Abstract
Background
Endothelial cell (EC) survival and regeneration are important determinants of the response to vascular injury that leads to neointimal hyperplasia and accelerated atherosclerosis. Nitric oxide (NO) is a key regulator of EC and endothelial progenitor cell function, but the pathophysiological mechanisms that regulate endothelial NO synthase in endothelial regeneration remain unclear.
Methods and Results
Endothelium-targeted overexpression of GTP cyclohydrolase (GCH) I increased levels of the endothelial NO synthase cofactor, tetrahydrobiopterin, in an EC-specific manner and reduced neointimal hyperplasia in experimental vein grafts in GCH/apolipoprotein E-knockout mice. These effects were mediated through enhanced donor-derived survival and recipient-derived repopulation of GCH transgenic ECs, revealed by tracking studies in Tie2-LacZ/GCH-Tg/apolipoprotein E-knockout recipient mice or donor grafts, respectively. Endothelial GCH overexpression increased endothelial NO synthase coupling and enhanced the proliferative capacity of ECs and circulating endothelial progenitor cell numbers after vascular injury.
Conclusions
These observations indicate that endothelial tetrahydrobiopterin availability modulates neointimal hyperplasia after vascular injury via accelerated EC repopulation and growth. Targeting tetrahydrobiopterin-dependent endothelial NO synthase regulation in the endothelium is a rational therapeutic target to enhance endothelial regeneration and reduce neointimal hyperplasia in vascular injury states.
Keywords: endothelial cells, endothelial progenitor cells, free radicals, nitric oxide synthase, remodeling
Loss of endothelial cells (ECs) in conditions of vascular injury is an important contributor to adverse vascular remodeling that leads to neointimal hyperplasia and accelerated atherosclerosis, for example, in venous bypass grafts, allograft vasculopathy, and after angioplasty or stenting.1 Re-endothelialization has been shown to be a key event in vascular repair. Endothelial nitric oxide synthase (eNOS) is essential for the normal function of ECs in the vascular wall and for the function of circulating endothelial progenitor cells (EPC).2–4 However, the molecular mechanisms relating eNOS regulation to endothelial loss, survival, and regeneration after vascular disease are uncertain. The eNOS cofactor, tetrahydrobiopterin (BH4), is a critical determinant of eNOS enzymatic activity and function. We and others have shown that in vascular disease states BH4 deficiency leads to eNOS uncoupling, whereby reduction of molecular oxygen by eNOS is no longer coupled to l-arginine oxidation and NO synthesis, and instead eNOS generates superoxide rather than NO.5
We hypothesized that BH4-dependent eNOS regulation may have an important role in endothelial loss and regeneration and in the development of neointimal hyperplasia after vascular injury. To investigate the specific role of EC BH4 availability, we compared apolipoprotein E (apoE)-knockout (KO) mice with or without transgenic endothelium-targeted overexpression of GTP cyclohydrolase I (GCH), the rate-limiting enzyme in endothelial BH4 synthesis,5,6 in a model of venous bypass grafting characterized by acute endothelial loss, neointimal hyperplasia, and endothelial regeneration2 and investigated how GCH and BH4 regulate primary EC survival and growth.
Methods
Detailed descriptions of methods may be found in the online-only Data Supplement.
Animals and Surgical Procedures
Mice were maintained in temperature-controlled (20°C–22°C) individually ventilated cages with a 12-hour light-dark cycle. Sterile water and standard chow diet were available ad libitum. Mice overexpressing human GCH targeted to the vascular endothelium under the Tie-2 promoter (GCH-Tg)5 were crossed onto C57BL/6J apoE-KO mice (Jackson Laboratories, Bar Harbor, MI) background. Some of these mice were further crossbred with Transgenic Tie2-LacZ mice (Jackson Laboratories, Bar Harbor, MI) expressing β-galactosidase (β-Gal) localized to the nucleus of ECs.7 All animal procedures were performed in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986. Vein graft surgery and femoral artery wire injury were performed in 18- to 22-week-old male mice as described previously.8,9 Four groups were compared with or without the Tie2-LacZ transgene: apoE-KO donor vein grafted into apoE-KO recipient, GCH/apoE-KO vein grafted into GCH/apoE-KO recipient, apoE-KO vein grafted into GCH/apoE-KO recipient, or GCH/apoE vein grafted into apoE-KO recipient.
Lipid and Lipoprotein Analysis
Total plasma cholesterol and triglyceride concentrations were measured using enzymatic assay (Roche, Indianapolis, IN) on a Cobas Mira Plus automated analyzer (Roche, Switzerland).
Tissue Preparation, Histology, and Lesion Quantification
Grafts were harvested at 28 (n=5–8/treatment group) or 56 days (n=5–8/treatment group) after surgery and perfusion fixed in situ with 4% phosphate-buffered paraformaldehyde.10 Grafts were sectioned at 150 μm from the midpoint, collecting 5 μm sections. Three representative sections, separated by 50 µm, were stained with Masson/Goldner stain for analysis. Lesion quantification was similar to that described previously.10
Biopterin Measurements
Total biopterins (BH4, dihydrobiopterin, and biopterin) and BH4 (total biopterin−dihydrobiopterin+biopterin) were measured using high-precision liquid chromatography analysis with fluorescent detection after differential iodine oxidation of tissue homogenate under acidic and alkaline conditions as described previously.5
Quantitative Real-Time Polymerase Chain Reaction
Total RNA (100 ng) was extracted from freshly harvested snap-frozen vein grafts harvested 28 days after surgery. Quantitative fluorescent real-time reverse transcription polymerase chain reaction analysis was performed to compare relative quantities of mRNA in vena cava and vein grafts using the Rotor-Gene system (Corbett Research Ltd, Cambridge, UK).
En Face X-Gal Staining
Freshly harvested vein segments or ECs were incubated at 37°C for 3 hours in PBS containing 1 mg/mL X-Gal (Sigma), 5 mmol/L potassium ferricyanide, 5 mmol/L potassium ferrocyanide, and 2 mmol/L MgCl2. Vessel segments were rinsed with 3% dimethyl sulfoxide in PBS and mounted with the endothelium up on paraffin base and analyzed as described previously.2
Tissue Preparation, Histology, and Lesion Quantification
Grafts were harvested at 28 (n=5–8/treatment group) or 56 days (n=5–8/treatment group) after surgery and perfusion-fixed in situ with 4% phosphate-buffered paraformaldehyde.10 Detailed procedures are described in Materials and Methods in the online-only Data Supplement .
Primary Murine ECs
Primary ECs were isolated from lungs. Lungs were finely minced and digested in DMEM containing 0.18 U/mL Liberase Blendzyme 3 (Roche) and 0.1 mg/mL DNase I (Roche) for 60 minutes at 37°C with gentle agitation. Positive selection for ECs was achieved using Dynabeads Sheep anti-Rat IgG (Invitrogen, USA) or MACS Goat anti-Rat IgG MicroBeads (Miltenyi Biotech, Germany) for flow cytometry experiments.
Western Blotting
Cell pellets were lyzed in radioimmunoprecipitation assay buffer (Tris 50 mmol/L, NaCl 150 mmol/L, SDS 0.1%, deoxycholate salt 0.5%, non-idet P40 1%, phenylmethylsulfonyl fluoride 1 mmol/L, dithiothreitol 1 mmol/L) containing protease inhibitors (Complete, Mini, EDTA-free. Roche, UK). NuPAGE LDS Sample Buffer and NuPAGE Reducing Agent (Invitrogen, UK) were added to the lysates, and the samples were heated at 70°C for 10 minutes right before gel electrophoresis.
Flow Cytometry Analysis
Data were acquired using a CyAnTM ADP flow cytometer (Beckman Coulter) and Summit software and analyzed using FlowJo software.
Bromodeoxyuridine Incorporation Assay
Primary mouse ECs were isolated from lungs as described above, and 10 μL of Bromodeoxyuridine (BrdU 1 mmol/L) was added to each milliliter of culture media, obtaining a final concentration of 10 μmol/L, and the cells were harvested after 16 hours.
Cell Viability Assay
The PE AnnexinV Apoptosis Detection Kit I (BD Bioscience, UK) was used to detect early and late apoptosis in CD31 Dynabead–isolated ECs directly after the isolation procedure.
β-Gal Activity Assay
The high-sensitivity β-galactosidase assay kit from Stratagene, USA, was used according to the manufacture’s instructions for measurement of β-Gal activity in primary ECs and lung tissue.
EPC Isolation and Characterization
Mononuclear cells (MNCs) were isolated from bone marrow of experimental animals by Histopaque 1083 (Sigma) through density gradient centrifugation or by lymphocyte separation medium 1077 (PAA, Austria). Cells were incubated for 72 hours at 37°C in EPC medium, after which nonadherant cells were removed by washing with Dulbecco’s phosphate-buffered saline, and resuspended in fresh EPC medium for a further 48 hours.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism software. Data are presented as mean±SEM. Data were subjected to the Kolmogorov–Smirnov test to determine distribution. An unpaired t test was used for comparison between 2 groups, and comparisons in experiments with ≥3 groups were performed with 1-way ANOVA and the Bonferonni post hoc test. P<0.05 was considered statistically significant.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the article as written.
Results
Effect of GCH Overexpression on Vascular Remodeling
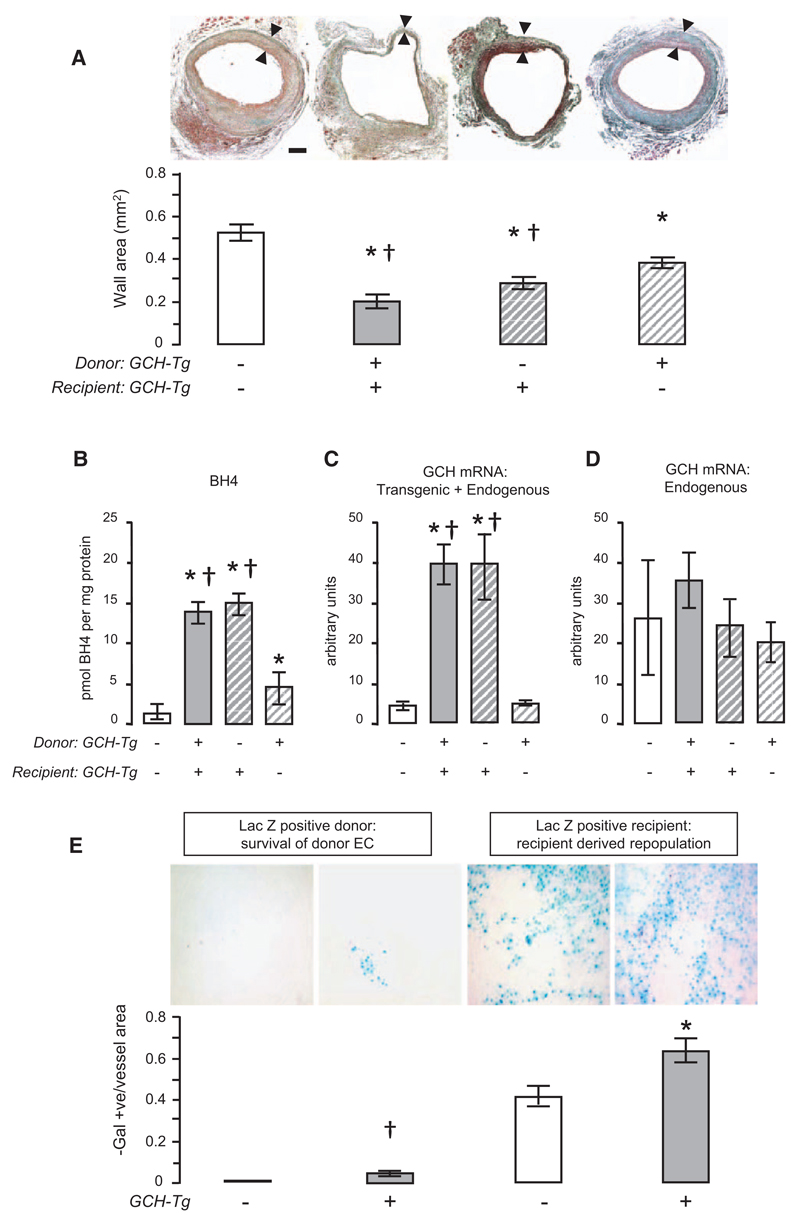
To investigate the effects of increased EC GCH expression on neointimal hyperplasia and vascular remodeling, we quantified vein graft wall area at both 28 and 56 days after vein graft surgery in GCH/apoE-KO and apoE-KO mice. Total cholesterol, triglyceride levels, and body weights were similar in GCH/apoE-KO and apoE-KO mice (Table I in the online-only Data Supplement). Mean vessel wall area was significantly reduced in GCH/apoE-KO mice 28 days after surgery compared with apoE-KO controls (61% reduction; P<0.001; Figure 1A), and this difference was sustained at 56 days (46% reduction in mean vessel wall area; P<0.001; Figure IA in the online-only Data Supplement). In contrast to the marked reduction of neointimal hyperplasia observed in GCH/apoE-KO vein grafts, mean aortic root atherosclerotic plaque area and lipid deposition in the descending aorta were similar in the 2 groups (Figure IB and IC in the online-only Data Supplement), suggesting that endothelial BH4 has little effect on native atherosclerosis during this time course, but exerts striking effects on vascular remodeling after vein graft surgery, where EC injury and loss are major features.
Figure 1. Endothelial overexpression of human GTP cyclohydrolase (GCH) reduces neointimal hyperplasia and enhances repopulation and survival of endothelial cell (EC) in vein grafts.
A, When vena cava was grafted into recipients of the same genotype, vein graft wall area at 28 days after surgery was significantly lower in GCH/apolipoprotein E (apoE)-knockout (KO; n=5) mice compared with apoE-KO mice (n=8). When vena cava of apoE-KO mice was grafted into GCH/apoE-KO recipients (n=6), the mean vessel wall area was similar to that seen when GCH/apoE-KO vena cava was grafted into GCH/apoE-KO recipients. When GCH/apoE-KO donor vena cava was grafted into apoE-KO recipients (n=8), there was a less marked, but still significant, reduction in lesion area compared with apoE-KO vena cava grafted into apoE-KO. Black bar represents 100 μm. One-way ANOVA overall P<0.0001. Bonferroni multiple comparison test *P<0.01 vs apoE-KO vein grafted into apoE-KO recipient and †P<0.05 vs GCH/apoE-KO vein grafted into apoE-KO. B, Tetrahydrobiopterin (BH4) levels in harvested vein grafts were significantly higher in GCH/apoE-KO compared with apoE-KO. When apoE-KO veins were grafted into GCH/apoE-KO mice, graft BH4 levels reached levels similar to the GCH/apoE-KO vein grafts. When GCH/apoE-KO veins were grafted into apoE-KO mice, BH4 levels were significantly higher than apoE-KO vein grafts grafted into matched recipients (n=5). One-way ANOVA overall P<0.0001. Bonferroni multiple comparison test *P<0.05 vs apoE-KO. †P<0.05 vs GCH/apoE-KO vein grafted into apoE-KO recipients. C, Vein graft mRNA levels of total GCH (transgenic+endogenous) were significantly higher in GCH/apoE-KO mice compared with apoE-KO. When apoE-KO veins were grafted into GCH/apoE-KO mice, mRNA levels were similar to the GCH/apoE-KO vein grafts. One-way ANOVA overall P<0.0001. Bonferroni multiple comparison test *P<0.001 vs apoE-KO. †P<0.001 vs GCH/apoE-KO vein grafted into apoE-KO recipients (n=4). D, Vein graft endogenous mouse GCH mRNA levels were not different in GCH/apoE-KO mice compared with apoE-KO, regardless of the source of the vein (n=4). E, When apoE-KO veins were grafted into apoE-KO/LacZ recipients, the presence of β-galactosidase (β-Gal)–positive staining (blue) on the surface of vein grafts at 28 days indicated recipient-derived EC repopulation (n=5). When GCH/apoE-KO veins were grafted into GCH/apoE-KO/LacZ recipients (n=6), EC coverage was increased compared with grafting apoE-KO veins into apoE-KO/LacZ recipients, indicating enhanced recipient-derived EC repopulation in GCH animals. Investigating EC survival, no β-Gal staining in apoE-KO/LacZ veins grafted into apoE-KO recipients could be detected. However, when GCH/apoE-KO/LacZ veins were grafted into GCH/apoE-KO recipients (n=5), a small proportion of β-Gal–positive cells remained evident on the luminal surface of the vein graft at 28 days, indicating enhanced EC survival from GCH transgenic donors. No β-Gal–positive cells were visualized in apoE-KO/LacZ veins grafted into apoE-KO recipients (n=5). Arrowhead denotes β-Gal–positive cell. Unpaired t test *P=0.02 vs apoE-KO/LacZ recipient. †P=0.02 vs apoE-KO recipient.
To determine whether reduced vein graft neointimal hyperplasia was the result of GCH overexpression in the endothelium of the grafted vein or in the ECs of the recipient animal, we mismatched grafted veins and vein graft recipients between apoE-KO and GCH/apoE-KO genotypes. When veins from apoE-KO mice were grafted into GCH/apoE-KO recipients, the reduction in lesion area was similar to that observed when GCH/apoE-KO veins were grafted into GCH/apoE-KO recipients (47%; P<0.001; Figure 1A). When GCH/apoE-KO donor veins were grafted into apoE-KO recipients, there was a less marked, but still significant, reduction in lesion area compared with apoE-KO controls (P<0.01; Figure 1A), suggesting that reduced neointimal hyperplasia is conferred by repopulating or surviving vein graft ECs overexpressing GCH.
Effect of GCH Overexpression on Biopterin Levels in Vein Grafts
We confirmed that increased endothelial GCH expression resulted in increased BH4 levels in vascular tissues and improved eNOS coupling (Results in the online-only Data Supplement and Figures II and III in the online-only Data Supplement).
To determine whether vein graft phenotype was directly modulated by endothelial GCH overexpression, we measured BH4 levels specifically in vein grafts harvested 28 days postoperatively. BH4 levels in the harvested vein grafts were 12-fold higher in GCH/apoE-KO mice compared with apoE-KO (Figure 1B). A similar increase in vein graft BH4 levels was observed when apoE-KO veins were grafted into GCH/apoE-KO mice, suggesting that recipient-derived transgenic ECs determine both vein graft BH4 level and the extent of neointimal hyperplasia (Figure 1B). When GCH/apoE-KO veins were grafted into apoE-KO mice, BH4 levels in the harvested vein graft were greatly reduced compared with GCH/apoE-KO vein grafts from GCH/apoE-KO recipients. However, BH4 levels in GCH/apoE-KO veins grafted into apoE-KO mice remained significantly higher than in the vena cava from the same animals and were increased 4-fold compared with apoE-KO vein grafts (Figure 1B), suggesting that a small proportion of GCH-transgenic ECs survived in the vein graft 28 days after surgery. Indeed, this observation was consistent with the reduced neointimal hyperplasia in these vein grafts in comparison with apoE-KO controls.
In keeping with these findings, when we quantified GCH mRNA in total RNA extracted from vein grafts, total GCH mRNA levels (using identical primers for both endogenous murine and transgenic human GCH mRNA) were 10-fold higher in GCH/apoE-KO vein grafts compared with apoE-KO vein grafts (Figure 1C). When apoE-KO veins were grafted into GCH/apoE-KO recipients, total GCH mRNA levels were similar to those measured when GCH/apoE-KO veins were grafted into GCH/apoE-KO recipients, supporting the hypothesis that the vein graft endothelium is repopulated from the recipient. When GCH/apoE-KO veins were grafted into apoE-KO recipients, no difference was detectable in total GCH mRNA levels compared with apoE-KO veins grafted into apoE-KO mice. Importantly, endogenous murine GCH expression in vein grafts was similar among the 4 groups (Figure 1D), indicating that increased GCH mRNA in GCH/apoE-KO vein grafts was the result of transgene overexpression and not the result of an alteration in endogenous GCH expression.
Taken together, these results suggest that the magnitude of neointimal hyperplasia in vein grafts is determined by endothelial BH4 levels. In turn, the endothelial phenotype that determines vein graft remodeling is conferred principally by the recipient animal, but with an additional component derived from persistence of graft-derived ECs.
Accelerated Regeneration and Improved Survival of GCH/apoE-KO ECs in Vein Grafts
To directly quantify the role of endothelial BH4 in EC loss after vein grafting and subsequent EC repopulation in vivo, we constructed vein grafts in apoE-KO mice with endothelium-targeted LacZ expression in either grafted veins or recipient animals, in each case with or without endothelial GCH overexpression. ECs in freshly harvested vena cava from both GCH/apoE-KO/LacZ and apoE-KO/LacZ mice demonstrated uniform β-Gal staining, whereas no β-Gal staining was evident in either GCH/apoE-KO or apoE-KO animals (Figure IIB in the online-only Data Supplement). When apoE-KO/LacZ veins were grafted into apoE-KO recipients, there was no β-Gal staining in vein grafts harvested at 28 days, demonstrating complete loss of ECs from the donor vein (Figure 1E). However, when GCH/apoE-KO/LacZ veins were grafted into GCH/apoE-KO recipients, a significant number of β-Gal–positive ECs were evident on the luminal surface of the vein graft, indicating survival of ECs from the grafted vein (Figure 1E). To investigate endothelial repopulation, apoE-KO veins were grafted into apoE-KO/LacZ recipients. β-Gal staining of vein grafts harvested 28 days later revealed repopulation of the graft with recipient-derived ECs (Figure 1E). When GCH/apoE-KO veins were grafted into GCH/apoE-KO/LacZ recipients, repopulation with recipient-derived ECs was significantly increased compared with apoE-KO/LacZ recipients (P=0.02; Figure 1E). These results suggest that increased endothelial BH4 enhances both local EC survival within the grafted vein and systemic EC repopulation of the vein graft, leading to reduced neointima formation.
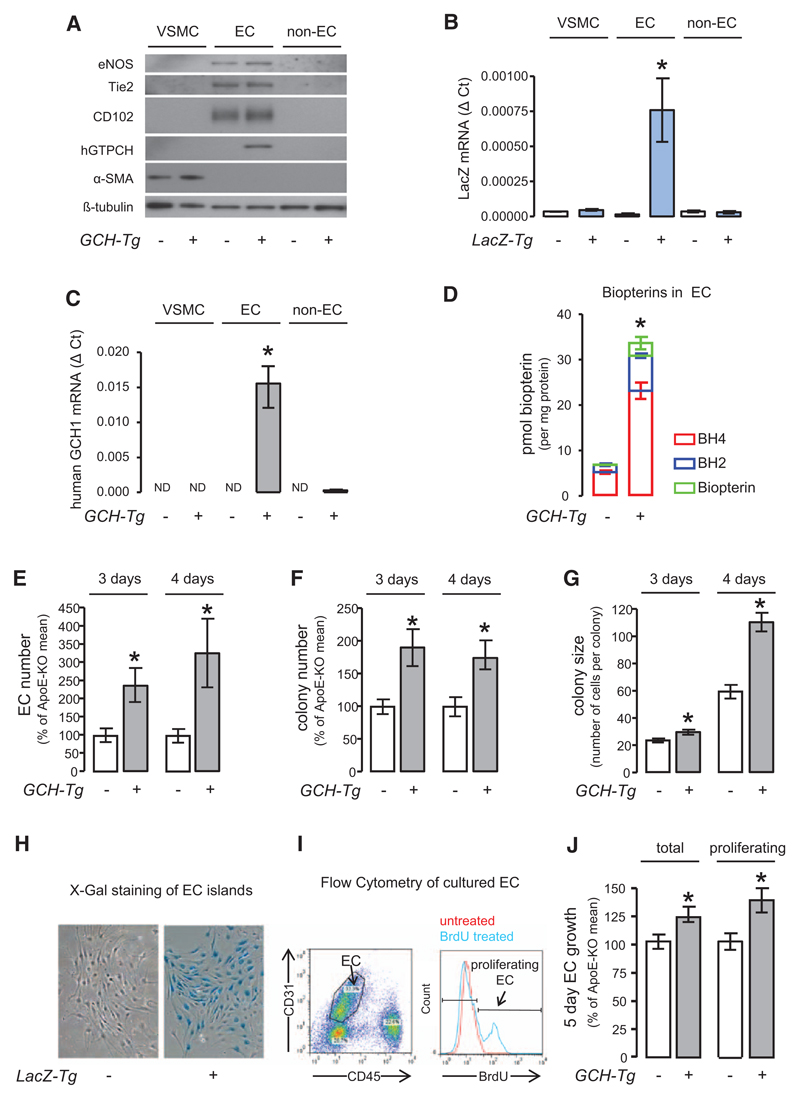
Endothelial-Specific Overexpression of Human GCH Increases Endothelial Biopterin Levels and Promotes Growth of Primary ECs
We next tested how endothelial GCH overexpression alters BH4 levels and cellular growth in ECs in vitro. Primary EC isolation from apoE-KO/LacZ and GCH/apoE-KO/LacZ mice was confirmed by the EC markers eNOS, Tie-2 and CD102 and by detection of mRNA for human GCH and LacZ (Figure 2A–2C). Furthermore, biopterin levels in freshly isolated primary ECs were increased 5-fold in GCH/apoE-KO ECs in comparison with apoE-KO (P<0.001; Figure 2D). To investigate the influence of GCH overexpression on primary EC proliferation in vitro, lung ECs were isolated from apoE-KO/LacZ and GCH/apoE-KO/LacZ mice. We excluded baseline differences in endothelial isolation, viability, and number by measuring β-Gal activity in lungs as an indicator for EC content and flow cytometry for viability and cell number (Figure IV in the online-only Data Supplement). The total number of ECs, the number of EC colonies, and average colony size were significantly increased in GCH/apoE-KO/LacZ cultures compared with apoE-KO/LacZ cultures (P<0.05; Figure 2E–2G), identified by staining with X-Gal (Figure 2H). We confirmed the increase in cell number by fluorescence-activated cell sorter analysis for CD31/CD45 and increased proliferation by incorporation of BrDU added to the culture media (Figure 2I and 2J). These results indicate that endothelial BH4 augmentation by GCH overexpression improves proliferation of ECs from apoE-KO mice, substantiating increased endothelial proliferation and thus regeneration as a mechanism of attenuated vein graft remodeling in GCH/apoE-KO mice.
Figure 2. Endothelial-specific overexpression of human GTP cyclohydrolase (GCH) increases endothelial biopterin levels and promotes in vitro growth of primary endothelial cells (ECs).
A, Western blotting showed that the EC markers endothelial nitric oxide synthase (eNOS), Tie-2, and CD102 were exclusively expressed in ECs. B, Reverse transcription polymerase chain reaction showed that the expression of human GCH mRNA was specific to GCH transgenic EC (unpaired t test with Welch’s correction *P<0.05 GCH transgenic EC [n=3] vs GCH transgenic non-EC [n=3], no human GCH1 detectable in vascular smooth muscle cell [VSMC] or apolipoprotein E [apoE]-knockout [KO] cells [n=4 each]). C, Expression of LacZ mRNA was specific to LacZ transgenic ECs (unpaired t test with Welch’s correction *P<0.05 LacZ transgenic EC [n=3] vs LacZ transgenic non-EC [n=3] and VSMC [n=4]). D, Biopterin levels in GCH/apoE-KO EC were increased by ≈5 fold compared with apoE-KO. Unpaired t test *P<0.001 GCH/apoE-KO (n=4) vs apoE-KO (n=4). Primary ECs were isolated from apoE-KO/LacZ and GCH/apoE-KO/LacZ animals and grown in tissue culture. The total number of EC (E), the number of EC colonies (F), and average colony size (G) were significantly increased in GCH/apoE-KO cultures both after 3 and 4 days in culture assessed by X-galactosidase (H). Unpaired t test with Welch’s correction *P<0.05 GCH/apoE-KO vs apoE-KO (n=5 per group for 3-day culture, n=6 per group for 4-day culture). Flow cytometry analysis of EC growth in culture. To identify proliferating EC, Bromodeoxyuridine (BrdU) was added to the culture media. I, Both total number of EC and number of proliferating EC were significantly increased in GCH/apoE-KO (J). Unpaired t test *P<0.05 GCH/apoE-KO vs apoE-KO (n=9 per group).
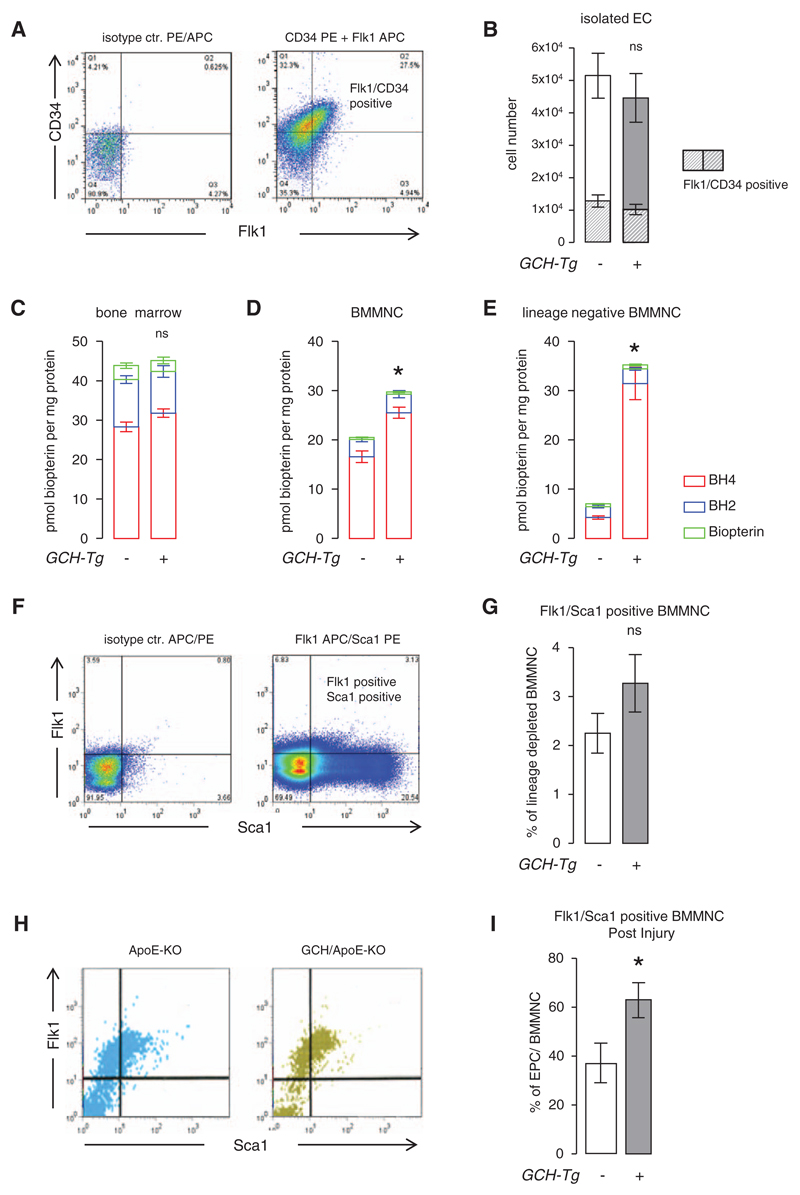
EPC Content in Primary Lung Endothelial Cells and Bone Marrow From ApoE-KO and GCH/apoE-KO Mice
Because endothelial regeneration and repair are mediated, in part, by circulating EPCs,2 we next investigated EPC levels in GCH/apoE-KO and apoE-KO mice. There was no difference between the genotypes under basal conditions (Figure 3A and 3B). Four different populations of cells within the CD31/CD45 population were identified (Figure VA in the online-only Data Supplement): CD31 high/CD45 dim, CD31 low/CD45-negative, CD31 dim/CD45-positive, and CD31-negative/CD45-negative cells. The CD31 high/CD45 dim population was identified as the population consisting of EC and EPC because these CD31+/CD34+/Flk-1+ cells (Figure VB–VE in the online-only Data Supplement) strongly expressed the EC markers eNOS, Tie-2, and CD102, as well as the human GCH1 transgene (Figure VF in the online-only Data Supplement), and formed typical EC islands in culture (Figure VG in the online-only Data Supplement).
Figure 3. Endothelial progenitor cells in apolipoprotein E (apoE)-knockout (KO) and GTP cyclohydrolase (GCH)/apoE-KO mice.
A and B, The proportion of freshly isolated endothelial cells (ECs) expressing the progenitor markers Flk1 and CD34 were determined by flow cytometry. There was no significant difference in the total number of ECs isolated between the genotypes at baseline. Unpaired t test *P>0.05 GCH/apoE-KO (n=5) vs apoE-KO (n=7). Biopterin levels in bone marrow were similar (C), whereas bone marrow–derived mononuclear cell (BMMNC; D) and lineage-depleted BMMNC (E) were increased in GCH/apoE-KO mice. Unpaired t test ***P<0.001 GCH/apoE-KO (n=5) vs apoE-KO (n=7). These results indicate that in preparations of bone marrow the difference in biopterin content because of transgenic GCH overexpression becomes more apparent the higher the expected content of endothelial progenitor cell (EPC) in the sample. F–G, There was no significant difference in the percentage of Flk1/Sca1-positive BMMNC (EPC) between apoE-KO (n=11) and GCH/apoE-KO (n=9) detectable at baseline Unpaired t test *P>0.05. However, after endothelial denudation by femoral artery wire-induced vascular injury, these numbers were significantly increased in GCH/apoE-KO mice (n=11) vs apoE-KO (n=9). Unpaired t test *P<0.05. APC/PE indicates allophycocyanin/phycoerythrin.
To investigate the influence of transgenic GCH overexpression on BH4 content in fractions of bone marrow containing increasing proportions of EPCs, biopterins were measured in whole bone marrow, bone marrow–derived MNCs (BMMNC), and lineage-depleted BMMNC, each isolated from both apoE-KO and GCH/apoE-KO mice. There was no difference in BH4 or total biopterin levels in whole bone marrow between apoE-KO and GCH/apoE-KO (Figure 3C). However, BMMNC from GCH/apoE-KO mice showed a 50% increase in BH4 levels compared with apoE-KO (Figure 3D), whereas BH4 levels in lineage-negative BMMNC from GCH/apoE-KO mice were increased 7-fold (Figure 3E). There was no significant difference in the percentage of Flk1/Sca1+ lineage-negative BMMNC between apoE-KO and GCH/apoE-KO detectable at baseline (Figure 3F and 3G). In contrast, 48 hours after wire endothelial denudation of the femoral artery, there was a significant increase in the percentage of Flk1/Sca1+ cells in GCH/apoE-KO animals (Figure 3H and 3I). These results indicate that increased Tie2-driven GCH overexpression augments BH4 synthesis in EPCs and promotes acceleration of endothelial regeneration.
Discussion
In this study, we have identified a key role for BH4 in the EC-specific impact on the remodeling response to vascular injury. Using a model of vein graft disease, incorporating crossovers between genetically modified mice overexpressing GCH specifically in ECs, we made the following key observations. First, increased endothelial BH4 synthesis resulted in markedly reduced vein graft neointimal hyperplasia at both 4 and 8 weeks after surgery. Second, endothelial GCH over-expression markedly increased BH4 and GCH mRNA levels in vascular tissues, including vein grafts, but not in plasma, excluding the potential for systemic effects of BH4. Third, experiments using mice also expressing endothelial β-Gal demonstrated that the vein graft remodeling phenotype was conferred by recipient-derived EPC/ECs that repopulated the vein graft. In transgenic GCH/apoE-KO animals, we found that EPC numbers were increased after vascular injury, reendothelialization was accelerated, and that these recipient-derived cells expressed the GCH transgene. Fourth, we found that some ECs from GCH transgenic mice survived the initial vascular injury and continued to produce increased levels of biopterins in vein grafts from GCH/apoE-KO donors. Finally, cell culture experiments revealed that the phenotype in GCH/apoE-KO vein grafts was likely because of a combination of enhanced proliferative and survival capacity of ECs and increased levels of EPCs, modulated through increased BH4. Together, these findings provide evidence that endothelial BH4 bioavailability regulates endothelial survival and regeneration after vascular injury and is an important determinant of vascular repair and remodeling.
Recent studies have identified EC repopulation of the vessel wall as an important aspect of the repair and remodeling response to vascular injury. Increasing levels of EPCs using statins,11 estrogen,12 physical exercise,13 granulocyte-macrophage colony-stimulating factor (GM-CSF),14 or erythropoietin15 accelerates re-endothelialization after vascular injury and leads to attenuation of the neointimal response. NO bioavailability seems to be a major mechanism regulating endothelialization and EPC function.4,12,13,15 However, the pathophysiological mechanisms that modulate the endothelial response to injury in vivo through regulation of eNOS remain unclear. We and others have shown previously16 that augmentation of BH4 preserves eNOS coupling in vascular disease states, reducing eNOS-derived superoxide production and promoting synthesis of NO. The present study now demonstrates the importance of BH4-dependent eNOS coupling as a critical mediator of endothelial repopulation after vascular injury. Indeed, in light of previous studies showing that statins,17 estrogen,18 physical exercise,19,20 and erythropoietin21 increase BH4 levels and that biopterins increase GM-CSF–induced hematopoietic cell proliferation and differentiation,22 BH4-dependent eNOS coupling may represent a unifying mechanism regulating re-endothelialization and vascular remodeling in vascular injury states.
Although previous studies have been performed in otherwise healthy wild-type mice, our work in apoE-KO mice demonstrates the potential importance of endothelial regeneration in the context of pre-existing vascular disease. Clinical studies have shown that levels of circulating EPCs correlate inversely with cardiovascular risk factors,23 in correlation with the finding of diminished endothelial regeneration in atherosclerotic apoE-KO mice.2 The observations that high oxidant stress impairs in vivo re-endothelialization in vascular disease states24 and that EPCs from healthy individuals express high levels of antioxidant enzymes25 raise the possibility that impaired function of antioxidant defense systems in apoE-KO mice may underlie their impaired re-endothelialization. The present study now demonstrates a mechanism whereby re-endothelialization can be enhanced in apoE-KO mice. This finding highlights a key role for BH4 and eNOS coupling in endothelial regeneration, with particular clinical relevance to atherosclerosis and other vascular disease states.
In contrast to the marked effects observed in vein graft remodeling shown here and aortic root atherosclerosis in high-fat–fed animals we reported previously,6 we found no difference in native atherosclerosis within the aortic root or descending thoracic aorta of apoE-KO and GCH/apoE-KO animals fed standard chow diet. This observation underscores the differences in the pathophysiology of native versus accelerated atherosclerosis, in which more subtle endothelial dysfunction is likely to be the mediator of native atherosclerosis development, compared with endothelial denudation in vascular injury states and severe endothelial dysfunction from high-fat feeding associated with accelerated atherosclerosis,1 where BH4-dependent eNOS regulation plays a more prominent role. Furthermore, shear stress may also play an important role in these findings. Distension of veins under arterial pressure increases vein diameter and reduces mean blood velocity, both favoring a shift in the shear-regulated production of several potent mitogens.26 Previous studies have shown that laminar shear stress dramatically increases GTP cyclohydrolase I (GTPCHI)-mediated BH4 synthesis.27 These findings may help explain our findings of 5-fold and 2-fold reductions in BH4 levels in apoE-KO vein grafts compared with aorta and vena cava, respectively (data not shown), and furthermore suggest a reason why overexpression of GTPCHI may inhibit neointimal hyperplasia and accelerated atherosclerosis more so than native atherosclerosis.
Loss of ECs is a key aspect of the response to injury in vein grafts and in other vascular injury states, such as transplant vasculopathy, angioplasty, and stenting. Previous studies of vein grafts in C57BL/6 mice have revealed that ≈70% of native ECs are lost within the first postoperative day and almost all by 3 days after surgery.2 The endothelial monolayer is subsequently repopulated by circulating and bone marrow–derived progenitors beginning as early as 24 hours after surgery, with complete re-endothelialization by 7 days. However, in the present experiments we used vein grafts in apoE-KO mice with hypercholesterolemia and atherosclerosis as a model of accelerated vein graft atherosclerosis in a setting of systemic endothelial dysfunction. Indeed, in apoE-KO mice re-endothelialization was markedly impaired, reaching only 40% coverage at 7 days after surgery, consistent with the known reduction of EPCs in these animals. We further observed that at 28 days after surgery the vein graft endothelial monolayer had still not achieved confluence. However, in mice with targeted endothelial overexpression of GCH, re-endothelialization of the vein graft significantly increased and neointimal hyperplasia significantly reduced 28 days after vein graft surgery.
In conclusion, we have shown that availability of BH4 within the endothelium critically modulates neointimal hyperplasia after vascular injury through effects on both survival of native endothelium and subsequent re-endothelialization of the injured vessel. Our study identifies BH4-dependent regulation of eNOS activity as a critical mediator of EC survival, re-endothelialization, and proliferation and a major determinant of vessel remodeling after vascular injury. Maintaining or augmenting endothelial BH4 levels is a rational therapeutic target for accelerating endothelial regeneration and enhancing vascular repair after vascular injury.
Sources of Funding
This work was funded by the British Heart Foundation, the Wellcome Trust (073213/Z/03/Z).
Footnotes
Presented at the 2012 American Heart Association meeting in Los Angeles, CA, November 3–7, 2012.
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.112.000249/-/DC1.
Disclosures
None.
Contributor Information
Ziad A. Ali, Center for Interventional Vascular Therapy, Division of Cardiology, New York Presbyterian Hospital and Columbia University, New York, NY; Cardiovascular Research Foundation, New York, NY; Department of Cardiovascular Medicine, John Radcliffe Hospital and University of Oxford, Oxford, United Kingdom.
Ruth Rinze, Department of Cardiovascular Medicine, John Radcliffe Hospital and University of Oxford, Oxford, United Kingdom.
Gillian Douglas, Department of Cardiovascular Medicine, John Radcliffe Hospital and University of Oxford, Oxford, United Kingdom.
Yanhua Hu, Cardiovascular Division, King’s College Hospital, London, United Kingdom.
Qingzhong Xiao, Cardiovascular Division, King’s College Hospital, London, United Kingdom.
Wei Qi, Center for Interventional Vascular Therapy, Division of Cardiology, New York Presbyterian Hospital and Columbia University, New York, NY.
Eileen McNeill, Department of Cardiovascular Medicine, John Radcliffe Hospital and University of Oxford, Oxford, United Kingdom.
Christina Bursill, Department of Cardiovascular Medicine, John Radcliffe Hospital and University of Oxford, Oxford, United Kingdom.
Isaac George, Center for Interventional Vascular Therapy, Division of Cardiology, New York Presbyterian Hospital and Columbia University, New York, NY.
David R. Greaves, Sir William Dunn School of Pathology, University of Oxford, Oxford, United Kingdom.
Qingbo Xu, Cardiovascular Division, King’s College Hospital, London, United Kingdom.
Keith M. Channon, Department of Cardiovascular Medicine, John Radcliffe Hospital and University of Oxford, Oxford, United Kingdom.
References
- 1.Ip JH, Fuster V, Badimon L, Badimon J, Taubman MB, Chesebro JH. Syndromes of accelerated atherosclerosis: role of vascular injury and smooth muscle cell proliferation. J Am Coll Cardiol. 1990;15:1667–1687. doi: 10.1016/0735-1097(90)92845-s. [DOI] [PubMed] [Google Scholar]
- 2.Xu Q, Zhang Z, Davison F, Hu Y. Circulating progenitor cells regenerate endothelium of vein graft atherosclerosis, which is diminished in ApoE-deficient mice. Circ Res. 2003;93:e76–e86. doi: 10.1161/01.RES.0000097864.24725.60. [DOI] [PubMed] [Google Scholar]
- 3.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 4.Landmesser U, Engberding N, Bahlmann FH, Schaefer A, Wiencke A, Heineke A, Spiekermann S, Hilfiker-Kleiner D, Templin C, Kotlarz D, Mueller M, et al. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–1939. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- 5.Alp NJ, Mussa S, Khoo J, Cai S, Guzik T, Jefferson A, Goh N, Rockett KA, Channon KM. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest. 2003;112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alp NJ, McAteer MA, Khoo J, Choudhury RP, Channon KM. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:445–450. doi: 10.1161/01.ATV.0000115637.48689.77. [DOI] [PubMed] [Google Scholar]
- 7.Schlaeger TM, Bartunkova S, Lawitts JA, Teichmann G, Risau W, Deutsch U, Sato TN. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc Natl Acad Sci U S A. 1997;94:3058–3063. doi: 10.1073/pnas.94.7.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao Q, Zeng L, Zhang Z, Margariti A, Ali ZA, Channon KM, Xu Q, Hu Y. Sca-1+ progenitors derived from embryonic stem cells differentiate into endothelial cells capable of vascular repair after arterial injury. Arterioscler Thromb Vasc Biol. 2006;26:2244–2251. doi: 10.1161/01.ATV.0000240251.50215.50. [DOI] [PubMed] [Google Scholar]
- 9.Zou Y, Dietrich H, Hu Y, Metzler B, Wick G, Xu Q. Mouse model of venous bypass graft arteriosclerosis. Am J Pathol. 1998;153:1301–1310. doi: 10.1016/S0002-9440(10)65675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietrich H, Hu Y, Zou Y, Huemer U, Metzler B, Li C, Mayr M, Xu Q. Rapid development of vein graft atheroma in ApoE-deficient mice. Am J Pathol. 2000;157:659–669. doi: 10.1016/S0002-9440(10)64576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werner N, Priller J, Laufs U, Endres M, Böhm M, Dirnagl U, Nickenig G. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 2002;22:1567–1572. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- 12.Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, Laufs K, Ghaeni L, Milosevic M, Böhm M, Nickenig G. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation. 2003;107:3059–3065. doi: 10.1161/01.CIR.0000077911.81151.30. [DOI] [PubMed] [Google Scholar]
- 13.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jürgens K, Miche E, Böhm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 14.Yoshioka T, Takahashi M, Shiba Y, Suzuki C, Morimoto H, Izawa A, Ise H, Ikeda U. Granulocyte colony-stimulating factor (G-CSF) accelerates reendothelialization and reduces neointimal formation after vascular injury in mice. Cardiovasc Res. 2006;70:61–69. doi: 10.1016/j.cardiores.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Urao N, Okigaki M, Yamada H, Aadachi Y, Matsuno K, Matsui A, Matsunaga S, Tateishi K, Nomura T, Takahashi T, Tatsumi T, et al. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res. 2006;98:1405–1413. doi: 10.1161/01.RES.0000224117.59417.f3. [DOI] [PubMed] [Google Scholar]
- 16.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- 17.Hattori Y, Nakanishi N, Akimoto K, Yoshida M, Kasai K. HMG-CoA reductase inhibitor increases GTP cyclohydrolase I mRNA and tetrahydrobiopterin in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:176–182. doi: 10.1161/01.atv.0000054659.72231.a1. [DOI] [PubMed] [Google Scholar]
- 18.Serova LI, Filipenko M, Schilt N, Veerasirikul M, Sabban EL. Estrogen-triggered activation of GTP cyclohydrolase 1 gene expression: role of estrogen receptor subtypes and interaction with cyclic AMP. Neuroscience. 2006;140:1253–1263. doi: 10.1016/j.neuroscience.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568(pt 3):1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizutani M, Hashimoto R, Ohta T, Nakazawa K, Nagatsu T. The effect of exercise on plasma biopterin levels. Neuropsychobiology. 1994;29:53–56. doi: 10.1159/000119063. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka J, Koshimura K, Sohmiya M, Murakami Y, Kato Y. Involvement of tetrahydrobiopterin in trophic effect of erythropoietin on PC12 cells. Biochem Biophys Res Commun. 2001;289:358–362. doi: 10.1006/bbrc.2001.6002. [DOI] [PubMed] [Google Scholar]
- 22.Aizawa S, Hiramoto M, Araki S, Negishi S, Kimura Y, Hoshi H, Kojima S, Wakasugi K. Stimulatory effects of neopterin on hematopoiesis in vitro are mediated by activation of stromal cell function. Hematol Oncol. 1998;16:57–67. doi: 10.1002/(sici)1099-1069(199806)16:2<57::aid-hon623>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 24.Sorrentino SA, Bahlmann FH, Besler C, Müller M, Schulz S, Kirchhoff N, Doerries C, Horváth T, Limbourg A, Limbourg F, Fliser D, et al. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus. Circulation. 2007;116:163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- 25.Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- 26.Cox JL, Chiasson DA, Gotlieb AI. Stranger in a strange land: the pathogenesis of saphenous vein graft stenosis with emphasis on structural and functional differences between veins and arteries. Prog Cardiovasc Dis. 1991;34:45–68. doi: 10.1016/0033-0620(91)90019-i. [DOI] [PubMed] [Google Scholar]
- 27.Widder JD, Chen W, Li L, Dikalov S, Thöny B, Hatakeyama K, Harrison DG. Regulation of tetrahydrobiopterin biosynthesis by shear stress. Circ Res. 2007;101:830–838. doi: 10.1161/CIRCRESAHA.107.153809. [DOI] [PubMed] [Google Scholar]