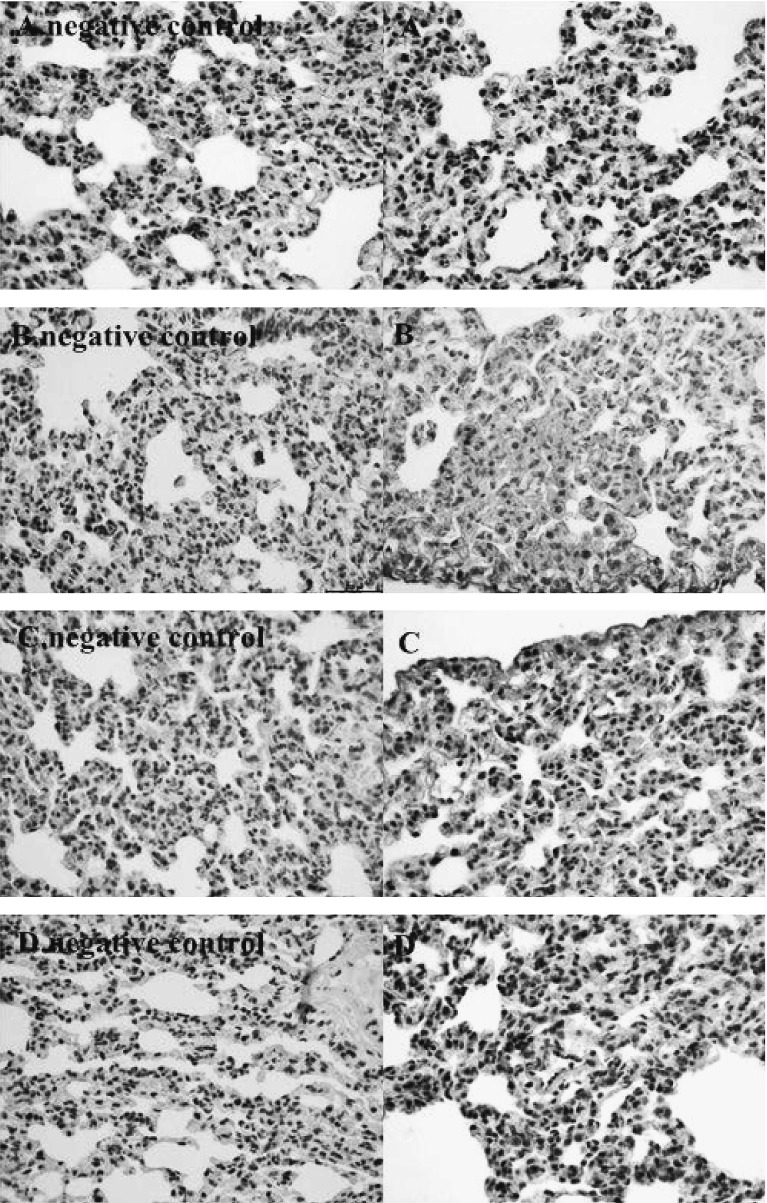
The 0 mg/kg group was used as the negative control and exposure to Ni powder (3 mg/kg) as the positive control. The expression of PI3K, p70S6k1, ERK, and HIF-1α in paraffin-embedded sections of rat lung was examined by immunohistochemistry (Fig. 4 and Table S. 1). The results indicated that Ni-smelting fumes increased the expression of PI3K, P70S6K1, ERK, and HIF-1α, but not p-AKT. The positive expression of PI3K (Fig. 4A), p70S6K1 (Fig. 4B), ERK (Fig. 4C), and HIF-1α (Fig. 4D) was significantly higher with Ni-smelting fumes compared with the negative control (*p<0.05).
Fig. 4.
Ni-smelting fumes activated PI3K, P70S6K1, ERK, and HIF-1α in paraffin embedded sections of rat lung (200 px). After expose to 3 mg/kg Ni-smelting fumes per rat every fifteen days for a total of two times, the strong positive expression of PI3K (A), P70S6K1 (B), ERK (C), HIF-1α (D). The experiment was repeated 3 times. The expression of the each protein in the high concentration group showed a strong positive expression (Table S.1), indicating that the expression of in the Ni-smelting fumes was high expression compared for the negative control (p<0.05).
Discussion
Ni is widely present in the environment, and low dose exposure may produce adverse effects in humans27). Due to the widespread use of Ni in modern industry, genetic toxicity and carcinogenicity of Ni have gained significant attention. Ni and its compounds can damage many organs, including liver, kidney, lung, the cardiovascular system, and blood. Excess Ni entering the body will cause corresponding functional and organic damage28). Numerous studies have demonstrated that a major cause of occupational lung cancer is metal exposure, and lungs have the highest Ni content in occupationally exposed populations29). Carcinogenesis due to Ni compounds has been correlated with oxidative stress, DNA damage, and the regulation of gene expression by activating specific transcription factors in corresponding signal transduction pathways; however, the specific mechanism is still unclear30). Copper is widely found in the environment and can stimulate the respiratory system nervous system and renal function31,32). While many studies have reported an increased risk of cancer among copper workers in industry, data on the genetic toxicity of copper are scarce. The carcinogenic potential of copper is weaker than that of Ni, and its carcinogenic potential may be weak or absent33). Lead, antimony, and cadmium play a very small role in the cytotoxicity of Ni-smelting fumes as the content of these elements in Ni-smelting fumes was less than 0.3%, and their harmful effects may not occur until a certain dose is reached. The regulation of Ni-smelting fumes involves a variety of metals, but is based on Ni. In subsequent experiments, we will conduct an analysis of chemical forms and detect the effect of associated elements.
Previously, our research group carried out a study on the cytotoxicity of Ni-smelting fumes on NIH/3T3 cells, and this laid the foundation for the present study26). We determined the ultrastructure of NIH/3T3 cells after exposure to Ni-smelting fumes and found that Ni-smelting fumes caused necrosis. The morphological changes in rat lung were also determined, which further confirmed the toxic effect of Ni refining dusts in vivo. Ni-smelting fumes affect the regulation of key oncogenic proteins, and their effect on the critical downstream molecule, HIF-1α, was studied. Our results showed that Ni-smelting fumes activated PI3K, p-AKT, p70S6K1, and ERK proteins and significantly increased the expression of HIF-1α in a time- and dose-dependent manner. In order to prove the activation of these proteins induced by Ni-smelting fumes not only in vitro but also in vivo, we determined the expression of these proteins in paraffin-embedded rat lung sections using immunohistochemistry following exposure to Ni-smelting fumes in vivo. To assess whether the PI3K and ERK pathways are necessary for Ni-smelting fumes-induced HIF-1α expression, NIH/3T3 cells were incubated with inhibitors of these pathways: LY290022, rapamycin, or U0126 before exposure to Ni-smelting fumes. The results showed that when NIH/3T3 cells were exposed to Ni-smelting fumes in advance, the activation of PI3K, p-AKT, p70S6k1, ERK was inhibited by the PI3K/AKT and ERK inhibitors. Suppression of these pathways significantly decreased HIF-1α level. This indicated that Ni-smelting fumes regulated HIF-1α expression through the PI3K/ERK pathway in NIH/3T3 cells; however, the specific mechanism remains to be determined. There was no significant difference between the positive control and the highest concentration of Ni-smelting fumes, which indicated that the main effect of smelting fumes was due to Ni. Transformation experiments proved that Ni-smelting fumes resulted in malignant transformation in NIH/3T3 cells, which proved that Ni-smelting fumes may be a potential carcinogen. Hematoxylin-eosin staining demonstrated that Ni-smelting fumes caused pathological changes in rat lungs in vivo. With increased concentrations of Ni-smelting fumes, the alveolar space widened and cell infiltration increased. Specific protein expression in vivo was shown by immunohistochemistry. The results indicated that the expression of proteins in the Ni-smelting fumes exposed group was stronger than that in the negative control group, and the difference was statistically significant (*p<0.05), but compared with the positive control group, the difference was not statistically significant.
The carcinogenesis mechanism of most metal compounds involves oxidative stress, regulation of DNA repair and interference of signal transduction34). Although the molecular mechanism of Ni compounds causing cancer is not clear, some studies suggest that it is related to oxidative stress, DNA damage, and the activation of specific transcription factors in corresponding signal transduction pathways to regulate gene expression30). In malignant tumor occurrence and development, apoptosis inhibition plays a larger role than excessive proliferation. Apoptosis may trigger further malignancies and protects the organism by eliminating damaged cells35).
The PI3K/AKT pathway regulates proliferation and survival of tumor cells. Abnormal activity not only causes malignant transformation but also tumor cell migration, adhesion, tumor angiogenesis, and degradation of extracellular matrix. The role of AKT in tumor signal transduction is to suppress apoptosis and promote tumor invasion and the cell cycle. ERK is an important transfer protein, which transfers the signal from the cell surface to the nucleus, and plays a significant role in the regulation of gene expression, cell proliferation, and cell death. Activation of the hypoxia signaling pathway is assumed to be the cause of human cancer due to soluble and insoluble Ni compounds. HIF-1α is present in many common cancers, particularly in the hypoxia zone. Following activation of the PI3K/AKT signaling pathway, p-AKT induces the expression of HIF; the P110 subunit is catalytic, and P110 alpha and gamma are protein kinases. The activity of P110 gamma can activate the MAPK pathway, which connects the two signal pathways. PI3K is activated downstream of P70S6K1 and eIF-4E, which increase the HIF-1α mRNA translated protein36). In a previous study, Ni compounds activated the PI3K/AKT signal pathway and the MAPK signal pathway37). Copper induced apoptosis through the PI3K and MAPK pathways in FL83B cells and increased the expression of HIF-1α38). Manganese can regulate the JNK/ERK and PI3K/AKT pathways in BV2 cells and up-regulate HIF-1α in Hep2 cells through the MAPK pathway39). Cadmium is a known carcinogen which can induce carcinogenesis through the PI3K/AKT and ERK signaling pathways in BEAS-2B cells40). In the present study, we found that there was no significant difference between the groups exposed to high concentrations of Ni-smelting fumes and the positive control group, which indicated that Ni plays a major role in Ni-smelting fumes. However, whether other metals also play a role requires further study. In this study, we only studied the effect of mixed fumes on the PI3K/ERK signaling pathway. As far as we know, this is the first study to show the effects of Ni-smelting fumes on the PI3K/ERK pathways and their downstream factor HIF-1α. In our study, Ni fallout beside the Ni smelter furnace in a factory was sampled instead of pure Ni compounds. This revealed the damage of Ni fumes in the occupational environment to mammalian cells which has more realistic significance. Further studies are needed to determine the specific mechanism involved in the effect of Ni-smelting fumes on HIF-1α.
Cell transformation in culture is a way of studying the mechanism of carcinogenesis. Following transformation, the cell traits can be passed on from generation to generation and can be maintained for a long time. Malignant transformation is a pre-cancerous state in cells, and the soft agar culture results and degree of cell malignancy were highly consistent41). Ni-smelting fumes caused malignant transformation of NIH/3T3 cells in the transformation experiment, which further proved that the activation of these proteins induced by Ni-smelting fumes caused cancer. This is consistent with the findings that almost all of the clones focus induced by Ni could be transformed into tumor in nude mice42). The transformation of NIH/3T3 cells following exposure to Ni-smelting fumes was the result of a variety of metals, but mainly Ni. The combined effects of Ni and other substances in the transformation of cells have also been reported. The coexistence of copper and Ni did not have a synergistic effect on the transformation of SHE cells43). The present study showed that Ni-smelting fumes could transform normal cells and transformed cells grew on soft agar. With increased concentrations of Ni-smelting fumes, cell conversion rate increased, which indicated that Ni-smelting fumes had high potential carcinogenicity.
In conclusion, this study aimed, from the perspective of the cell signal transduction of critical oncogenic proteins, to determine the possible mechanism of carcinogenic mechanism induced by Ni-smelting fumes in mammalian cell. This study not only simulate the occupational environment but also has great significance of occupational population exposed to Ni-smelting fumes. Regulation of the proteins induced by Ni-smelting fumes was the result of a variety of metals; however, the specific mechanism involved is still not clear.
Table S.1.
The positive expression of PI3K, P70S6K1, ERK, and HIF-1α in paraffin embedded sections of rat lung. Compared with the negative group, *P<0.05.
| DOSE (mg/kg) | N | PI3K | P70S6K1 | ERK | HIF-1α | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | – | + | – | + | – | + | – | |||||
| 0.00 | 15 | 0 | 15 | 0 | 15 | 0 | 15 | 0 | 15 | |||
| 0.50 | 15 | 3 | 12 | 2 | 13 | 5* | 10* | 0 | 15 | |||
| 1.00 | 15 | 6* | 9* | 7* | 8* | 8* | 7* | 2 | 13 | |||
| 2.00 | 15 | 11* | 4* | 11* | 4* | 11* | 4* | 10* | 5* | |||
| 3.00 | 15 | 15* | 0* | 15* | 0* | 15* | 0* | 15* | 0* | |||
| nickel | 15 | 15 | 0 | 15 | 0 | 15 | 0 | 15 | 0 | |||
Table S.2.
The affects of smelting furnace dust on the colony of NIH/3T3 cells.
| Ni-smelting fumes ((μg/mL) | Inoculated cell number (×103/hole) | Holes | Colony number | colony forming efficiency (10-3) |
|---|---|---|---|---|
| 0.00 | 2.5 | 4 | 0 | 0.0 |
| 12.50 | 2.5 | 4 | 27 | 2.7 |
| 25.00 | 2.5 | 4 | 31 | 3.1 |
| 50.00 | 2.5 | 4 | 37 | 3.7 |
| 100.00 | 2.5 | 4 | 48 | 4.8 |
Table S.3.
The affects of smelting furnace dust on the colony of NIH/3T3 cells.
| Ni-smelting fumes ((μg/mL) | Inoculated cell number (×103/hole) | Holes | Colony number | colony forming efficiency (10-3) |
|---|---|---|---|---|
| 0.00 | 2.5 | 4 | 0 | 0.0 |
| 12.50 | 2.5 | 4 | 28 | 2.8 |
| 25.00 | 2.5 | 4 | 32 | 3.2 |
| 50.00 | 2.5 | 4 | 38 | 3.8 |
| 100.00 | 2.5 | 4 | 50 | 5.0 |
Acknowledgments: We wish to thank all the subjects who participated in this study. This study was supported by grants from the National Natural Science Foundation of China (81372965).
References
- 1). Freitas M, Barcellos-de-Souza P, Barja-Fidalgo C, Fernandes E. Nickel induces apoptosis in human neutrophils. Biometals 2013; 26: 13-21. [DOI] [PubMed] [Google Scholar]
- 2). Oller AR, Costa M, Oberdorster G. Carcinogenicity assessment of selected nickel compounds. Toxicol Appl Pharmacol 1997; 143: 152-166. [DOI] [PubMed] [Google Scholar]
- 3). Chromium, Nickel and Welding. IARC monographs on the Evaluation of Carcinogenic Risks to Human/World Health Organization, International Agency for Research on Cancer 1990; 49: 1-648, 677-691. [PMC free article] [PubMed] [Google Scholar]
- 4). Wang YF, Shyu HW, Chang YC, et al. Nickel (II)-induced cytotoxicity and apoptosis in human proximal tubule cells through a ROS- and mitochondria-mediated pathway. Toxicol Appl Pharmacol 2012; 259: 177-186. [DOI] [PubMed] [Google Scholar]
- 5). Chen F, Ding M, Castranova V, Shi X. Carcinogenic metals and NF-kappaB activation. Mol Cell Biochem 2001; 222: 159-171. [PubMed] [Google Scholar]
- 6). Kročková JZ, Massányi P, Sirotkin AV, et al. Nickel induced structural and functional alterations in mouse Leydig cells in vitro. J Trace Elem Med Biol 2011; 25: 14-18. [DOI] [PubMed] [Google Scholar]
- 7). Liden C, Skare L, Vahter M. Release of nickel from coins and deposition onto skin from coin handling-comparing euro coins and SEK. Contact Dermatitis 2008; 59: 31-37. [DOI] [PubMed] [Google Scholar]
- 8). Zhicheng S. Nickel carbonyl: toxicity and human health. Sci Total Environ 1994; 148: 293-298. [DOI] [PubMed] [Google Scholar]
- 9). Gawkrodger DJ, Lewis FW, Shah M. Contact sensitivity to nickel and other metals in jewelry reactors. J Am Acad Dermatol 2000; 43: 31-36. [DOI] [PubMed] [Google Scholar]
- 10). Grimsrud TK, Berge SR, Martinsen JI, Andersen A. Lung cancer incidence among Norwegian nickel-refinery workers 1953-2000. J Environ Monit 2003; 5: 190-197. [DOI] [PubMed] [Google Scholar]
- 11). Costa M, Salnikow K, Cosentino S, Klein CB, Huang X, Zhuang Z. Molecular mechanisms of nickel carcinogenesis. Environ Health Perspect 1994; 3: 127-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Velagapudi C, Bhandari BS, Abboud-Werner S, Simone S, Abboud HE, Habib SL. The tuberin/mTOR pathway promotes apoptosis of tubular epithelial cells in diabetes. Journal of the American Society of Nephrology 2011; 22: 262-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Testa JR, Tsichlis PN. Akt signaling in normal and malignant cells. Oncogene 2005; 24: 7391-7393. [DOI] [PubMed] [Google Scholar]
- 14). Shinohara M, Chung YJ, Saji M, Ringel MD. Akt in thyroid tumorigenesis and progression. Endocrinology 2007; 148: 942-947. [DOI] [PubMed] [Google Scholar]
- 15). Paes JE, Ringel MD. Dysregulation of the phosphatidylinositol 3-kinase pathway in thyroid neoplasia. Endocrinol Metab Clin North Am 2008; 37: 375-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Chalhoub N, Baker SJ. PTEN and the Pi3-kinase pathway in cancer. Annu Rev Pathol 2009; 4: 127-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Chin YR, Toker A. Function of akt/pkb signaling to cell motility, invasion and the tumor stroma in cancer. Cell Signal 2009; 21: 470-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Tessier DM, Pascal LE. Activation Of MAP Kinases by hexavalent chromium, manganese and nickel in human lung epithelial cells. Toxicol Lett 2006; 167: 114-121. [DOI] [PubMed] [Google Scholar]
- 19). Yang Y, Zhao W, Xu QW, Wang XS, Zhang Y, Zhang J. IQGAP3 promotes EGFR-ERK signaling and the growth and metastasis of lung cancer cells. PLoS One 2014; 9: e97578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Zheng J, Bird IM, Melsaether AN, Magness RR. Activation of the mitogen-activated protein kinase cascade is necessary but not sufficient for basic fibroblast growth factor- and epidermal growth factorstimulated expression of endothelial nitric oxide synthase in ovine fetoplacental artery endothelial cells. Endocrinology 1999; 140: 1399-1407. [DOI] [PubMed] [Google Scholar]
- 21). Risbud MV, Guttapalli A, Albert TJ, Shapiro IM. Hypoxia activates MAPK activity in rat nucleus pulposus cells: regulation of integrin expression and cell survival. Spine 2005; 30: 2503-2509. [DOI] [PubMed] [Google Scholar]
- 22). Gerasimovskaya EV, Tucker DA, Stenmark KR. Activation of phosphatidylinositol 3-kinase, Akt, and mammalian target of rapamycin is necessary for hypoxia-induced pulmonary artery adventitial fibroblast proliferation. J Appl Physiol 2005; 98: 722-731. [DOI] [PubMed] [Google Scholar]
- 23). Minet E, Arnould T, Michel G, et al. ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett 2000; 468: 53-58. [DOI] [PubMed] [Google Scholar]
- 24). Dunnick JK, Elwell MR, Radovsky AE, et al. Comparative carcinogenic effects of nickel subsulfide, nickel oxide, or nickel sulfate hexahydrate chronic exposures in the lung. CANCER RESEARCH 1995; 55: 5251-5256. [PubMed] [Google Scholar]
- 25). Zhao G, Wang Y, Wang SY, Ba JC, Wu YH. Application of ICP-MS to Detecting Nickel production occupation exposure to dust in metal content. Chi Sciencepaper Online 2013. [Google Scholar]
- 26). Jin YT, Wu YH, Hu FL, Hu XY. Transformation and apoptosis of NIH/3T3 cells treated with nickel-smelting fumes. J Toxicol Environ Health A 2009; 72(11-12): 733-739. [DOI] [PubMed] [Google Scholar]
- 27). Kasprzak KS, Sunderman FW, Salnikow K. Nickel carcinogenesis. Mutat Res 2003; 533: 67-97. [DOI] [PubMed] [Google Scholar]
- 28). Doll R. Reports of the International Committee on Nickel Carcinogenesis in Man. Scand J Work Environ Health 1990; 16: 9-82. [DOI] [PubMed] [Google Scholar]
- 29). Jockel KH, Ahrens W, Wichman HE, et al. Occupational and environmental hazards associated with lung cancer. Int J Epidemiol 1992; 21: 202-213. [DOI] [PubMed] [Google Scholar]
- 30). Lu H, Shi X, Costa M, Huang C. Carcinogenic effect of nickel compounds. Mol Cell Biochem 2005; 279: 45-67. [DOI] [PubMed] [Google Scholar]
- 31). Blom S, Lagerkvist B, Linderholm H. Arsenic exposure to smelter workers. Clinical and neurophysiological studies. Scand J Work Environ Health 1985; 11: 265-269. [DOI] [PubMed] [Google Scholar]
- 32). Lilis R, Valciukas JA, Weber JP, Malkin J, Selikoff IJ. Epidemiologic study of renal function in copper smelter workers. Environ Health Perspect 1984; 54: 181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Trotta A, Paulsen AB, Silvestri A, Ruisi G, Girasolo MA, Barbieri R. The dynamics of 57fe nuclei in feiii-dna condensates. Journal of Inorganic Biochemistry 2002; 88: 14-18. [DOI] [PubMed] [Google Scholar]
- 34). Beyersmann D, Hartwig A. Carcinogenic metal compounds:recent insight into molecular and cellular mechanisms. Arch Toxicol 2008; 82: 493-512. [DOI] [PubMed] [Google Scholar]
- 35). Ding J, Zhang X, Li J, et al. Nickel compounds render anti-apoptotic effect to human bronchial epithelial Beas-2B cells by induction of cyclooxygenase-2 through an IKKbeta/p65-dependent and IKKalpha-andp50-independent pathway. The J biol chem 2006; 281: 39022-39032. [DOI] [PubMed] [Google Scholar]
- 36). Sun XJ, Huang CZ. PI3K-AKT signal pathway and tumor. Word Chinese Journal of Digestology 2006; 14: 306-311. [Google Scholar]
- 37). Tessier DM, Pascal LE. Activation Of MAP Kinases by hexavalent chromium, manganese and nickel in human lung epithelial cells. Toxicol Lett 2006; 167: 114-121. [DOI] [PubMed] [Google Scholar]
- 38). Hsiao-Ling T, Chia-Jung L, Lin-Huang H, et al. Quercetin 3-o-methyl ether protects fl83b cells from copper induced oxidative stress through the pi3k/akt and mapk/erk pathway. Toxicology & Applied Pharmacology 2012; 264: 104-113. [DOI] [PubMed] [Google Scholar]
- 39). Shin HJ, Choi MS, Ryoo NH, et al. Manganese-mediated up-regulation of HIF-1alpha protein in Hep2 human laryngeal epithelial cells via activation of the family of MAPKs. Toxicology in Vitro An International Journal Published in Association with Bibra 2010; 24: 1208-1214. [DOI] [PubMed] [Google Scholar]
- 40). Son YO, Wang L, Poyil P, et al. Cadmium induces carcinogenesis in BEAS-2B cells through ROS-dependent activation of PI3K/AKT/GSK-3β/β-catenin signaling. Toxicology\s&\sapplied Pharmacology 2012; 264: 153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Gruenert DC, Finkbeiner WE, Widdicombe JH. Culture and transformation of human airway epithelial cells. Am J Physiol 1995; 268(3pt1): L347-360. [DOI] [PubMed] [Google Scholar]
- 42). Costa M, Nye JS, Sunderman FW, Allpass PR, Gondos B. Induction of sarcomas in nude mice by implantation of Syrian hamster fetal cells exposed in vitro to nickel sub-sulfide. Cancer Res 1979; 39: 3591-3597. [PubMed] [Google Scholar]
- 43). Clemens F, Landolph JR. Genotoxicity of samples of nickel refinery dust. Toxicological sciences: an official journal of the Society of Toxicology 2003; 73: 114-123. [DOI] [PubMed] [Google Scholar]