Abstract
Migraine is a disabling brain disorder involving abnormal trigeminovascular activation and sensitization. Fasting or skipping meals is considered a migraine trigger and altered fasting glucose and insulin levels have been observed in migraineurs. Therefore peptides involved in appetite and glucose regulation including insulin, glucagon and leptin could potentially influence migraine neurobiology. We aimed to determine the effect of insulin (10 U·kg− 1), glucagon (100 μg·200 μl− 1) and leptin (0.3, 1 and 3 mg·kg− 1) signaling on trigeminovascular nociceptive processing at the level of the trigeminocervical-complex and hypothalamus. Male rats were anesthetized and prepared for craniovascular stimulation. In vivo electrophysiology was used to determine changes in trigeminocervical neuronal responses to dural electrical stimulation, and phosphorylated extracellular signal-regulated kinases 1 and 2 (pERK1/2) immunohistochemistry to determine trigeminocervical and hypothalamic neural activity; both in response to intravenous administration of insulin, glucagon, leptin or vehicle control in combination with blood glucose analysis. Blood glucose levels were significantly decreased by insulin (p < 0.001) and leptin (p < 0.01) whereas glucagon had the opposite effect (p < 0.001). Dural-evoked neuronal firing in the trigeminocervical-complex was significantly inhibited by insulin (p < 0.001), glucagon (p < 0.05) and leptin (p < 0.01). Trigeminocervical-complex pERK1/2 cell expression was significantly decreased by insulin and leptin (both p < 0.001), and increased by glucagon (p < 0.001), when compared to vehicle control. However, only leptin affected pERK1/2 expression in the hypothalamus, significantly decreasing pERK1/2 immunoreactive cell expression in the arcuate nucleus (p < 0.05). These findings demonstrate that insulin, glucagon and leptin can alter the transmission of trigeminal nociceptive inputs. A potential neurobiological link between migraine and impaired metabolic homeostasis may occur through disturbed glucose regulation and a transient hypothalamic dysfunction.
Abbreviations: ARC, hypothalamic arcuate nucleus; BBB, blood brain barrier; CNS, central nervous system; CSD, cortical spreading depression; DMN, hypothalamic dorsomedial nucleus; DMH, dorsomedial hypothalamic area; ERK1/2, extracellular signal-regulated kinase 1/2; LRP1, lipoprotein receptor-related protein 1; MAP, mitogen-activated protein; MMA, middle meningeal artery; ObRb, long form of the leptin receptor; pERK1/2, phosphorylated extracellular signal-regulated kinase 1/2; PVN, hypothalamic paraventricular nucleus; TCC, trigeminocervical complex; TNC, trigeminal nucleus caudalis; VMH, ventral medial hypothalamic area; WDR, wide dynamic range
Keywords: Glucose, Appetite, Migraine, Trigeminovascular activation, Headache, pERK1/2
Highlights
-
•
Insulin and glucagon inhibit trigeminal nociception.
-
•
Inhibition by leptin is dose dependent suggesting potential multiple targets.
-
•
Insulin, glucagon and leptin directly modulate pERK1/2 expression within the TCC.
-
•
TCC neurons are glucose-responsive, potentially linking blood glucose levels to pain processing.
1. Introduction
Migraine is a severe and disabling brain condition (Goadsby et al., 2002), characterized by attacks of unilateral throbbing head pain, with hypersensitivity to movement, visual and auditory inputs (Headache Classification Committee of the International Headache Society, 2013). Approximately one-third of migraine patients suffer attacks that are associated with cortical perturbations, namely migraine aura (Charles, 2013). Additional symptoms in the premonitory phase, such as changes of appetite, thirst, tiredness, irritability and reduced concentration, can precede the headache by up to 48 h (Giffin et al., 2003).
Migraine pathophysiology is thought to involve activation and sensitization of trigeminovascular nociceptive pathways that innervate the cranial vasculature, and activation of hypothalamic and brain stem nuclei (Akerman et al., 2011). In addition, some studies report that inducing cortical spreading depression (CSD), the animal correlate of aura in humans, can also activate the trigeminovascular system and therefore, the authors suggest migraine can originate outside the TCC (Moskowitz and Cutrer, 1993, Zhang et al., 2011). Migraine attacks can start centrally in the brain as evidenced by hypothalamic changes (Denuelle et al., 2007, Maniyar et al., 2014) and trigeminocervical complex (TCC) alterations (Stankewitz et al., 2011) during the pre-ictal phase. The migraine brain is extremely susceptible to perturbations of internal and external cues and their alteration likely leads to activation of the pain processing trigeminovascular system that includes the pseudounipolar trigeminal ganglion and its afferent projections to the trigeminal nucleus caudalis (TNC) and C1 and C2 regions in the medullary and cervical spinal cord (the TCC), and its peripheral afferent projections mainly from the ophthalmic division of the trigeminal nerve to the cranial blood vessels including the pain-sensitive dura mater (Akerman et al., 2011).
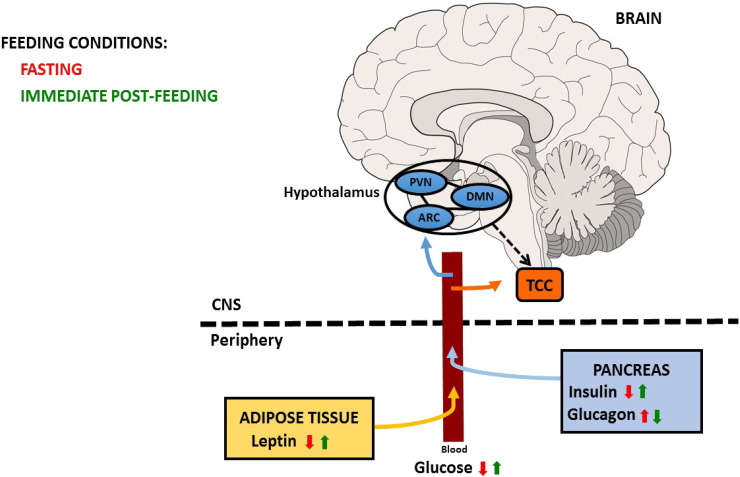
Fasting or skipping meals is one of the most consistently reported migraine triggers in susceptible individuals and appetite change is reported during the premonitory phase (Blau and Cumings, 1966, Giffin et al., 2003, Kelman, 2007). The hypothalamus is a major appetite center and imaging studies in humans show activations in the region of the hypothalamus before (Maniyar et al., 2014) and during migraine headache (Denuelle et al., 2008). Several studies and clinical observations highlight a clear association between migraine, feeding behavior and metabolic disorders. Clinically, some patients report loss of appetite during attacks, potentially via hypothalamic mechanisms (Malick and Burstein, 2001); and common premonitory symptoms and/or migraine triggers include hunger or skipping meals (Giffin et al., 2003), suggesting disruption of common regulatory networks early in the attack phase. Obesity is a risk factor for migraine chronification (Bigal et al., 2006) and attack frequency and severity increase with body mass index (Bigal et al., 2006). Moreover, a higher migraine prevalence in metabolic syndrome patients was demonstrated comparing to the general population (Guldiken et al., 2009). On the other hand, there is also a potential association with eating disorders, with a high prevalence of migraine in woman affected by anorexia or bulimia nervosa (D'Andrea et al., 2012). In rodents, neuropeptides like orexin and neuropeptide Y involved in the regulation of feeding have been proposed to regulate the trigeminovascular system (Hoffmann et al., 2015, Holland et al., 2006, Martins-Oliveira et al., 2016). Therefore, the gut-brain pathway that enables the communication between the endocrine system and CNS may underlie the observed interaction of appetite regulation, glucose homeostasis and migraine (Fig. 1).
Fig. 1.
Schematic representation of neuroendocrine signaling in healthy individuals during two different feeding conditions (fasting and immediate post-feeding).
Leptin is released from adipose tissue and insulin and glucagon are release by the pancreas into the blood circulation. These peptides are able to cross the blood brain barrier (BBB) and act in the hypothalamus to regulate appetite and blood glucose levels. There is a potential hypothalamic output (dashed arrow) towards the trigeminocervical complex (TCC) and these peptides may additionally act directly at the TCC, modulating nociceptive trigeminovascular activation. PVN: hypothalamic paraventricular nucleus; ARC: hypothalamic arcuate nucleus; DMN: hypothalamic dorsomedial nucleus; CNS: Central nervous system.
Insulin is a dipeptide hormone secreted by the β-cells of the pancreatic islets of Langerhans and maintains normal blood glucose levels by facilitating cellular glucose uptake, regulating carbohydrate, lipid and protein metabolism (Wilcox, 2005). The insulin-secreting β-cells, which respond to a rise in extracellular glucose with membrane depolarization, are the best understood glucose-sensing excitable cells (Burdakov et al., 2005). Headache is associated with high fasting glucose blood levels and migraine is specifically associated with higher insulin levels, after fasting and after the oral glucose tolerance test (Bernecker et al., 2010, Cavestro et al., 2007, Rainero et al., 2005). In addition, a significant prevalence of insulin resistance has been observed in chronic migraineurs (Fava et al., 2014). Glucagon is a polypeptide synthesized and secreted from pancreatic α-cells, the levels of which increase during meals creating postprandial satiety (Geary, 1990). Glucagon has been previously administered in migraineurs resulting in a lowered hyperglycemic effect compared to controls (De Silva et al., 1974), suggesting a defect or failure of mechanisms which normally counteract hypo- and hyperglycemia in migraineurs. Leptin, a peptide hormone mainly secreted by white adipocytes, crosses the blood-brain barrier (BBB) (Friedman and Halaas, 1998) and activates neurons in the paraventricular nucleus (PVN), arcuate nucleus (ARC), ventromedial hypothalamic (VMH) and dorsomedial hypothalamic (DMH) areas. Of particular interest lipoprotein receptor-related protein 1 (LRP1), which regulates leptin signaling and energy homeostasis (Liu et al., 2011), has been identified as a migraine risk variants in several genome-wide association studies (Chasman et al., 2011, Freilinger et al., 2012).
As these are the major peripheral hormones regulating glucose homeostasis both systemically and centrally, it is important to investigate their actions within the trigeminovascular system. In this study, we first measured blood glucose levels following systemic administration of insulin, glucagon and leptin throughout a 60 min cohort. Using a valid animal model to study migraine pathophysiology (Bergerot et al., 2006, Messlinger and Ellrich, 2001), we then administered the same peptides and performed in vivo electrophysiological recordings within the TCC following dural-evoked nociceptive trigeminovascular activation.
Given that in some migraineurs appetite is altered and glucose metabolism might be impaired, the question remains as to whether peripheral signals are able to modify the activity of TCC and hypothalamic neurons and if these neurons are sensitive to systemic glucose changes. Parts of this work have been presented previously in preliminary form at Headache meetings (Martins-Oliveira et al., 2014a, Martins-Oliveira et al., 2014b, Martins-Oliveira et al., 2013)
2. Material and Methods
2.1. General
All experiments were conducted in agreement with the guidelines of Institutional Animal Care and Use Committee (UCSF) and the UK Home Office Animals (Scientific Procedures) Act 1986, and in agreement with the ARRIVE guidelines and the guidelines of the Committee for Research and Ethical Issues of International Association for the Study of Pain (Zimmermann, 1983). Animals were maintained under standard conditions with food and water available ad libitum.
2.1.1. Drugs
Male Sprague Dawley rats were tested with human insulin (10 U·kg− 1; NovolinR, NovoNordisk), glucagon (100 μg·200 μl− 1; Sigma-Aldrich) or rat recombinant leptin (0.3, 1 and 3 mg·kg− 1; Sigma-Aldrich). All drugs were dissolved in sterile water, which was additionally used as vehicle control in the sham group. Doses were chosen based on previous studies demonstrating hypothalamic activity following peripheral administration (Elias et al., 2000, Mighiu et al., 2013) and the induction of only mild hypoglycemia or transient hyperglycemia prevented any neuronal damage as is seen with extremely low or high blood glucose levels (Auer, 2004). Regarding insulin, in preliminary studies, we have tested different doses of insulin (3 U·kg− 1, 5 U·kg− 1 and 20 U·kg− 1; data not shown) to optimize mild hypoglycemia in our animal model. We thus decided to test the 10 U·kg− 1.
2.2. Blood glucose levels
The effect of intravenous treatment with insulin (10 U·kg− 1), glucagon (100 μg·200 μl− 1) and leptin (1 mg·kg− 1) or sham control (0.2–0.3 ml sterile water) on blood glucose levels was studied (n = 6 per group). Briefly, adult male Sprague Dawley rats (275–365 g; n = 24) were anesthetized with 5% (v/v) isoflurane (Abbott Laboratories, USA) for induction, cannulation of one femoral artery and both femoral veins for blood pressure measurement, maintenance anesthesia and drug infusion, respectively. Anesthesia was maintained with propofol (PropoFlo, 25–30 mg·kg− 1·h− 1, i.v. infusion). Following a 60 min rest period, drugs were infused over 2 min and physiological monitoring (body temperature, blood pressure, respiratory rate, etc.), was performed through 60 min, as well as quantification of blood glucose levels using tail vein blood samples and a glucometer (Freestyle Lyte, Abbott Diabetes Care, USA) at specific time-points: before surgery, before infusion of drugs and at 15, 30, 45 and 60 min post infusion.
2.3. In vivo electrophysiology
The surgical preparation and recording setup has been reported in detail recently (Akerman et al., 2013). Briefly, male Sprague Dawley rats (267–386 g, n = 47) were anesthetized with sodium pentobarbitone (60 mg·kg− 1, i.p.) for induction and anesthesia maintained with a propofol solution (PropoFlo, 25–30 mg·kg− 1·h− 1, i.v. infusion). Body temperature, respiratory rate, end-tidal CO2 and blood pressure were continuously monitored. After placement in a stereotaxic frame rats were ventilated with oxygen-enriched air (2–2.5 ml, 80–100 strokes/min).
To access the dura mater and middle meningeal artery (MMA), a craniotomy was performed with a saline-cooled drill and the underlying dura mater covered in mineral oil. To access the TCC, muscles of the dorsal neck were separated, a cervical laminectomy performed and the dura mater incised to expose the brainstem at the level of the caudal medulla oblongata. A piezo-electric microelectrode positioner was used to locate the optimal recording site within the TCC. After completion of the surgery, animals were left to stabilize for at least 60 min before recordings.
Following removal of the parietal bone a bipolar stimulating electrode connected to a stimulus isolation unit was placed on the intact dura mater adjacent to the MMA for electrical stimulation of the perivascular afferents of the trigeminal nerve (Fig. 2A). Stimulation of primary trigeminal afferents was performed with supramaximally square wave pulses generated by a Grass S88 stimulator. Dural nociceptive neurons in the TCC were identified via electrical stimuli (9–15 V, 0.15–0.3 ms, 0.4–0.5 Hz, 20 sweeps) to the dura mater, activating trigeminal Aδ-fibers with approximate latencies between 3 and 20 ms; by using low stimulation parameters, we were only able to activate fibers in the range of Aδ-fibers.
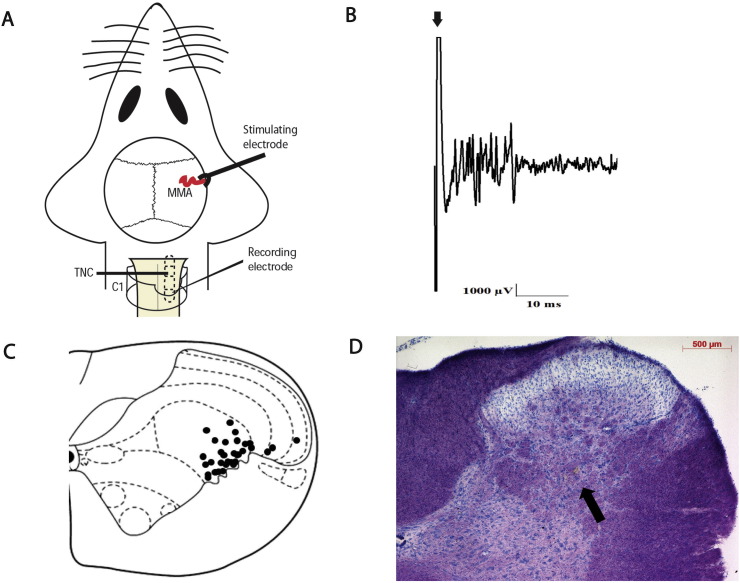
Fig. 2.
Overview of the experimental setup and neuronal characteristics.
A) Experimental setup with dural electrical stimulation and recording of neurons in the TCC. MMA: middle meningeal artery; TCC: trigeminocervical complex; C1: spinal cord cervical 1; TNC: trigeminal nucleus caudalis.
B) An original tracing from a typical unit (second-order neurons) responding to electrical stimulation of the dura mater adjacent to the MMA (latencies in the Aδ-fiber range). Black arrow represents stimulus artefact.
C) The location of recording sites in the TCC from which recordings of nociceptive neurons, receiving convergent input from the dura mater and facial receptive field, were made. The locations were reconstructed from lesions (•) and are located in laminae II–V, predominantly in lamina V.
D) A histological example for the lesion mark (brown lesion as indicated by the arrow) of the recording site in the TCC (lamina V), marked by electrothermolytic lesion (4–6 μA for 60 s). The section was counterstained with thionin.
Extracellular recordings were made from neurons in the TCC, activated by dural stimulation, with cutaneous facial receptive fields in the ophthalmic dermatome, using tungsten microelectrodes (0.5–1.0 MΩ). The signal from the recording electrode attached to a high-impedance head-stage preamplifier was fed via an AC preamplifier, band-pass filtered from 300 Hz to 20 kHz, and passed through a noise eliminator (Humbug, Quest Scientific, Vancouver Canada) for removal of line interference before further amplification using an AC-DC second-stage amplifier with a gain range of × 20–× 90. The obtained electrical signal was then fed through a gated amplitude discriminator and an analog-to-digital converter and to a microprocessor-based personal computer using Spike 2 v5.21 software where the signal was processed and stored. Additionally it was fed to a loudspeaker via a power amplifier for audio monitoring and displayed on analog and digital storage oscilloscopes to assist isolation of action potentials from adjacent cell activity and noise.
Neurons were characterized for their cutaneous and deep receptive fields. The receptive field was assessed for both non-noxious (brush) and noxious inputs (pinch). When a neuron sensitive to stimulation of the ophthalmic dermatome of the trigeminal nerve was identified it was tested for convergent input from the dura mater.
Trains of 20 stimuli were delivered at 5 min intervals to assess the baseline response to dural electrical stimulation. Responses were analyzed using post-stimulus histograms with a sweep length of 100 ms and a bin width of 1 ms that separated Aδ-fiber-activated firing (3–20 ms). Spontaneous activity (spikes per second, Hz) was recorded for 150 s preceding the dural stimulation using peri-stimulus histograms. Background activity was analyzed as cumulative rate histograms in which neuronal activity gated through the amplitude discriminator was collected into successive bins. When stable baseline values of the stimulus-evoked responses were achieved (average of 3 stimulation series) and cutaneous and deep receptive field inputs from the ophthalmic division of the trigeminal nerve were obtained, responses were tested for up to 60 min following intervention.
TCC neuronal responses to electrical dural afferent stimulation were tested with human insulin (10 U·kg− 1), glucagon (100 μg·200 μl− 1) or rat recombinant leptin (0.3, 1 and 3 mg·kg− 1). Sterile water was used as vehicle in all experiments.
At the end of each experiment animals were euthanized by a lethal dose of pentobarbital and phenytoin sodium and an electrothermolytic lesion was made in the TCC (4–6 μA for 60 s) to confirm the location of the recording electrode. The spinal cord was removed, serial 40 μm thick coronal sections were cut, stained with thionin and visualized under the light microscope (Axioplan Microscope; Carl Zeiss) (Paxinos and Watson, 2005). All analyses were conducted on the TCC, which includes the trigeminal nucleus caudalis and the upper two cervical spinal levels.
2.4. Immunohistochemistry
Using the same cohort of animals of blood glucose measurements, we studied the effect of intravenous treatment with insulin (10 U·kg− 1), glucagon (100 μg·200 μl− 1) and leptin (1 mg·kg− 1) or sham control (0.2–0.3 ml sterile water) on pERK1/2 expression within the TCC and the hypothalamus. At the end of each experiment, animals were euthanized with pentobarbital sodium (60 mg/kg, i.v.; Euthatal®, 20% w/v, JML) and perfused with 250 ml of heparinized (1%) phosphate-buffered saline (PBS) through the ascending aorta, followed by 300 ml of a fixative solution containing 4% paraformaldehyde in 0.01 M phosphate buffer, pH 7.4. The brain and upper spinal cord were removed, immersed in the same fixative for 2–4 h followed by 30% sucrose in 0.01 M PBS and 0.01% sodium azide, at 4 °C, and sliced at 40 μm in coronal orientation on a cryostat. Tissue was then processed for free-floating immunohistochemistry. One in four sequential transverse brain sections containing the hypothalamus and the TCC from each rat were collected, washed, blocked and incubated with a rabbit antiserum against the phosphorylated ERK1 and ERK2 isoforms (pERK1/2; 1:1000; 48 h at 4–8 °C: Neuromics, USA). Immunodetection was achieved with a biotinylated swine anti-rabbit antiserum (1:200; 1 h; Dako, Denmark), followed by an ABC solution (1:200, 1 h; ABC Elite kit, Vector Laboratories, UK) and a colorimetric reaction with 3,3-diaminobenzidine tetrahydrochloride (DAB) in 0.05 M Tris-HCl buffer containing 0.003% hydrogen peroxide. Sections were then washed in PBS, mounted on gelatin-coated glass slides, cleared in xylene, cover-slipped with Eukitt® and analyzed by light microscopy. Histological delineation of the TCC and the hypothalamus was made using the rat brain atlas (Paxinos and Watson, 2005).
The expression of pERK1/2 was identified by the accumulation of a brown labelling for DAB in cell bodies, as described (Cruz et al., 2005). For data presentation, TNC, C1 and C2 were plotted together as TCC and included an average of 35 sections per animal. Quantification of the expression of pERK1/2 in the TCC (laminae I–V) was performed by visual counting using an optical light microscope (Axioplan Microscope; Carl Zeiss). Values of pERK1/2 staining are expressed as the median per animal across all the sections analyzed.
In the hypothalamus, we saw patterns of pERK1/2 labelling which consisted of a brown precipitate in perikarya and dendrites of neurons in the ARC, PVN and caudal dorsomedial nuclei (DMN). The PVN was divided in levels according to bregma, level I (− 1.08 mm and − 1.20 mm; 1–3 sections per animal, n = 22), level II (− 1.44 mm and − 1.56 mm; 1–3 sections per animal, n = 24) and level III (− 1.72 mm and − 1.80 mm; 1–3 sections per animal, n = 24). Quantification in the PVN was performed by visual counting and values of pERK1/2 staining are expressed as the average per animal.
In the hypothalamic ARC and caudal DMN, a distinct pERK1/2 fiber labelling intensity was visually detected, thus separate quantification was performed as described (Borges et al., 2013) and values of pERK1/2 staining were expressed as optical density ratio using the free access software (ImageJ 1.49v). Hypothalamic ARC was divided in four levels according to bregma: level I (− 1.72 mm, − 1.80 mm and − 1.92 mm; 2 sections per animal, n = 23), level II (− 2.16 mm, − 2.28 mm and − 2.40 mm; 1–2 sections per animal, n = 24), level III (− 3.00 mm, − 3.12 mm and − 3.24 mm; 2 sections per animal, n = 24) and level IV (− 3.60 mm, − 3.72 mm and − 3.84 mm; 2 sections per animal, n = 23). The caudal DMN was selected according to bregma (− 3.60 mm, − 3.72 mm and − 3.84 mm; 2 sections per animal, n = 23).
Quantification was performed using a blinded method and we only quantified pERK1/2 labelling on one side. As leptin, insulin and glucagon were given systemically we would anticipate that labelling would be similar on either side. However, for the purposes of side consistency with respect to the blood glucose assay/immunohistochemistry experimental group (no unilateral noxious stimulus) and the experimental group of stimulus evoked electrophysiology (different animals used in each group), a mark was made in the hypothetical contralateral side (to stimulus from electrophysiology studies) of the brain/spinal cord before sectioning and quantifications were performed in the hypothetical ipsilateral side.
2.5. Statistical analysis
Statistical analysis was performed using IBM SPSS 22.0 software. Regarding electrophysiology experiments, data collected for Aδ-fibers represent the normalized data for the number of cells firing over a 10 ms time period in the region 4–20 ms post-stimulation over the 20 sweeps, and expressed as mean ± SEM. Spontaneous activity was measured in cell firings per second (Hz). In order to compare electrophysiology data with the blood glucose levels experimental group (baseline and 4 time-points), neuronal recording responses were averaged for 5, 10 and 15 min (shown as 15 min time-point), as well as for 20, 25 and 30 min (shown as 30 min time-point). For electrophysiology and blood glucose experiments, to detect whether there was a significant effect over time we used ANOVA for repeated measures with Bonferroni post-hoc correction for multiple comparisons. If Mauchly's test of sphericity was violated, appropriate corrections to the degrees of freedom were made according to Greenhouse–Geisser (Field, 2013). Student's paired t-test (two-tailed) was used for post-hoc analysis of individual time points comparing to pre-injection values, using the average of the three baselines for electrophysiology data. For immunohistochemistry, we first tested normality of the four groups using the Kolmogorov-Smirnov test within a region of interest. According to the nature of data, we either used one-way ANOVA with Bonferroni post-hoc correction for multiple comparisons and independent Student's t-test (two-tailed) was used for comparison with sham control group (data expressed as mean ± SEM); or used Kruskal-Wallis test with Monte Carlo exact test post-hoc correction (CI of 95%) and Mann Whitney U test for comparison with sham control group (data are reported as a median with interquartile, 25%,–75% ranges), where a Bonferroni post-hoc correction was applied.
3. Results
3.1. Glucose metabolism
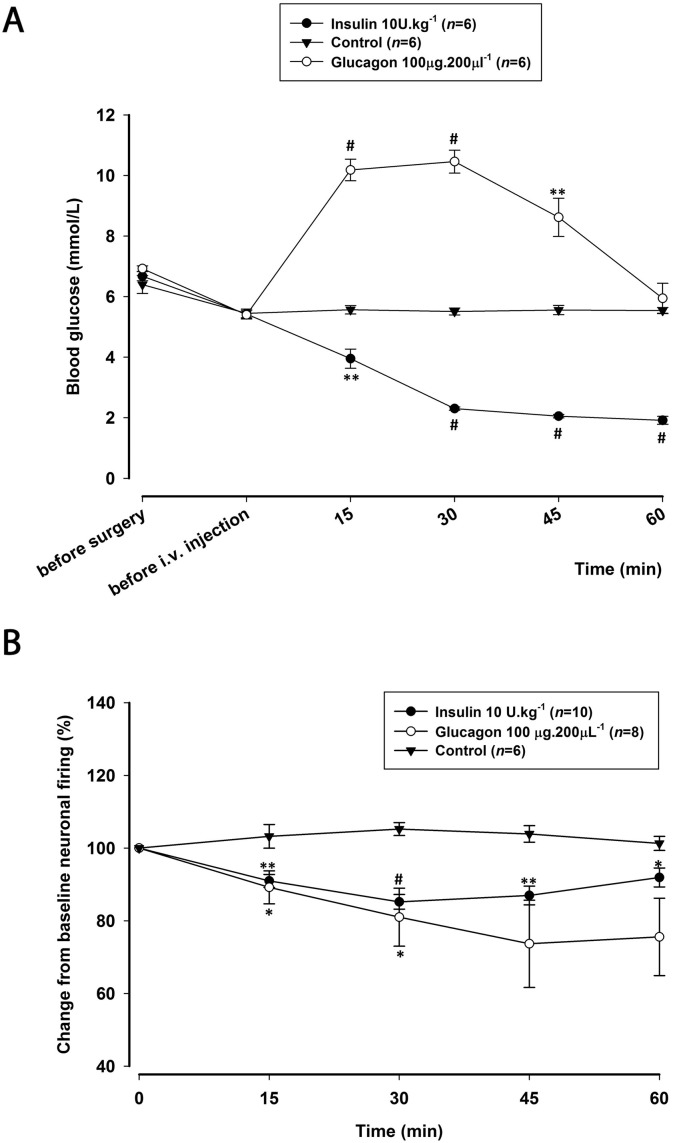
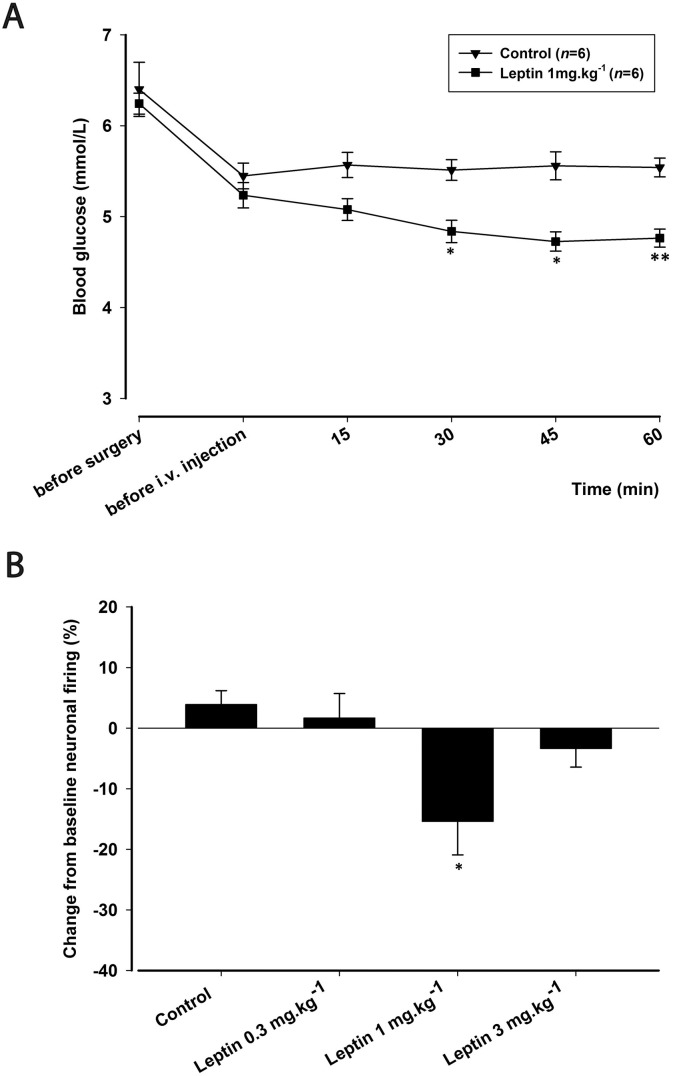
Animals studied had a mean body weight of 289 ± 3.3 g and were provided with water and food ad libitum before the experiment. In all experiments, blood glucose was within physiological levels before surgery (6.6 ± 0.1 mmol/L) and before injections (5.4 ± 0.1 mmol/L). Intravenous administration of insulin (10 U·kg− 1; n = 6) significantly decreased blood glucose levels (F1.8,9.0 = 91.6; p = 0.000), reaching a maximum decrease of 65% (t5 = 20.755; p = 0.000) at 60 min post-injection (Fig. 3A). Glucagon (100 μg·200 μl− 1; n = 6) significantly increased blood glucose levels (F1.7,8.6 = 46.801; p = 0.000), reaching a maximum increase of 94% (t5 = 15.960; p = 0.000) at 30 min post-injection, returning to baseline levels by 60 min (Fig. 3A). Leptin (1 mg·kg− 1; n = 6) significantly decreased blood glucose levels (F2.1,10.5 = 7.469; p = 0.009), reaching a maximum decrease of 10% (t5 = 2.985; p = 0.031) at 45 min that continued over the 60 min period (Fig. 4A). Sham control (n = 6) had no significant effects on blood glucose levels throughout the 60 min cohort (F2.1,10.7 = 0.395; p = 0.698).
Fig. 3.
Effects of insulin and glucagon on blood glucose levels and on dural-evoked neuronal firing in the trigeminocervical complex (TCC).
A) Time course changes in blood glucose levels before surgery, before administration of drugs and following intravenous administration of insulin (10 U·kg− 1) and glucagon (100 μg·200 μl− 1) throughout the 60 min post-injection. Insulin (10 U·kg− 1) significantly decreased blood glucose levels to a maximum of 1.9 mmol/L. Glucagon (100 μg·200 μl− 1) significantly increased blood glucose levels to a maximum of 10.4 mmol/L at 30 min and returned to pre-injection levels at 60 min. Vehicle control had no effect on responses and blood glucose levels were kept in the physiological range of 5.4–5.5 mmol/L.
B) Time course changes in the average response of dural-evoked Aδ-fiber trigeminal neuronal firing in response to insulin (10 U·kg− 1) and glucagon (100 μg·200 μl− 1), which significantly decreased neuronal responses across the 60 min study. Vehicle control had no effect on responses.
*p < 0.05; **p < 0.01; #p < 0.001.
Fig. 4.
Effects of leptin on blood glucose levels and on dural-evoked neuronal firing in the trigeminocervical complex (TCC).
A) Time course changes in blood glucose levels before surgery, before administration of drugs and following intravenous administration of leptin (1 mg·kg− 1) throughout the 60 min post-injection. Leptin (1 mg·kg− 1) significantly decreased blood glucose levels to a maximum of 4.7 mmol/L, respectively. Vehicle control had no effect on responses and blood glucose levels were kept in the physiological range of 5.4–5.5 mmol/L.
B) Bar graph of the maximum effect of leptin (0.3, 1 and 3 mg·kg− 1) at the ‘45 min time point’ of the change from baseline of dural-evoked Aδ-fiber activity in the TCC. Leptin (1 mg·kg− 1) significantly inhibited dural-evoked Aδ-fiber activity in the TCC, demonstrating an optimal dose effect for leptin. Vehicle control had no effect on responses.
*p < 0.05; **p < 0.01; #p < 0.001.
3.2. In vivo electrophysiology experiments
3.2.1. Electrophysiological data
Recordings were made from a total of 47 neuronal clusters in 47 rats (mean body weight of 313 ± 4 g). Extracellular recordings in the TCC were made from wide dynamic range (WDR) neurons, responsive to dural MMA stimulation (Fig. 2A), and with cutaneous receptive fields in the ophthalmic division of the trigeminal nerve. Neurons responding to dural electrical stimulation responded with an average latency of 10.3 ± 0.2 ms (range 4 to 20 ms, an example of evoked neuronal firing can be seen in Fig. 2B) and hence were classified as Aδ fibers. Very few C-fiber latency (beyond 20 ms) responses were observed, and therefore we were only able to quantify Aδ-fiber responses. The majority of neurons were located in lamina V of the dorsal horn at the level of the cervicomedullary junction, with 4 neurons in lamina II–IV, at an average depth of 538 ± 26 μm and the electrode placement was confirmed in all animals via an electrothermolytic lesion in the TCC (Fig. 2C, D). The mean ongoing spontaneous firing rate was 30.9 ± 3.1 Hz with the majority of neurons responding between 10 and 20 Hz; this is within the same range as that demonstrated in previous studies (Akerman et al., 2013). Intravenous injection of sterile water (n = 6) had no significant effect on Aδ-fiber responses (F2.0,10.5 = 1.6; p = 0.242) and ongoing spontaneous activity (F4,20 = 1.6; p = 0.203) of trigeminal second-order neurons during the 60 min time period.
3.2.2. Pancreatic peptides are able to modulate nociceptive evoked trigeminovascular activation
Following the observed changes of blood glucose levels by pancreatic peptides (Fig. 3A), we then investigated the impact of insulin and glucagon on TCC neurons. Insulin (10 U·kg− 1; n = 10) significantly inhibited dural-evoked responses (F3.0,27.4 = 9.4; p = 0.000), with a significant maximal inhibition of 15% at 30 min (t9 = 6.320; p = 0.000; Fig. 3B). At this dose there was no significant effect on spontaneous neuronal firing (F4,36 = 2.0; p = 0.113). Glucagon (100 μg·200 μl− 1; n = 8) significantly reduced nociceptive neuronal firing within the TCC (F4,28 = 2.7; p = 0.049; Fig. 3B). A significant maximal inhibition of 18% was achieved at 30 min post-injection (t7 = 2.603; p = 0.035). Glucagon had no significant effect on the ongoing spontaneous activity (F4,28 = 0.7; p = 0.560).
3.2.3. Leptin shows an optimal dose effect on the nociceptive activation of the trigeminovascular system
Because leptin also has the potential to reduce blood glucose levels (Fig. 4A), we investigated if leptin is able to modulate TCC neuronal firing by testing three doses (0.3, 1 and 3 mg·kg− 1). Leptin (1 mg·kg− 1; n = 8) significantly reduced Aδ-fiber responses to dural stimulation within the TCC (F3,21 = 5.3; p = 0.007). Maximal inhibition of 15% was achieved at 45 min when compared with baseline (t7 = 2.526; p = 0.039; Fig. 4B). Conversely, there was no significant effect on spontaneous neuronal firing (F3,21 = 1.2; p = 0.315) with this dose. Leptin (0.3 mg·kg− 1; n = 7) had no significant effect on dural-evoked nociceptive firing in the TCC (F2.1,12.8 = 0.8; p = 0.471) or ongoing spontaneous activity (F3,18 = 1.4; p = 0.265). Leptin (3 mg·kg− 1; n = 8) had no significant effect on dural-evoked nociceptive responses (F1.7,12.2 = 1.3; p = 0.287) and ongoing spontaneous neuronal activity (F3,21 = 2.5; p = 0.082) in the TCC. These results suggest leptin displays an optimal dose inhibition of dural-evoked trigeminovascular activation, as a nociceptive inhibitory response was only seen with 1 mg·kg− 1.
3.2.4. Blood pressure
In all experiments, blood pressure was kept at physiological levels (95.3 ± 3.2 mm Hg) before injections. Intravenous administration of insulin (10 U·kg− 1) significantly increased mean arterial blood pressure after injection, reaching a maximum increase of 16% (t9 = 5.719; p = 0.000). Blood pressure values recovered to pre-injection values after 11 ± 2 min (t9 = 5.801; p = 0.000) post-injection. Glucagon (100 μg·200 μl− 1) significantly decreased the mean arterial blood pressure, maximally by 11% (t7 = 4.899; p = 0.002), returning to baseline after 2 ± 0.2 min (t7 = 8.039; p = 0.000). Leptin (1 mg·kg− 1) significantly increased mean arterial blood pressure after injection, reaching a maximum increase of 7% (t7 = 2.834; p = 0.025). Blood pressure values slowly recovered to pre-injection values after approximately 8 ± 1 min (t7 = 4.537; p = 0.003) post-injection. Leptin (0.3 mg·kg− 1) and leptin (3 mg·kg− 1) had no significant effects on blood pressure (t6 = 0.499; p = 0.636 and t7 = 2.319; p = 0.053, respectively), likewise sterile water administration (t5 = 2.392; p = 0.062).
3.2.5. Immunohistochemistry
In general, a baseline labelling was observed in control sham group and this is likely due to normal circadian variations in pERK1/2 expression (Goldsmith and Bell-Pedersen, 2013) or the result of the manipulation of the animals on the experimental day.
3.2.5.1. Trigeminocervical complex
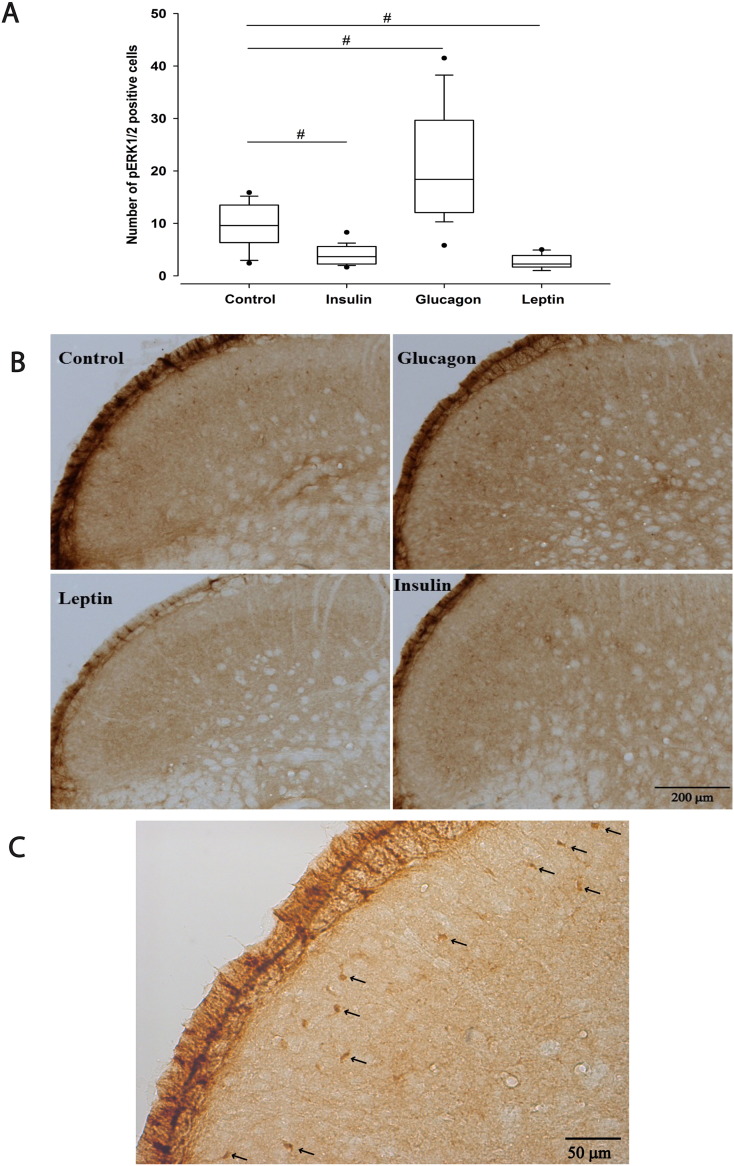
In the TCC, there was an overall significant effect between groups (χ2(3) = 53.647; p = 0.000; Fig. 5A, B). Comparing to sham control animals with a median of 10 cells (range 7–13), the levels of pERK1/2 positive cells were significantly decreased by insulin (median = 4, range 2–5; U = 34.0; p = 0.000) and leptin (median = 2, range 2–4; U = 11.0; p = 0.000). Conversely, glucagon significantly increased pERK1/2 expression levels (median = 18, range 12–28; U = 51.5; p = 0.000; Fig. 5A, B, C). Besides of the overall increase, we observed a reorganization of pERK1/2 expression within laminae, most noticeable within lamina III, where glucagon (median = 7, range 3–10) significantly increased pERK1/2 expression (U = 23; p = 0.003) comparing to sham control (median = 2, range 1–4).
Fig. 5.
Effect of intravenous administration of insulin, glucagon and leptin on pERK1/2 cell expression within the trigeminocervical complex (TCC).
A) Box plots summarising pERK1/2 expression in the TCC, consisting of the trigeminal nucleus caudalis (TNC) and C1 and C2 dorsal horn laminae I–V. Insulin (10 U·kg− 1) and leptin (1 mg·kg− 1) significantly decreased pERK1/2 labelling within the TCC, as oppose to glucagon (100 μg·200 μl− 1), which significantly increased pERK1/2 expression, when compared to control sham group, 60 min post-injection.
B) Photomicrographs showing the localization of pERK1/2 staining in the TCC.
C) Photomicrograph showing pERK1/2 stained neurons in the TCC of an animal administered with glucagon (100 μg·200 μl− 1). Arrows indicate cell bodies.
*p < 0.05; **p < 0.01; #p < 0.001.
3.2.5.2 Hypothalamic regions
In the hypothalamic ARC, there was an overall significant effect between groups (F3,20 = 5.5; p = 0.006; Fig. 6A, B, C). Comparing to sham control animals, pERK1/2 expression was significantly decreased by 24.3% following leptin administration (t10 = 2.856; p = 0.017). However, insulin (t10 = 0.295; p = 0.774) or glucagon (t10 = 2.160; p = 0.056) had no significant effect. Moreover, in other hypothalamic nuclei analyzed, there was no significant overall difference in the PVN (F3,20 = 2.1; p = 0.127) (Fig. 6D) and caudal DMN (χ2(3) = 0.846; p = 0.869).
Fig. 6.
Effect of intravenous administration of insulin, glucagon and leptin on pERK1/2 cell expression within the hypothalamic arcuate nucleus (ARC) and paraventricular nucleus (PVN)
A) Leptin (1 mg·kg− 1) significantly reduced pERK1/2 cell expression within the ARC, while insulin (10 U·kg− 1) and glucagon (100 μg·200 μl− 1) did not significantly affect pERK1/2 expression, when compared to control sham group, 60 min post-injection.
B) Photomicrographs showing the localization of pERK1/2 staining in the hypothalamic ARC. Dotted line shows the ARC nucleus; 3V: third ventricle.
C) Photomicrograph showing the localization of pERK1/2 staining in the hypothalamic ARC of an animal administered with glucagon (100 μg·200 μl− 1). Arrows indicate cell bodies; 3V: third ventricle.
D) Photomicrographs displaying the localization of pERK1/2 staining in the PVN showing no significant difference between groups.
*p < 0.05; **p < 0.01; #p < 0.001.
3.2.5.3. Non-hypothalamic regions
Considering other brain areas of potential interest in the context of both appetite and pain pathophysiology: there was no significant overall difference between groups in the midbrain periaqueductal gray (χ2(3) = 3.000; p = 0.392) and no pERK1/2 expression in the ventral tegmental area (χ2(3) = 0.000; p = 1.000). Likewise, there was no significant overall difference between groups in the nucleus accumbens (χ2(3) = 3.000; p = 0.392).
4. Discussion
Our data demonstrates that peptides normally produced by the endocrine system and involved in feeding mechanisms activate pathways capable of modulating neuronal responses in the TCC following dural nociceptive activation. These changes are likely mediated through coordinated actions as part of a homeostatic feedback mechanism whose perturbation can influence the trigeminovascular system.
4.1. Glucose homeostasis and nociceptive activation of the trigeminovascular system
Pancreatic peptides insulin and glucagon both inhibited nociceptive trigeminovascular activation, yet display opposite effects on blood glucose levels. These results suggest acute changes of systemic glucose levels can alter TCC nociceptive responses in healthy animals by perturbation of normal physiology. The insulin dose used in our study induced a mild hypoglycemic state, reducing blood glucose levels, which has been previously shown to normalize beyond 60 min (data not shown). Interestingly, the maximum insulin-induced inhibition of dural-evoked TCC neuronal firing occurred after 30 min. In the case of glucagon, blood glucose levels increased maximally at 30 min, which matched the maximum inhibition of Aδ-fiber responses to dural stimulation, with responses no different to pre-injection levels by 60 min. Indeed, both insulin and glucagon activate a counter regulatory mechanism that involves a tight regulation of glucose sensing (Thorens, 2011), suggesting that modulation of glucose-excited or glucose-inhibited neurons in the brain (Karnani and Burdakov, 2011, Routh, 2002), may be a crucial interface between migraine and appetite regulation. The data are consistent with a view that abnormal nociceptive trigeminovascular activation may be triggered by a transient dysfunction of glucose homeostasis.
In addition, our study adds new information by confirming leptin modulation of dural-evoked TCC neuronal activity with an optimal dose effect. It has long been known that endogenous leptin-resistant mechanisms are present in obese individuals (Considine et al., 1996), and previous studies in migraine animal models have shown that obesity causes abnormal sensory processing within the trigeminovascular system (Rossi et al., 2016, Rossi et al., 2013). These studies and our results support the involvement of leptin in migraine pathophysiology.
4.2. ERK signaling cascade in the TCC and the hypothalamus: role in nociception and energy homeostasis control
Our immunohistochemistry data shows a distinct pattern of pERK1/2 expression in the TCC and in the hypothalamus following peripheral administration of insulin, glucagon and leptin. We demonstrate that insulin and leptin are able to decrease pERK1/2 cell expression in the TCC, as opposed to glucagon, which significantly increased pERK1/2 levels. Interestingly, there is as correlation between neuronal pERK1/2 expression in the TCC and blood glucose changes: both insulin and leptin decreased blood glucose levels and pERK1/2 expression in the TCC at 60 min and glucagon increases pERK1/2 neuronal expression in the TCC at 60 min, however inducing a transient increase in blood glucose levels. This might indicate that TCC neurons are glucose-responsive through the ERK signaling cascade.
The pERK1/2 expression study adds further information with more precise localization of activated neurons in the TCC, complementing the results obtained with the electrophysiology assay. An interesting reorganization within the TCC laminae was observed with glucagon, namely an increased pERK1/2 expression in lamina III (in addition to overall laminae increased expression). This can be related to the considerable interlaminar communication that influences laminae and their sensory input (Cramer and Darby, 2014). Many of the laminae communicate with each other via profuse dendritic branching and the axonal projections of their interneurons, providing a mechanism by which incoming sensory signals may be processed and modulated before ascending to higher centers (Cramer and Darby, 2014). Perhaps, this particular pathway and its corresponding interneurons are activated to allow a selective response to unexpected increase of blood glucose levels. Our results demonstrate that insulin, glucagon and leptin directly modulate nociceptive trigeminal traffic within the TCC. Consequently, this pathway might be impaired in migraineurs, contributing to abnormal sensitive responses to glucose homeostasis regulation.
Regarding the hypothalamus, we observed that pERK1/2 cell expression in the ARC was significantly altered between groups, but no changes were observed in the other hypothalamic nuclei. At the tested dose, leptin significantly decreased pERK1/2 cell expression in the ARC, yet this was unaffected by insulin and glucagon. Besides being involved in a number of physiological effects, ERKs are associated with synaptic plasticity in the brain and spinal cord during induction of several pain conditions (Cruz et al., 2005). In other migraine experimental models, increased pERK expression has been demonstrated in the TNC and related to sensitization of the trigeminovascular system (Dong et al., 2015) (Zhang et al., 2013). Generally, the main target region of leptin is the ventrobasal hypothalamus (Elmquist et al., 1997) and our study shows that leptin acts in the ARC by inhibiting pERK1/2 expression. The fact that fasting induces a reduction in circulating leptin levels and it is a reported trigger of migraine supports our results showing that leptin has an anti-nociceptive role in a migraine animal model. However, previous studies using the same intravenous dose of leptin, in animals fed ad libitum show reduced Fos protein expression in VMH, PVN and DMN (Elmquist et al., 1997) and in the ARC (Elias et al., 2000). This may be related to differences in the point at which perfusion was performed; 2 h after leptin administration in previous studies and 1 h here. We also observed pERK1/2 labelling in other hypothalamic nuclei, such as the PVN and DMN, but pERK1/2 cell expression was not significantly different between groups. Indeed, studies in migraine animal models have suggested the involvement of PVN and DMN (Malick and Burstein, 2001, Robert et al., 2013), while the lack of significant differences of pERK1/2 expression within the PVN or DMN in our study may reflect a time-course difference of activation.
4.3. Fasting and skipping meals relevance as migraine trigger
Our study shows that TCC neurons are glucose-responsive and regardless of systemic glucose levels, the output is the same, i.e., inhibition of Aδ-fiber trigeminal responses to dural-nociceptive stimulation in healthy animals. Fasting or skipping a meal for longer than the recommended guidelines, followed by refeeding, will rapidly shift glucose homeostasis pathways, and it is this sudden physiological response that is likely to trigger migraines in susceptible individuals. In addition, and according to the suggested insulin-resistance observed in migraineurs, insulin will not be able to perform its homeostatic role in glucose metabolism and, perhaps, will not be able to modulate nociceptive inputs.
Furthermore, we have shown that peptides involved in glucose homeostasis affect Aδ-fiber responses to dural stimulation, however, we cannot rule out potential effects on C-fiber neural responses. Ongoing spontaneous TCC neuronal firing was unaffected suggesting their action is nociceptive-specific. Preservation of glucose homeostasis during fasting requires a fine balance in glucose and appetite metabolism, including physiological feedback inhibition loops, and this might explain the optimal dose effect of leptin observed herein. Fasting or skipping meals has been consistently described as a migraine trigger and clinical studies also report perturbations in peptide levels in migraineurs: increased leptin and insulin levels (Bernecker et al., 2010), but lower serum levels interictally (Guldiken et al., 2008); and higher insulin levels compared to controls (Cavestro et al., 2007, Rainero et al., 2005) and a significant prevalence of insulin resistance in chronic migraineurs (Fava et al., 2014). Interestingly, a significant association between a polymorphism of five single nucleotides of the gene encoding for insulin receptors in migraineurs has been found (McCarthy et al., 2001), which may cause lower insulin receptor activation in susceptible migraineurs. The relevance of these studies is to acknowledge that modulatory cross-talks between insulin, glucagon and leptin may be impaired in migraineurs. These results might indicate that fasting or skipping meals should not be interpreted as an isolated trigger of migraine, but rather part of a “cumulative triggering”, for instance along with dehydration, changes of sleep patterns or disruption of the stress hormones feedback loop within the hypothalamic-pituitary-adrenal axis. Therefore, it is the combination of triggering factors that increases the likelihood of a migraine attack.
5. Conclusion
Considered altogether, the data are consistent with a view that off target effects of peptides involved in blood glucose homeostasis may increase the predisposition to triggering migraine through altered feeding schedule. In addition, pharmacological pERK inhibition may constitute a putative tool to control nociceptive-induced mechanisms related to migraine pathophysiology and further studies should be considered. Furthermore, since endocrine peptides are clearly involved in migraine pathophysiology, skipping meals should be taken into consideration as soon as the premonitory phase of the attack is identified by the migraineur.
Conflict of interest statement
MMO (margarida.martins_oliveira@kcl.ac.uk; margarida.oliveira.m@gmail.com) reports no conflicts of interest.
SA (simon.akerman@nyu.edu) reports, unrelated to this report, grants from Electrocore LLC.
PRH (philip.holland@kcl.ac.uk) reports, unrelated to this report, grants from Amgen and honoraria and travel expenses in relation to educational duties from Allergan and Almirall
JRH (j-r.hoffmann@uke.de) reports no conflicts of interest.
IT (isatav@med.up.pt) reports no conflicts of interest.
PJG reports, unrelated to this report, grants and personal fees from Allergan, grants and personal fees from eNeura Inc., personal fees from Autonomic Technologies Inc., grants and personal fees from Amgen Inc., personal fees from Alder Biopharmaceuticals, personal fees from Pfizer Inc., personal fees from Dr. Reddy's Laboratories, personal fees from Zosano Pharma Corporation, personal fees from Colucid Pharmaceuticals, Ltd., personal fees from Eli-Lilly and Company, personal fees from Avanir Pharmaceuticals, personal fees from WL Gore & Associates, personal fees from Heptares Therapeutics, personal fees from Nupathe Inc., personal fees from Teva, personal fees from Cipla Ltd., personal fees from Ajinomoto Pharmaceuticals Co., personal fees from Akita Biomedical, personal fees from Wells Fargo, personal fees from Ethicon, US, personal fees from EMKinetics, personal fees from Promius Pharma, personal fees from Supernus, personal fees and other from Trigemina, personal fees from MedicoLegal work, personal fees from Journal Watch, personal fees from Up-to-Date, outside the submitted work; In addition, Dr. Goadsby has a patent Magnetic stimulation for headache pending.
Author contributions
All the authors have read and approved the manuscript. Margarida Martins-Oliveira performed and designed experiments, conducted data analyses and data interpretation, and wrote the manuscript. Simon Akerman designed experiments, assisted in in vivo electrophysiology experiments, contributed to interpretation of data and revised critically the manuscript. Philip R. Holland and Jan R. Hoffmann assisted in in vivo electrophysiology experiments, contributed to interpretation of data and revised critically the manuscript. Isaura Tavares designed experiments, assisted in immunohistochemistry experiments, contributed to interpretation of data and revised critically the manuscript. Peter J. Goadsby designed experiments, assisted in in vivo electrophysiology experiments, contributed to interpretation of data and revised critically the manuscript.
Acknowledgments
Margarida Martins-Oliveira is grateful to the Portuguese Fundação para a Ciência e a Tecnologia (FCT) for its support with an individual PhD grant (SFRH/BD/77127/2011). The work was supported by the EUROHEADPAIN European Union FP7 (602633) and the Wellcome Trust (104033). The authors would like to thank Michele P. Lasalandra for technical advice on the postsurgical examination of tissue; Marta Louçano for helpful technical assistance with immunohistochemistry experiments; and Gisela S. Borges for valuable advice on pERK1/2 immunohistochemistry.
Footnotes
Completed “The ARRIVE Guidelines Checklist” for reporting animal data in this manuscript. Supplementary data associated with this article can be found in the online version, at http://dx.doi.org/10.1016/j.nbd.2017.01.005.
Appendix A. Supplementary data
The following is the supplementary data related to this article.
Supplementary material
References
- Akerman S. Diencephalic and brainstem mechanisms in migraine. Nat. Rev. Neurosci. 2011;12:570–584. doi: 10.1038/nrn3057. [DOI] [PubMed] [Google Scholar]
- Akerman S. Endocannabinoids in the brainstem modulate dural trigeminovascular nociceptive traffic via CB1 and ‘triptan’ receptors: implications in migraine. J. Neurosci. 2013;33:14869–14877. doi: 10.1523/JNEUROSCI.0943-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer R.N. Hypoglycemic brain damage. Metab. Brain Dis. 2004;19:169–175. doi: 10.1023/b:mebr.0000043967.78763.5b. [DOI] [PubMed] [Google Scholar]
- Bergerot A. Animal models of migraine. Looking at the component parts of a complex disorder. Eur. J. Neurosci. 2006;24:1517–1534. doi: 10.1111/j.1460-9568.2006.05036.x. [DOI] [PubMed] [Google Scholar]
- Bernecker C. GLP-2 and leptin are associated with hyperinsulinemia in non-obese female migraineurs. Cephalalgia. 2010;30:1366–1374. doi: 10.1177/0333102410364674. [DOI] [PubMed] [Google Scholar]
- Bigal M.E. Obesity and migraine: a population study. Neurology. 2006;66:545–550. doi: 10.1212/01.wnl.0000197218.05284.82. [DOI] [PubMed] [Google Scholar]
- Blau J.N., Cumings J.N. Method of precipitating and preventing migraine attacks. Br. Med. J. 1966;II:1242–1243. doi: 10.1136/bmj.2.5524.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges G.S. Extracellular signal-regulated kinase activation in the chronic constriction injury model of neuropathic pain in anaesthetized rats. Eur. J. Pain. 2013;17:35–45. doi: 10.1002/j.1532-2149.2012.00181.x. [DOI] [PubMed] [Google Scholar]
- Burdakov D. Glucose-sensing neurons of the hypothalamus. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2005;360:2227–2235. doi: 10.1098/rstb.2005.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavestro C. Insulin metabolism is altered in migraineurs: a new pathogenic mechanism for migraine? Headache. 2007;47:1436–1442. doi: 10.1111/j.1526-4610.2007.00719.x. [DOI] [PubMed] [Google Scholar]
- Charles A. Migraine: a brain state. Curr. Opin. Neurol. 2013;26:235–239. doi: 10.1097/WCO.0b013e32836085f4. [DOI] [PubMed] [Google Scholar]
- Chasman D.I. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat. Genet. 2011;43:695–698. doi: 10.1038/ng.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine R.V. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Cramer G.D., Darby S.A. St. Louis; MO: 2014. Clinical Anatomy of the Spine, Spinal Cord and ANS. [Google Scholar]
- Cruz C.D. Inhibition of ERK phosphorylation decreases nociceptive behaviour in monoarthritic rats. Pain. 2005;116:411–419. doi: 10.1016/j.pain.2005.05.031. [DOI] [PubMed] [Google Scholar]
- D'Andrea G. Is migraine a risk factor for the occurrence of eating disorders? Prevalence and biochemical evidences. Neurol. Sci. 2012;33(Suppl. 1):S71–S76. doi: 10.1007/s10072-012-1045-6. [DOI] [PubMed] [Google Scholar]
- De Silva K.L. Blood sugar response to glucagon in migraine. J. Neurol. Neurosurg. Psychiatry. 1974;37:105–107. doi: 10.1136/jnnp.37.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denuelle M. Hypothalamic activation in spontaneous migraine attacks. Headache. 2007;47:1418–1426. doi: 10.1111/j.1526-4610.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- Denuelle M. Posterior cerebral hypoperfusion in migraine without aura. Cephalalgia. 2008;28:856–862. doi: 10.1111/j.1468-2982.2008.01623.x. [DOI] [PubMed] [Google Scholar]
- Dong X. Role of phosphorylated extracellular signal-regulated kinase, calcitonin gene-related peptide and cyclooxygenase-2 in experimental rat models of migraine. Mol. Med. Rep. 2015;12:1803–1809. doi: 10.3892/mmr.2015.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias C.F. Chemical characterization of leptin-activated neurons in the rat brain. J. Comp. Neurol. 2000;423:261–281. [PubMed] [Google Scholar]
- Elmquist J.K. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology. 1997;138:839–842. doi: 10.1210/endo.138.2.5033. [DOI] [PubMed] [Google Scholar]
- Fava A. Chronic migraine in women is associated with insulin resistance: a cross-sectional study. Eur. J. Neurol. 2014;21:267–272. doi: 10.1111/ene.12289. [DOI] [PubMed] [Google Scholar]
- Field A. SAGE Publications; London: 2013. Discovering Statistics Using SPSS. [Google Scholar]
- Freilinger T. Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat. Genet. 2012;44:777–782. doi: 10.1038/ng.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J.M., Halaas J.L. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Geary N. Pancreatic glucagon signals postprandial satiety. Neurosci. Biobehav. Rev. 1990;14:323–338. doi: 10.1016/s0149-7634(05)80042-9. [DOI] [PubMed] [Google Scholar]
- Giffin N.J. Premonitory symptoms in migraine: an electronic diary study. Neurology. 2003;60:935–940. doi: 10.1212/01.wnl.0000052998.58526.a9. [DOI] [PubMed] [Google Scholar]
- Goadsby P.J. Migraine- current understanding and treatment. N. Engl. J. Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- Goldsmith C.S., Bell-Pedersen D. Diverse roles for MAPK signaling in circadian clocks. Adv. Genet. 2013;84:1–39. doi: 10.1016/B978-0-12-407703-4.00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldiken B. Low leptin levels in migraine: a case control study. Headache. 2008;48:1103–1107. doi: 10.1111/j.1526-4610.2008.01152.x. [DOI] [PubMed] [Google Scholar]
- Guldiken B. Migraine in metabolic syndrome. Neurologist. 2009;15:55–58. doi: 10.1097/NRL.0b013e31817781b6. [DOI] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society The international classification of headache disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- Hoffmann J. Evidence for orexinergic mechanisms in migraine. Neurobiol. Dis. 2015;74:137–143. doi: 10.1016/j.nbd.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Holland P.R. Modulation of nociceptive dural input to the trigeminal nucleus caudalis via activation of the orexin 1 receptor in the rat. Eur. J. Neurosci. 2006;24:2825–2833. doi: 10.1111/j.1460-9568.2006.05168.x. [DOI] [PubMed] [Google Scholar]
- Karnani M., Burdakov D. Multiple hypothalamic circuits sense and regulate glucose levels. Am. J. Phys. Regul. Integr. Comp. Phys. 2011;300:R47–R55. doi: 10.1152/ajpregu.00527.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394–402. doi: 10.1111/j.1468-2982.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- Liu Q. Lipoprotein receptor LRP1 regulates leptin signaling and energy homeostasis in the adult central nervous system. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malick A., Burstein R. A neurohistochemical blueprint for pain-induced loss of appetite. Proc. Acad. Sci. (U.S.A.) 2001;98:9930–9935. doi: 10.1073/pnas.171616898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniyar F.H. Brain activations in the premonitory phase of nitroglycerin triggered migraine attacks. Brain. 2014;137:232–242. doi: 10.1093/brain/awt320. [DOI] [PubMed] [Google Scholar]
- Martins-Oliveira M. Modulation of trigeminovascular activity by leptin: a novel antinociceptive mechanism? J. Headache Pain. 2013;14:P76. [Google Scholar]
- Martins-Oliveira M. Modulation of trigeminovascular nociceptive inputs by peptides involved in sleep and appetite homeostatic synchronization: systemic effects of neuropeptide Y, leptin and insulin. Headache. 2014;54:5. [Google Scholar]
- Martins-Oliveira M. Peptides involved in sleep and appetite homeostatic regulation and its effects in the modulation of trigeminovascular nociceptive activation. J. Headache Pain. 2014;15:F18. [Google Scholar]
- Martins-Oliveira M. Neuropeptide Y inhibits the trigeminovascular pathway through NPY Y1 receptor: implications for migraine. Pain. 2016;157:1666–1673. doi: 10.1097/j.pain.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy L.C. Single-nucleotide polymorphism alleles in the insulin receptor gene are associated with typical migraine. Genomics. 2001;78:135–149. doi: 10.1006/geno.2001.6647. [DOI] [PubMed] [Google Scholar]
- Messlinger K., Ellrich J. Meningeal nociception: electrophysiological studies related to headache and referred pain. Microsc. Res. Tech. 2001;53:129–137. doi: 10.1002/jemt.1077. [DOI] [PubMed] [Google Scholar]
- Mighiu P.I. Hypothalamic glucagon signaling inhibits hepatic glucose production. Nat. Med. 2013;19:766–772. doi: 10.1038/nm.3115. [DOI] [PubMed] [Google Scholar]
- Moskowitz M.A., Cutrer F.M. SUMATRIPTAN: a receptor-targeted treatment for migraine. Annu. Rev. Med. 1993;44:145–154. doi: 10.1146/annurev.me.44.020193.001045. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. Elsevier Academic Press; San Diego: 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Rainero I. Insulin sensitivity is impaired in patients with migraine. Cephalalgia. 2005;25:593–597. doi: 10.1111/j.1468-2982.2005.00928.x. [DOI] [PubMed] [Google Scholar]
- Robert C. Paraventricular hypothalamic regulation of trigeminovascular mechanisms involved in headaches. J. Neurosci. 2013;33:8827–8840. doi: 10.1523/JNEUROSCI.0439-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi H.L. Obesity increases nociceptive activation of the trigeminal system. Eur. J. Pain. 2013;17:649–653. doi: 10.1002/j.1532-2149.2012.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi H.L. Abnormal trigeminal sensory processing in obese mice. Pain. 2016;157:235–246. doi: 10.1097/j.pain.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh V.H. Glucose-sensing neurons: are they physiologically relevant? Physiol. Behav. 2002;76:403–413. doi: 10.1016/s0031-9384(02)00761-8. [DOI] [PubMed] [Google Scholar]
- Stankewitz A. Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J. Neurosci. 2011;31:1937–1943. doi: 10.1523/JNEUROSCI.4496-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B. Brain glucose sensing and neural regulation of insulin and glucagon secretion. Diabetes Obes. Metab. 2011;13(Suppl. 1):82–88. doi: 10.1111/j.1463-1326.2011.01453.x. [DOI] [PubMed] [Google Scholar]
- Wilcox G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005;26:19–39. [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Activation of central trigeminovascular neurons by cortical spreading depression. Ann. Neurol. 2011;69:855–865. doi: 10.1002/ana.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Vascular extracellular signal-regulated kinase mediates migraine-related sensitization of meningeal nociceptors. Ann. Neurol. 2013;73:741–750. doi: 10.1002/ana.23873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material