Abstract
Manganese is essential to life, and humans typically absorb sufficient quantities of this element from a normal healthy diet; however, chronic, elevated ingestion or inhalation of manganese can be neurotoxic, potentially leading to manganism. Although imaging of large amounts of accumulated Mn(II) is possible by MRI, quantitative measurement of the biodistribution of manganese, particularly at the trace level, can be challenging. In this study, we produced the positron-emitting radionuclide 52Mn (t1/2 = 5.6 d) by proton bombardment (Ep<15 MeV) of chromium metal, followed by solid-phase isolation by cation-exchange chromatography. An aqueous solution of [52Mn]MnCl2 was nebulized into a closed chamber with openings through which mice inhaled the aerosol, and a separate cohort of mice received intravenous (IV) injections of [52Mn]MnCl2. Ex vivo biodistribution was performed at 1 h and 1 d post-injection/inhalation (p.i.). In both trials, we observed uptake in lungs and thyroid at 1 d p.i. Manganese is known to cross the blood-brain barrier, as confirmed in our studies following IV injection (0.86%ID/g, 1 d p.i.) and following inhalation of aerosol, (0.31%ID/g, 1 d p.i.). Uptake in salivary gland and pancreas were observed at 1 d p.i. (0.5 and 0.8%ID/g), but to a much greater degree from IV injection (6.8 and 10%ID/g). In a separate study, mice received IV injection of an imaging dose of [52Mn]MnCl2, followed by in vivo imaging by positron emission tomography (PET) and ex vivo biodistribution. The results from this study supported many of the results from the biodistribution-only studies. In this work, we have confirmed results in the literature and contributed new results for the biodistribution of inhaled radiomanganese for several organs. Our results could serve as supporting information for environmental and occupational regulations, for designing PET studies utilizing 52Mn, and/or for predicting the biodistribution of manganese-based MR contrast agents.
Introduction
Manganese is a micronutrient that is essential for human life in only trace amounts [1, 2], but deficiency is rare [3]. Existing primarily in the Mn(IV) oxidation state in the earth’s crust [4], manganese is the third most abundant transition metal in the crust [4] and is taken up by plants, where it is utilized in green leaves as an oxidizer in photosynthesis [4]. Manganese(II) is by far the most electrochemically stable oxidation state for manganese [4] and the state that is predominantly used as an enzymatic cofactor. Manganese in the blood is found as Mn(III) bound to binding sites for Fe(III) on transferrin [5, 6] or as Mn(II) bound to other serum proteins [7], but the vast majority of manganese leaves the blood quickly and is accumulated in various organs or excreted [8]. The necessity of manganese as a nutrient is sometimes overshadowed by its neurotoxic effects that can result in manganism [2], a condition that was first described in 1837 [9]. In mangansim, early-stage patients often present with psychiatric symptoms [10, 11] that are sometimes referred to as “manganese madness” [12]. Later-stage patients develop symptoms of parkinsonism, including intellectual impairment, tremors, and rigidity similar to idiopathic Parkinson’s disease [9, 12–14], with a few key differences [12].
In addition to studying the neurotoxicity of manganese, an additional motivation for understanding the biodistribution of free manganese is that Mn(II) can be utilized in contrast agents for manganese-enhanced magnetic resonance imaging (MEMRI), which was studied early in the history of MRI by P. Lauterbur and colleagues [15] and later achieved in vivo [16]. Manganese(II) is “high-spin” from its five unpaired valence electrons, which shorten the spin-lattice time constant (T1) for nearby 1H nuclei, resulting in brighter signal (when using a T1-weighted MR pulse sequence) [8, 17–19]. In fact, T1-weighted MRI may be used as an indicator of manganese-induced parkinsonism [20, 21]. Additionally, chelated Mn(II) could serve as an alternative to Gd(III), which is currently the most commonly used cation core in T1-shortening contrast agents for MRI, since free Gd(III) is excreted through the kidneys, but free Mn(II) is excreted almost exclusively in feces, primarily from bile from the liver [22–25].
Thus far, the only manganese-based MR contrast agent to reach the market in the United States was Teslascan® (mangafodipir, manganese(II)-dipyridoxyl diphosphate, Mn-DPDP) (Amersham plc, Amersham, Buckinghamshire, United Kingdom), a chelated form of Mn(II) that reduces cardiac toxicity, by slowing the availability of free Mn(II) [17, 26–28] due to dissociation [29]. Mn-DPDP was officially indicated for detecting liver metastases of colorectal cancer [26, 27], but it no longer on the market in the United States. Various other chelated forms of Mn(II) and Mn(III) [30] are being developed, numerous kinds of Mn-doped inorganic and organic nanoparticles are in the in vitro and pre-clinical stages [19], and oral administration of liquids containing Mn(II) has even been tested in humans [31, 32]. However, since MRI is not inherently as quantitative as PET, it is valuable to develop surrogates that are radiolabeled with a positron-emitting radioisotope of manganese [27, 28, 33, 34] for quantitative confirmation of their biodistribution.
Although imaging of large amounts of accumulated Mn(II) is possible by T1-weighted MRI, true quantification of the biodistribution of non-radioactive manganese in vivo is difficult. However, pre-clinical imaging and ex vivo biodistribution using tracer amounts of radiomanganese can provide a route to quantify the amount of manganese as a percentage of administered dose in animal models. Past studies have examined the biodistribution of manganese in various forms administered by various routes, mostly in rodents, and have generally found that manganese is distributed to many tissues throughout the body. Several of these studies have used the radiotracers 52Mn (t1/2 = 5.6 d [35]), and 54Mn (t1/2 = 312 d [35]), or by administering stable manganese (55Mn: 100% natural abundance [35]) followed by various techniques for quantifying non-radioactive metals in the tissues [17, 36–38]. More recently, researchers at the University of Wisconsin have published results for ex vivo biodistribution of IV 52Mn(II) in rodents: Graves, et al. [39] have investigated biodistribution in tumor-bearing mice at 96 h post-injection (p.i.), as well as by in vivo PET at timepoints 4–96 h p.i. Additionally, Brunnquell, et al. [40] have investigated biodistribution in rats at 4 and 48 h p.i.
The half-life of 52Mn is long enough for in vivo imaging at timepoints as long as days or even weeks p.i., and 52Mn emits positrons at a sufficient branching ratio (Iβ+ = 29.6% [35]) for in vivo medical imaging by positron emission tomography (PET). Manganese-52 provides the additional benefit of low-energy positron emission (Eβ+ = 242 keV [35]) (resulting in better spatial resolution in PET). Manganese-52 can be produced efficiently by the 52Cr(p,n) or natCr(p,x) reactions (52Cr: 83.8% natural abundance) by bombarding target material with low-energy protons (6<Ep<20 MeV [41, 42]). Thus, 52Mn can be produced at medical centers using protons from a low-energy cyclotron without expensive isotopically enriched target material. So far, 52Mn has been used in several ex vivo and in vivo studies, including the biodistribution of Mn(II) [43], cell tracking [44], antibody imaging [39], and as a positron-emitting surrogate of a MEMRI contrast agent [34].
In this work, we utilized the radioisotope 52Mn to study the biodistribution of free Mn(II) in sixteen different tissues in mice following intravenous injection or inhalation in saline solution.
Materials and methods
Materials
Copper sheet (0.762 mm thick, 99.9% purity) was purchased from ESPI Metals (Ashland, Oregon, United States), and natural abundance chromium was electroplated by Four Star Finishing (Saint Louis, Missouri, United States). Nitrogen (N2) gas was purchased from Cee Kay Supply (Saint Louis), and all other chemicals were purchased from Sigma-Aldrich (Saint Louis), unless otherwise indicated.
Production of 52Mn
Manganese-52 was produced from the natCr(p,x) reaction using the CS-15 cyclotron at Washington University School of Medicine in Saint Louis as previously reported [41, 42]). We developed a method for fabricating batches of thin chromium foils that would fit into our target holders [41], similar to the one used by Tanaka and Furukawa [45]. Non-enriched chromium was electroplated onto copper metal sheet in an industrial “hard chrome” electroplating bath (Four Star Finishing, Saint Louis, United States). Following electroplating, the copper backing was removed by repeatedly digesting it in diluted nitric acid in a large glass pan, eventually leaving the electrodeposited layer of chromium as a batch of thin metal foils [41, 45]. In previously published results [41], the only contaminants >100 ppm (within uncertainty) were Sn (1195 ppm) and Se (134 ppm) in the final product. These elemental impurities were discovered by ICP-MS in a dissolved chromium foil that was never irradiated but was produced in a similar manner to the foils used in this work. For each production of 52Mn, one or more foils of this chromium metal was placed in a target holder and bombarded with protons from the CS-15 cyclotron (The Cyclotron Corporation, Berkeley, California, United States) [41]. Gamma-ray spectroscopy results from a non-dissolved chromium foil from a previous bombardment showed a radionuclidic purity of >99.5% (activity%) 52Mn (<0.5% 54Mn).
Several separation procedures have been published for 52Mn from a chromium metal target [39, 43, 46–52]. Following a method published by Buchholz, et al. in 2013 [50], cation-exchange chromatography was used to separate 52Mn from the chromium metal target after proton bombardment. The irradiated chromium metal was dissolved in diluted hydrochloric acid (for the biodistribution-only study: 5.5 mL of 1:10 (v/v) c. HCl:H2O). The solution was repeatedly evaporated and resuspended in sulfuric acid to replace the chloride anions with sulfate, then diluted to ~0.1 M sulfuric acid. Next, the solution was transferred to a simple gravity-flow column (inner diameter = 1.0 cm) consisting of AG 50W-X8 cation-exchange resin (Bio-Rad, Hercules, California, United States) that was pre-conditioned in 0.1 M sulfuric acid. The majority of the Cr(III) passed through the column and was further eluted in 0.1 M sulfuric acid, while 52Mn(II) was immobilized on the column. Of the 52Mn that was measured in the top chromium foil, >90.9% was eluted in 6 M HCl. The resulting solution was brought to dryness using heat and/or (N2) gas, and redissolved in saline. If necessary, solutions of hydrochloric acid, sodium hydroxide, and/or ammonium hydroxide were added to adjust the pH of the resuspended saline product to pH 4.0–5.0. When manganese is dissolved in HCl, it is expected to form manganese(II) chloride, which is soluble in water; manganese(IV) is much less soluble in water, and we did not observe any insolubility of our radiomanganese. For the inhalation study, the final solution that was placed inside the nebulizer was between 13.1–13.3 MBq (355–360 μCi) in 600 μL. The isotonicity of both the IV and inhaled doses was not evaluated in the current study, so it is possible that the concentrations of metal cation contaminants were great enough to influence the pharmacokinetics of the 52Mn(II). Metal impurities in our 52Mn will be addressed in future studies.
Administration of 52Mn
All animals were purchased from Charles River Laboratories (Wilmington, Massachusetts, United States) and allowed to eat and drink ad libitum. All experiments involving animal subjects were performed under a protocol that was approved by the Animal Studies Committee of Washington University in St. Louis. Male CD-1 mice were used for biodistribution studies for IV injection and aerosol inhalation.
For the cohorts that received IV injection, mice (n = 4) were anesthetized by inhalation of isoflurane in a chamber before each mouse was removed and injected in the tail vein with ~100 μL volume, ~0.2–0.4 MBq (~5–10 μCi) of 52Mn. One extra syringe was prepared as a standard for later determining the count rate per volume of injectate using the gamma counter (see below). Each syringe, including the standard syringe, was weighed pre- and post-injection to determine the mass of injectate that was actually expelled.
In the inhalation studies, doses of 52Mn salt solutions were administered to mice as previously reported by our research group [53, 54] for other radiolabeled compounds. Solutions were loaded into a Small Volumetric Mean Diameter (VMD) Nebulizer Unit (Aeroneb Lab, Aerogen, Dangan, Galway, Ireland) with the following specifications: >0.1 mL·min-1 flow rate, 4.0–6.0 μm VMD, <0.2 mL residual volume. The exit port of the nebulizer was inserted into a clear plastic chamber (20.3×20.3×11.4 cm l×w×h). Four mice for each timepoint were inserted individually, without any anesthesia, into four tubes with a plastic plunger to gently push the body to the front of the tube and snout through a small opening at the opposite end of the tube. The 52Mn solution was pipetted into the liquid reservoir of the nebulizer and then the nebulizer was powered on, producing an aerosol that filled the chamber. The four mice were allowed to inhale the aerosol for ~4–7 min., then the tubes were detached, and the mice were removed. One of the mice from each inhalation group of four mice was euthanized immediately following removal from its tube to serve as the standard “dose mouse” for the dose received by that group, leaving three mice alive from each administration by inhalation.
Ex vivo biodistribution
At each timepoint post-administration (by injection or inhalation), animals were euthanized, dissected, and then each tissue was weighed and analyzed for radioactivity using a sodium iodide gamma-ray counter (Beckman 8000, Beckman Instruments, Irvine, California, United States). For the injection study, the injectate from the standard syringe was diluted, and one unique aliquot of this dilution was assayed after the tissues from each animal. For the inhalation study, the whole carcass of the “dose mouse” from each inhalation group was assayed once by the gamma counter. For the samples from each administration route, a single background measurement was performed, and the count rate (counts per minute, CPM) from each sample of tissue (or of diluted standard) was corrected by background subtraction and by decay correction (neglecting decay during gamma counting). The corrected CPM from the diluted standard was used to estimate the (corrected) CPM from the same mass of undiluted standard. The corrected CPM from each tissue sample was normalized both to the mass of the tissue sample (in grams, g) and to the estimated CPM from the mass of undiluted injectate that was actually delivered to the corresponding animal (injected dose, ID). Thus, the relative concentration of 52Mn in each tissue sample was calculated as %ID/g.
Statistical and uncertainty analysis
For each tissue and timepoint, results were averaged across animals—n = 4 in the injection study and n = 3 in the inhalation study—and statistical analysis was performed. The uncertainty in the final result for each tissue reflects only sample standard deviation across animals for that tissue. Statistical significance between the biodistribution at 1 d after injection or inhalation was determined using Welch’s t-test, which is a correction to Student’s t-test that is applied when performing a hypothesis test on sample means from two unpaired samples of different size and variance, as was the case in this comparison. This test was administered using the TTEST function in Microsoft Excel for Mac (various versions, Microsoft, Redmond, Washington, United States) for a two-tailed t-test between two samples of heteroscedastic (unequal variance) data.
Results
For the biodistribution-only study, bombardment of natCr metal foils with ~13.4 MeV protons for a total of 27 μA·h (12 μA), which produced 41 MBq (1.1 mCi) (decay-corrected to end-of-bombardment), which was 69.6% of theoretical yield based on our published cross-section results [41]. Fig 1 and Table 1 show the results from ex vivo biodistribution of 52Mn in saline administered via intravenous injection or by inhalation. Manganese-52 was cleared rapidly from the blood, as demonstrated by its rapid decrease from 1 h to 1 d p.i., and the activity in the gastrointestinal tract—stomach and intestines—also decreased rapidly between these timepoints. At 1 h p.i., the highest activity was found, in decreasing order, in the liver, kidney, lung, heart, pancreas, spleen, and salivary glands (Table 1); however, by 1 d p.i., the concentration of 52Mn in all of these organs of high initial uptake had decreased substantially, except for the salivary glands, pancreas, and kidney, as shown in Fig 1. Among the other tissues, which had lower uptake at 1 h p.i., 52Mn was retained to various degrees in the brain, bone, and thyroid.
Fig 1. Plot showing results from ex vivo biodistribution of saline solutions containing 52Mn administered via intravenous injection or inhalation.
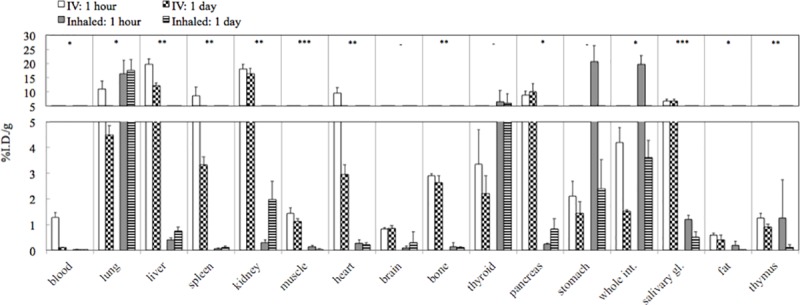
The timepoints shown represent the time after administration of the dose. Results for the p-values from Student’s t-test with Welch’s correction at 1 d p.i. only: *: p<5%; **: p<0.1%; ***: p<0.01%; -: p≥5%. Sample sizes for each timepoint were n = 4 mice for injection and n = 3 for inhalation. Uncertainties: one absolute standard deviation in units of %ID/g. Error bars were only drawn in the positive direction for visual clarity. The plotted data is presented in numerical form in Table 1.
Table 1. Results from ex vivo biodistribution of saline solutions containing 52Mn administered via intravenous injection or inhalation.
| Uptake of 52Mn (%ID/g) | t-test (p) | ||||
|---|---|---|---|---|---|
| Intravenous | Inhalation | ||||
| 1 h | 1 d | 1 h | 1 d | 1 d | |
| Blood | 1.3 ± 0.2 | 0.10±0.02 | 0.019±0.008 | 0.007±0.003 | 0.0011 |
| Lung | 11 ± 3 | 4.5±0.4 | 16±5 | 18±4 | 0.026 |
| Liver | 20 ± 2 | 12±1 | 0.39±0.09 | 0.7±0.2 | 0.00013 |
| Spleen | 9 ± 3 | 3.3±0.3 | 0.07±0.03 | 0.12±0.05 | 0.00025 |
| Kidney | 18 ± 2 | 17±2 | 0.31±0.09 | 2.0±0.7 | 0.00012 |
| Muscle | 1.4 ± 0.2 | 1.13±0.09 | 0.14±0.05 | 0.04±0.03 | 0.000028 |
| Heart | 10 ± 2 | 2.9±0.4 | 0.3±0.1 | 0.23±0.08 | 0.020 |
| Brain | 0.8±0.1 | 0.9±0.1 | 0.09±0.07 | 0.3±0.4 | 0.00034 |
| Bone | 2.90±0.08 | 2.6±0.3 | 0.1±0.2 | 0.11±0.03 | 0.14 |
| Thyroid | 3±1 | 2.2±0.7 | 6±4 | 6±3 | 0.00026 |
| Pancreas | 9±1 | 10±3 | 0.25±0.02 | 0.8±0.4 | 0.17 |
| Stomach | 2.1±0.6 | 1.4±0.5 | 21±6 | 2±1 | 0.0073 |
| Whole Intestine | 4.2±0.6 | 1.51±0.06 | 20±3 | 3.6±0.7 | 0.00029 |
| Salivary Gland | 6.7±0.8 | 6.8±0.6 | 1.2±0.2 | 0.5±0.2 | 0.29 |
| Fat | 0.58±0.09 | 0.4±0.2 | 0.2±0.2 | 0.004±0.001 | 0.031 |
| Thymus | 1.3±0.2 | 0.9±0.1 | 1±1 | 0.1±0.1 | 0.000074 |
The timepoints shown represent time after administration of the dose. Sample sizes for each timepoint were n = 4 mice for injection and n = 3 for inhalation. Uncertainties: one absolute standard deviation in units of %ID/g (i.e., percentage does not imply relative sample standard deviation). All values for uncertainty were rounded to one significant digit, and the mean value was rounded to a matching number of decimal places. Results for the p-values from Student’s t-test with Welch’s correction are shown rounded (up or down) to two significant digits. The numerical results in this table have been plotted in Fig 1.
Comparing the biodistribution of 52Mn(II) at 1 d after IV injection or inhalation, we considered two sample means to be statistically significant if the p-value from the t-test was <0.05. Applying the t-test and this cutoff value, nearly all tissues that we measured showed a statistically significant difference, with the exception of brain, thyroid, and stomach, all of which demonstrated large relative sample standard deviations (%RSD). Salivary glands and muscles demonstrated especially strong statistical significance (p<0.0001). S1 Fig (Supplementary Information) shows co-registered PET/CT images of two mice (species: C57-Black-6) from a separate but similar study in which larger doses of [52Mn]MnCl2 were administered for in vivo PET imaging. These images demonstrate a distribution of PET signal in many of the same tissues with high uptake in the current ex vivo biodistribution results, and these images suggest the potential utility of 52Mn for further quantitative PET/CT studies.
Discussion
For the comparison of IV injection to inhalation of 52Mn in mice, the concentration of 52Mn in most tissues at both 1 h and 1 d timepoints is higher than in the inhalation results. The activity in the gastrointestinal tract was much greater in the inhalation results, suggesting that some of the dose may have been swallowed directly from the aerosol or inhaled, cleared by mucociliary clearance, and then swallowed. Also in our studies, we observed uptake in the pancreas and brain, while 52Mn that entered the stomach or intestines did not appear to be retained, although the one hour amounts were much higher in the inhalation groups likely due to putative swallowing of some of the dose. Retention of 52Mn in the brain, thyroid, and thymus was observed in results from both injection and inhalation. We also found significant uptake of 52Mn at 1 d in the bone (2.6%ID/g) resulting from injection, but not from inhalation.
For both injection and inhalation, 52Mn was cleared rapidly from the blood, and the injection data showed 52Mn in the liver and kidney in <1 h. The inhalation data also suggest accumulation in the liver and kidneys, but this accumulation took much longer than one hour. Although manganese appears to accumulate in both liver and kidneys, results from the literature [22–25] suggest that very little manganese is excreted in urine. Instead, the vast majority of manganese is excreted in feces, primarily due to release of manganese from the liver into the enterohepatic circulation. In other studies, animals that were administered manganese or radiomanganese intravenously [17, 22, 55–58], high uptake was generally observed in the kidney, pancreas, liver, adrenal glands, and intestine, as well as comparatively small amounts in the brain. For intratracheal instillation, Heilig, et al. [59] and Brain, et al. [60] reported that [54Mn]MnCl2 was retained in the lungs to large extent, but was also found in significant amounts in the intestines, liver, kidneys, and in small amounts in the brain. These intratracheal instillation studies also showed that Mn can enter the bloodstream from the lungs, possibly by ion channels, but not by the divalent metal transporter-1 (DMT-1) in that particular tissue [59, 60]. These general trends in manganese distribution agree with studies that focused more narrowly on the in vitro or in vivo uptake of manganese by cells or tissues that included liver [25, 61], kidney [61], pancreas [61], intestines [60, 62], salivary glands [17, 63], myocardium [58], and bone [64].
Owing to its neurotoxic effects, particular interest has been paid to uptake of manganese in the brain by various routes. Kanayama, et al. [65] administered to mice by eight different routes a solution containing sixteen different radiotracers, mostly transition metals, and, for most administration routes, 54Mn(II) had higher brain uptake than most of the other tracers. In several other studies, rodents received radiomanganese by carotid injection or continuous in situ brain perfusion brain. These studies suggest that manganese enters the brain by more than one mechanism, including carrier-mediated uptake of Mn-citrate [66], by store-operated calcium channels as Mn(II) [67], by transferrin-receptor mediated endocytosis as Mn-transferrin [68, 69], or by other unspecified mechanism(s) that are faster than simple diffusion [69, 70]. In a comparison of 54Mn species administered by in situ brain perfusion, Mn-citrate was transported across the blood-brain barrier faster than either free Mn(II) or Mn-transferrin, suggesting that Mn-citrate might be an important route for manganese uptake in the brain [66]. Furthermore, it is likely that manganese accumulates in the brain because it effluxes at a rate that is consistent with simple diffusion [71, 72].
Since certain inhaled substances can enter the brain directly from the olfactory bulb—without entering the bloodstream—there have been a considerable number of studies examining the possibility of manganese entering the brain by such mechanisms. Characterization of this mechanism(s) would be particularly relevant to the problem of inhaled manganese in metal workers. Animal studies using radiomanganese [73, 74] or MEMRI [75, 76], suggested that Mn(II) can be transported from the olfactory bulb and into olfactory neurons, transported through those neurons, across synaptic junctions, into secondary olfactory neurons, and then into the diencephalon and cerebrum, and (in rats) into the spinal cord. Thompson, et al. [77] tested uptake in rats with defective divalent metal transporter-1 (DMT-1) proteins and confirmed that this transporter is involved in manganese uptake in the rat olfactory bulb based on higher uptake in healthy control rats. In rats, ninety minute nose-only inhalation of aerosolized [54Mn]MnCl2 [78] or [54Mn]MnHPO4 [79] solution leads to uptake in the olfactory bulb and olfactory tubercle. In both studies, they observed uptake in lungs, liver, kidney, and pancreas, and—at much lower amounts—in the striatum of the brain. In a similar study, Lewis, et al. [38] exposed mice and rats to nebulized, non-radioactive MnCl2 for 10 nose-only doses of 6 hours each. Sample analysis by proton induced X-ray emission (PIXE) revealed elevated manganese in trigeminal ganglia, suggesting this nerve as a possible pathway for brain entry of manganese. Along with these published examples of brain uptake of Mn cations, the present study has shown uptake of 52Mn(II) in the mouse brain following administration by IV injection or inhalation or aerosol. The magnitude of uptake in the brain that we observed was low compared to some other tissues, with uptake being greater following IV injection than from inhalation. However, the presence of an exogenous substance in the brain in any amount demonstrates an ability to cross the blood-brain barrier, which is an important result in itself.
In our studies, relatively high uptake was observed in the lung and thyroid following either route of administration. Although several papers confirmed thyroid uptake of manganese from different administration routes in animal models [80–83], the biology of uptake of manganese in the thyroid does not seem clear [83]. Interestingly, electron spin resonance (ESR) has shown that only a small fraction of the manganese naturally found in the rat thyroid is in the 2+ oxidation state [37], perhaps suggesting that it is bound to serum protein(s) as Mn(III) and not present as a free Mn(II). Additionally, following subcutaneous injection of Mn(II)Cl2, in guinea pigs [81], manganese rapidly accumulated in thyroid by 1 d p.i., but then 70% of the manganese in the thyroid at 1 d had cleared by 2 d p.i.; and then 85%, by 96 hours p.i. Inhaled manganese uptake in salivary glands was in agreement with observations in welders exposed to manganese [84]. Interestingly, our results showed that injection of [52Mn]MnCl2 exhibited higher and faster uptake in salivary gland than in the inhalation study. Our results demonstrate uptake of 52Mn in the brain, thyroid, and pancreas in all trials. Interestingly, uptake from IV administration was higher in brain and pancreas, but lower in thyroid, compared to inhalation results. Importantly, Graves, et al. [85] have shown that anesthesia by isoflurane can significantly decrease uptake of Mn(II) in the pancreas in fasted mice. This study concluded that isoflurane initiates a sequence in pancreatic β-cells that reduces opening of voltage-dependent calcium channels (VDCCs)—the channels through which Ca(II), Mn(II), and potentially other divalent cations can enter the pancreatic beta cells. In one part of this work, 52Mn(II) in aqueous sodium acetate was administered IV with and without anesthesia by isoflurane, and the uptake in the pancreas at 1 h p.i. under anesthesia was similar to our result (in %ID/g). However, the uptake in the pancreas without anesthesia was ~3x greater than with anesthesia. Since our study only used anesthesia during IV administration of 52Mn(II), and not during inhalation, we expect that in the absence of anesthesia in both routes of administration the difference would be even greater in uptake in the pancreas between IV injection and inhalation.
Along with recently renewed interest in manganese for signal in both MRI and PET, Brunnquell, et al. [40] have published results for brain uptake of manganese in female rats following tail vein infusion at 2 mL/h of non-carrier-added 52Mn(II) in radiotracer concentrations. In addition to monitoring brain uptake of 52Mn(II), this study included results for biodistribution from several tissues at 4 and 48 h p.i. Compared to Brunnquell, et al., our biodistribution results were roughly 10x greater for many tissues (in %ID/g), likely due to a roughly 10x lower body mass of the mice in our study compared to rats, along with different timepoints and gender. However, the relative changes from 4 to 48 h p.i. in this study was similar to our results at 1 h and 1 d, including brain, spleen, liver, intestine, heart, lung, and muscle, while results for blood, pancreas, thymus, kidney, and bone demonstrated results that were less similar to our results, based on tissue concentrations relative to other tissues and/or relative changes from 4 to 48 h. Manganese-52 might someday be used as a PET agent for niche clinical research applications, but our biodistribution results in mice can be used, in conjunction with other published results, to inform regulations for safe levels of environmental and occupational exposure to manganese. The primary objective of this work was to contribute to basic research with regards to the in vivo behavior of manganese cations, which are increasingly being studied preclinically for PET agents and MRI contrast agents—with only the possibility for future translation to the clinic.
Conclusion
In this work, we have produced 52Mn via the natCr(p,x) reaction, chemically isolated this radioisotope of manganese from the chromium metal target material, and reported new data for the biodistribution of manganese in mice following administration by IV injection or inhalation. Manganese-52 was produced by cyclotron bombardment with low-energy protons on chromium metal, and subsequent chemical separation of 52Mn(II) was performed by cation-exchange chromatography and re-dissolved in saline solution for ex vivo biodistribution studies in mice. The doses were administered by IV injection or inhalation, always resulting in uptake of 52Mn in the brain, thyroid, and pancreas. Interestingly, uptake from IV administration was higher in brain and pancreas, but lower in thyroid, compared to inhalation results. While 52Mn might find use as a PET agent in preclinical studies, our biodistribution results in mice may be used to inform regulations for safe levels of environmental and occupational exposure to Mn(II) and the predicted biodistribution of Mn(II) following exposure by IV injection or inhalation.
Disclaimer
“This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or limited, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.”
Supporting information
At 1 h p.i., 52Mn was observed in the digestive tract, kidneys, and likely the pancreas. At 3 d p.i., 52Mn had cleared from the digestive tract, while it is retained in the kidneys, as well as liver, pancreas, and thyroid gland. Manganese-52 was eluted from the cation-exchange column in 0.067 M ammonium oxalate solution. The ammonium oxalate product was heated to dryness, and then the heat was increased to burn away the ammonium oxalate. Manganese-52 was resuspended by adding water followed by a drop of 6 M hydrochloric acid, then heated to dryness. Evaporation and resuspension in water was repeated and then the 52Mn was finally resuspended in water, resulting in a solution with pH of ~6.5. Mice (n = 2; C57-Black-6; male) were anesthetized by isoflurane (1–2% induction), injected in the tail vein with ~41 μCi of 52Mn in 50 μL total volume, and imaged simultaneously side-by-side at 1 h and 3 d p.i. At the imaging timepoints, anatomic images were obtained by non-contrast CT using an Inveon small animal PET/CT scanner (Siemens Preclinical Solutions, Knoxville, Tennessee, United States), and PET data were acquired using either the Inveon PET/CT scanner or a microPET Focus 220 scanner (Siemens Preclinical Solutions). Static PET data were acquired for 30 minutes at the 1-hour timepoint and for 1 hour at the 3-day timepoint. Attenuation maps for each subject were obtained from either the CT scan in the Inveon scanner or by a transmission scan on the Focus 220 scanner. Images were analyzed using Inveon Research Workplace software (Siemens Preclinical Solutions).
(TIF)
This file is a workbook made using Microsoft Excel for Mac and contains multiple sheets.
(XLSX)
This file is a workbook made using Microsoft Excel for Mac and contains multiple sheets.
(XLSX)
This file is a workbook made using Microsoft Excel for Mac and contains multiple sheets. The workbook includes results from S1 File and S2 File, comparison of these results by plotting, and hypothesis testing of these results by Student’s t-test with Welch’s correction at 1 d p.i. only.
(XLSX)
Acknowledgments
The authors gratefully acknowledge: Four Star Finishing (Saint Louis) for suggesting the method for fabricating batches of thin chromium metal foils and for performing the electroplating of chromium; the Instrument Machine Shop at WUSM for machine shop work and related advice; the Electronics Shop at WUSM for performing soldering; the Cyclotron Facility at WUSM/MIR for operation and maintenance of the CS-15 cyclotron, for other services, and for helpful advice; and the Pre-Clinical Imaging Facility at WUSM/MIR for conducting injections, dissections, gamma-ray counting of tissues, and data analysis.
Data Availability
The data for Fig 1 are presented in Table 1. The data behind both Fig 1 and Table 1 are found in supplementary files as Excel workbooks.
Funding Statement
The authors gratefully acknowledge funding for this work from: National Institute of Biomedical Imaging and Bioengineering (NIBIB; nibib.nih.gov), National Institutes of Health (NIH; 1T32EB14855-01; ALW); Office of Defense Nuclear Nonproliferation (DNN), National Nuclear Security Administration (NNSA; nnsa.energy.gov), United States Department of Energy (DOE; DE-NA0000979; ALW, BCL, research expenses); Office of Science (SC; science.energy.gov), DOE (SEL); internal funds from Mallinckrodt Institute of Radiology (MIR; mir.wustl.edu), Washington University School of Medicine in St. Louis (WUSM; research costs, TAA, RBG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Averill BA, Eldredge P. General Chemistry: Principles, Patterns, and Applications. Published online: Flat World; 2011. [Google Scholar]
- 2.Avila DS, Puntel RL, Aschner M. Manganese in Health and Disease. Met Ions Life Sci. 2013;13:199–227. 10.1007/978-94-007-7500-8_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higdon J, Drake VJ. “Manganese” [Peer-reviewed webpage]. Corvalis, Oregon, United States: Micronutrient Information Center, Linus Pauling Institute, Oregon State University; 2010. [updated Mar. 2010; cited 11 Jun. 2015]. Available from: http://lpi.oregonstate.edu/mic/minerals/manganese. [Google Scholar]
- 4.Greenwood NN, Earnshaw A. Chemistry of the Elements, 2nd ed. Oxford, United Kingdom: Butterworth-Heinemann; 1997. p. 1041. [Google Scholar]
- 5.Gibbons RA, Dixon SN, Hallis K, Russell AM, Sansom BF, Symonds HW. Manganese metabolism in cows and goats. Biochim Biophys Acta. 1976;444(1):1–10. [DOI] [PubMed] [Google Scholar]
- 6.Scheuhammer AM, Cherian MG. Binding of manganese in human and rat plasma. Biochim Biophys Acta. 1985;840(2):163–9. [DOI] [PubMed] [Google Scholar]
- 7.Lau SJ, Sarkar B. Comparative studies of manganese(II)-, nickel(II)-, zinc(II)-, copper(II)-, cadmium(II)-, and iron(III)-binding components in human cord and adult sera. Can J Biochem Cell Biol. 1984;62(6):449–55. [DOI] [PubMed] [Google Scholar]
- 8.Wolf GL, Burnett KR, Goldstein EJ, Joseph PM. Contrast Agents for Magnetic Resonance Imaging. In: Kressel H, editor. Magnetic Resonance Annual. New York: Raven; 1985. p. 231 [PubMed] [Google Scholar]
- 9.Couper J. On the effects of black oxide of manganese when inhaled into the lungs. Br Ann Med Pharm Vital Stat Gen Sci. 1837;1:41–2. [Google Scholar]
- 10.Sjögren B, Gustavsson P, Hogstedt C. Neuropsychiatric symptoms among welders exposed to neurotoxic metals. Br J Ind Med. 1990;47(10):704–7. PubMed Central PMCID: PMC1012030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laohaudomchok W, Lin X, Herrick RF, Fang SC, Cavallari JM, Shrairman R, et al. Neuropsychological effects of low-level manganese exposure in welders. Neurotoxicology. 2011;32(2):171–9. PubMed Central PMCID: PMC3049839. 10.1016/j.neuro.2010.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences. Neurology. 1994;44(9):1583–6. [DOI] [PubMed] [Google Scholar]
- 13.Rodier J. Manganese poisoning in Moroccan miners. Br J Ind Med. 1955;12(1):21–35. PubMed Central PMCID: PMC1037597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cersosimo MG, Koller WC. The diagnosis of manganese-induced parkinsonism. Neurotoxicology. 2006;27(3):340–6. 10.1016/j.neuro.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 15.Lauterbur PC, Mendonça-Dias M, Rudin A. Augmentation of tissue water proton spin-lattice relaxation rates by in vivo addition of paramagnetic ions In: Dutton P, Leigh JS, Scarpa A, editors. Frontiers of Biological Energetics. Vol. 1 New York: Academic Press; 1978. p. 752–9. [Google Scholar]
- 16.Wolf GL, Baum L. Cardiovascular toxicity and tissue proton T1 response to manganese injection in the dog and rabbit. Am J Roentgenol. 1983;141(1):193–7. [DOI] [PubMed] [Google Scholar]
- 17.Ni Y, Petré C, Bosmans H, Miao Y, Grant D, Baert AL, et al. Comparison of manganese biodistribution and MR contrast enhancement in rats after intravenous injection of MnDPDP and MnCl2. Acta Radiol. 1997;38(5):700–7. [DOI] [PubMed] [Google Scholar]
- 18.Vargas ER, Chen JW. Magnetic resonance imaging agents In: Weissleder R, Ross BD, Rehemtulla A, Gambhir SS, editors. Molecular imaging: principles and practice. Shelton, Connecticut, United States: People's Medical Publishing House-USA; 2010. p. 389–404. [Google Scholar]
- 19.Pan D, Caruthers SD, Senpan A, Schmieder AH, Wickline SA, Lanza GM. Revisiting an old friend: manganese-based MRI contrast agents. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3(2):162–73. PubMed Central PMCID: PMC3157601. 10.1002/wnan.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, et al. Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology. 2005;64(12):2033–9. 10.1212/01.WNL.0000167411.93483.A1 [DOI] [PubMed] [Google Scholar]
- 21.Criswell SR, Perlmutter JS, Huang JL, Golchin N, Flores HP, Hobson A, et al. Basal ganglia intensity indices and diffusion weighted imaging in manganese-exposed welders. Occup Environ Med. 2012;69(6):437–43. PubMed Central PMCID: PMC3651997. 10.1136/oemed-2011-100119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato M. Distribution and excretion of radiomanganese administered to the mouse. Q J Exp Physiol Cogn Med Sci. 1963;48(4):355–69. [DOI] [PubMed] [Google Scholar]
- 23.Bertinchamps AJ, Miller ST, Cotzias GC. Interdependence of routes excreting manganese. Am J Physiol. 1966;211(1):217–24. [DOI] [PubMed] [Google Scholar]
- 24.Papavasiliou PS, Miller ST, Cotzias GC. Role of liver in regulating distribution and excretion of manganese. Am J Physiol. 1966;211(1):211–6. [DOI] [PubMed] [Google Scholar]
- 25.Cikrt M. Biliary excretion of 203Hg, 64Cu, 52Mn, and 210Pb in the rat. Br J Ind Med. 1972;29(1):74–80. PubMed Central PMCID: PMC1009354. [PMC free article] [PubMed] [Google Scholar]
- 26.Rocklage SM, Cacheris WP, Quay SC, Hahn FE, Raymond KN. Manganese(II) N,N'-dipyridoxylethylenediamine-N,N'-diacetate 5,5'-bis(phosphate). Synthesis and characterization of a paramagnetic chelate for magnetic resonance imaging enhancement. Inorg Chem. 1989;28(3):477–85. [Google Scholar]
- 27.Elizondo G, Fretz CJ, Stark DD, Rocklage SM, Quay SC, Worah D, et al. Preclinical evaluation of MnDPDP: new paramagnetic hepatobiliary contrast agent for MR imaging. Radiology. 1991;178(1):73–8. 10.1148/radiology.178.1.1898538 [DOI] [PubMed] [Google Scholar]
- 28.Hustvedt SO, Grant D, Southon TE, Zech K. Plasma pharmacokinetics, tissue distribution and excretion of MnDPDP in the rat and dog after intravenous administration. Acta Radiol. 1997;38(5):690–9. [DOI] [PubMed] [Google Scholar]
- 29.Gallez B, Bacic G, Swartz HM. Evidence for the dissociation of the hepatobiliary MRI contrast agent Mn-DPDP. Magn Reson Med. 1996;35(1):14–9. [DOI] [PubMed] [Google Scholar]
- 30.Pierre VC, Allen MJ, Caravan P. Contrast agents for MRI: 30+ years and where are we going? J Biol Inorg Chem. 2014;19(2):127–31. PubMed Central PMCID: PMC4075138. 10.1007/s00775-013-1074-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiraishi K, Narabayashi I, Fujita O, Yamamoto K, Sagami A, Hisada Y, et al. Blueberry juice: preliminary evaluation as an oral contrast agent in gastrointestinal MR imaging. Radiology. 1995;194(1):119–23. 10.1148/radiology.194.1.7997537 [DOI] [PubMed] [Google Scholar]
- 32.Brismar TB, Kartalis N, Kylander C, Albiin N. MRI of colorectal cancer liver metastases: comparison of orally administered manganese with intravenously administered gadobenate dimeglumine. Eur Radiol. 2012;22(3):633–41. PubMed Central PMCID: PMC3269572. 10.1007/s00330-011-2288-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein ATJ, Rosch F, Coenen HH, Qaim SM. Labelling of manganese-based magnetic resonance imaging (MRI) contrast agents with the positron emitter 51Mn, as exemplified by manganese-tetraphenyl-porphin-sulfonate (MnTPPS4). Appl Radiat Isot. 2005;62(5):711–20. 10.1016/j.apradiso.2004.09.009 [DOI] [PubMed] [Google Scholar]
- 34.Vanasschen C, Brandt M, Ermert J, Coenen HH. Radiolabelling with isotopic mixtures of 52g/55Mn(II) as a straight route to stable manganese complexes for bimodal PET/MR imaging. Dalton Trans. 2016;45(4):1315–21. 10.1039/c5dt04270d [DOI] [PubMed] [Google Scholar]
- 35.National Nuclear Data Center, Brookhaven National Laboratory. Upton, New York, United States. [Multiple online databases that compile nuclear data from published articles, government agencies, and other sources.] Available from: http://www.nndc.bnl.gov/.
- 36.Cotzias GC, Papavasiliou PS, Miller ST. Neutron activation analysis: clinical and biological studies of manganese. Symposium on Radioactivation Analysis and its Application to the Biological Sciences; 1963; Saclay, France. Published: 1962.
- 37.Sakurai H, Nishida M, Yoshimura T, Takada J, Koyama M. Partition of divalent and total manganese in organs and subcellular organelles of MnCl2-treated rats studied by ESR and neutron activation analysis. Biochim Biophys Acta. 1985;841(2):208–14. [PubMed] [Google Scholar]
- 38.Lewis J, Bench G, Myers O, Tinner B, Staines W, Barr E, et al. Trigeminal uptake and clearance of inhaled manganese chloride in rats and mice. Neurotoxicology. 2005;26(1):113–23. 10.1016/j.neuro.2004.06.005 [DOI] [PubMed] [Google Scholar]
- 39.Graves SA, Hernandez R, Fonslet J, England CG, Valdovinos HF, Ellison PA, et al. Novel preparation methods of Mn for immunoPET imaging. Bioconjugate Chem. 2015;26:2118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brunnquell CL, Hernandez R, Graves SA, Smit-Oistad I, Nickles RJ, Cai W, et al. Uptake and retention of manganese contrast agents for PET and MRI in the rodent brain. Contrast Media Mol Imaging. 2016;11(5):371–80. 10.1002/cmmi.1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wooten AL, Lewis BC, Lapi SE. Cross-sections for (p,x) reactions on natural chromium for the production of 52,52m,54Mn radioisotopes. Appl Radiat Isot. 2015;96:154–61. 10.1016/j.apradiso.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cross-Section Information Storage and Retrieval System (CSISRS), National Nuclear Data Center, Brookhaven National Laboratory. Upton, New York, United States. Available from: http://www.nndc.bnl.gov/exfor/exfor.htm. Original data source: International Network of Nuclear Reaction Data Centres (NRDC). [NRDC compiles data on nuclear reactions from published articles, government agencies, and other sources.]
- 43.Topping GJ, Schaffer P, Hoehr C, Ruth TJ, Sossi V. Manganese-52 positron emission tomography tracer characterization and initial results in phantoms and in vivo. Med Phys. 2013;40(4):042502 10.1118/1.4793756 [DOI] [PubMed] [Google Scholar]
- 44.Lewis CM, Graves SA, Hernandez R, Valdovinos HF, Barnhart TE, Cai W, et al. 52Mn production for PET/MRI tracking of human stem cells expressing divalent metal transporter 1 (DMT1). Theranostics. 2015;5(3):227–39. PubMed Central PMCID: PMC4279187. 10.7150/thno.10185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka S, Furukawa M. Excitation functions for (p,n) reactions with titanium, vanadium, chromium, iron and nickel up to Ep = 14 MeV. J Phys Soc Jpn. 1959;14(10):1269–75. [Google Scholar]
- 46.Daube ME, Nickles RJ. Development of myocardial perfusion tracers for positron emission tomography. Int J Nucl Med Biol. 1985;12(4):303–14. [DOI] [PubMed] [Google Scholar]
- 47.Klein ATJ, Rösch F, Qaim SM. Investigation of 50Cr(d,n)51Mn and natCr(p,x)51Mn processes with respect to the production of the positron emitter 51Mn. Radiochim Acta. 2000;88(5):253–64. [Google Scholar]
- 48.Klein ATJ, Rösch F, Coenen HH, Qaim SM. Production of the positron emitter 51Mn via the 50Cr(d, n) reaction: targetry and separation of no-carrier-added radiomanganese. Radiochim Acta. 2002;90(3):167–77. [Google Scholar]
- 49.Lahiri S, Nayak D, Korschinek G. Separation of no-carrier-added 52Mn from bulk chromium: a simulation study for accelerator mass spectrometry measurement of 53Mn. Anal Chem. 2006;78(21):7517–21. 10.1021/ac0607459 [DOI] [PubMed] [Google Scholar]
- 50.Buchholz M, Spahn I, Scholten B, Coenen HH. Cross-section measurements for the formation of manganese-52 and its isolation with a non-hazardous eluent. Radiochim Acta. 2013;101(8):491–9. [Google Scholar]
- 51.Buchholz M, Spahn I, Coenen Heinz H. Optimized separation procedure for production of no-carrier-added radiomanganese for positron emission tomography. Radiochim Acta. 2015;103(12):893. [Google Scholar]
- 52.Fonslet J, Tietze S, Jensen AI, Graves SA, Severin GW. Optimized procedures for manganese-52: production, separation and radiolabeling. Appl Radiat Isot. 2017;121:38–43. 10.1016/j.apradiso.2016.11.021 [DOI] [PubMed] [Google Scholar]
- 53.Aweda TA, Ikotun O, Mastren T, Cannon CL, Wright B, Youngs WJ, et al. The use of Ag as a tool for studying biological distribution of silver-based antimicrobials. Med Chem Comm. 2013;4(6):1015–7. PubMed Central PMCID: PMC3733397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aweda TA, Zhang S, Mupanomunda C, Burkemper J, Heo GS, Bandara N, et al. Investigating the pharmacokinetics and biological distribution of silver-loaded polyphosphoester-based nanoparticles using 111Ag as a radiotracer. J Labelled Comp Radiopharm. 2015;58(6):234–41. PubMed Central PMCID: PMC4457551. 10.1002/jlcr.3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fore H, Morton RA. Manganese in rabbit tissues. Biochem J. 1952;51(5):600–3. PubMed Central PMCID: PMC1197903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koshida Y, Kato M, Hara T. Autoradiographic observations of manganese in adult and embryo mice. Q J Exp Physiol Cogn Med Sci. 1963;48:370–8. [DOI] [PubMed] [Google Scholar]
- 57.Hughes ER, Miller ST, Cotzias GC. Tissue concentrations of manganese and adrenal function. Am J Physiol. 1966;211(1):207–10. [DOI] [PubMed] [Google Scholar]
- 58.Chauncey DM Jr., Schelbert HR, Halpern SE, Delano F, McKegney ML, Ashburn WL, et al. Tissue distribution studies with radioactive manganese: a potential agent for myocardial imaging. J Nucl Med. 1977;18(9):933–6. [PubMed] [Google Scholar]
- 59.Heilig EA, Thompson KJ, Molina RM, Ivanov AR, Brain JD, Wessling-Resnick M. Manganese and iron transport across pulmonary epithelium. Am J Physiol Lung Cell Mol Physiol. 2006;290(6):L1247–59. 10.1152/ajplung.00450.2005 [DOI] [PubMed] [Google Scholar]
- 60.Brain JD, Heilig E, Donaghey TC, Knutson MD, Wessling-Resnick M, Molina RM. Effects of iron status on transpulmonary transport and tissue distribution of Mn and Fe. Am J Respir Cell Mol Biol. 2006;34(3):330–7. PubMed Central PMCID: PMC2644198. 10.1165/rcmb.2005-0101OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kodama H, Shimojo N, Suzuki KT. Distribution of manganese in rat pancreas and identification of its primary binding protein as pro-carboxypeptidase B. Biochem J. 1991;278(3):857–62. PubMed Central PMCID: PMC1151425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leblondel G, Allain P. Manganese transport by Caco-2 cells. Biol Trace Elem Res. 1999;67(1):13–28. 10.1007/BF02784271 [DOI] [PubMed] [Google Scholar]
- 63.Seshadri M, Hoy A. Manganese-enhanced MRI of salivary glands and head and neck tumors in living subjects. Magn Reson Med. 2010;64(3):902–6. 10.1002/mrm.22452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fore H, Morton RA. The manganese in bone. Biochem J. 1952;51(5):598–600. PubMed Central PMCID: PMC1197902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kanayama Y, Tsuji T, Enomoto S, Amano R. Multitracer screening: brain delivery of trace elements by eight different administration methods. Biometals. 2005;18(6):553–65. 10.1007/s10534-005-4775-6 [DOI] [PubMed] [Google Scholar]
- 66.Crossgrove JS, Allen DD, Bukaveckas BL, Rhineheimer SS, Yokel RA. Manganese distribution across the blood-brain barrier I. Evidence for carrier-mediated influx of managanese citrate as well as manganese and manganese transferrin. Neurotoxicology. 2003;24(1):3–13. [DOI] [PubMed] [Google Scholar]
- 67.Crossgrove JS, Yokel RA. Manganese distribution across the blood-brain barrier IV. Evidence for brain influx through store-operated calcium channels. Neurotoxicology. 2005;26(3):297–307. 10.1016/j.neuro.2004.09.004 [DOI] [PubMed] [Google Scholar]
- 68.Aschner M, Aschner JL. Manganese transport across the blood-brain barrier: relationship to iron homeostasis. Brain Res Bull. 1990;24(6):857–60. [DOI] [PubMed] [Google Scholar]
- 69.Aschner M, Gannon M. Manganese (Mn) transport across the rat blood-brain barrier: saturable and transferrin-dependent transport mechanisms. Brain Res Bull. 1994;33(3):345–9. [DOI] [PubMed] [Google Scholar]
- 70.Rabin O, Hegedus L, Bourre JM, Smith QR. Rapid brain uptake of manganese(II) across the blood-brain barrier. J Neurochem. 1993;61(2):509–17. [DOI] [PubMed] [Google Scholar]
- 71.Yokel RA. Brain uptake, retention, and efflux of aluminum and manganese. Environ Health Perspect. 2002;110(Suppl 5):699–704. PubMed Central PMCID: PMC1241228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yokel RA, Crossgrove JS, Bukaveckas BL. Manganese distribution across the blood-brain barrier II. Manganese efflux from the brain does not appear to be carrier mediated. Neurotoxicology. 2003;24(1):15–22. [DOI] [PubMed] [Google Scholar]
- 73.Tjälve H, Mejàre C, Borg-Neczak K. Uptake and transport of manganese in primary and secondary olfactory neurones in pike. Pharmacol Toxicol. 1995;77(1):23–31. [DOI] [PubMed] [Google Scholar]
- 74.Tjälve H, Henriksson J, Tallkvist J, Larsson BS, Lindquist NG. Uptake of manganese and cadmium from the nasal mucosa into the central nervous system via olfactory pathways in rats. Pharmacol Toxicol. 1996;79(6):347–56. [DOI] [PubMed] [Google Scholar]
- 75.Pautler RG, Silva AC, Koretsky AP. In vivo neuronal tract tracing using manganese-enhanced magnetic resonance imaging. Magn Reson Med. 1998;40(5):740–8. [DOI] [PubMed] [Google Scholar]
- 76.Serrano F, Deshazer M, Smith KD, Ananta JS, Wilson LJ, Pautler RG. Assessing transneuronal dysfunction utilizing manganese-enhanced MRI (MEMRI). Magn Reson Med. 2008;60(1):169–75. 10.1002/mrm.21648 [DOI] [PubMed] [Google Scholar]
- 77.Thompson K, Molina RM, Donaghey T, Schwob JE, Brain JD, Wessling-Resnick M. Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. FASEB J. 2007;21(1):223–30. PubMed Central PMCID: PMC2432183. 10.1096/fj.06-6710com [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brenneman KA, Wong BA, Buccellato MA, Costa ER, Gross EA, Dorman DC. Direct olfactory transport of inhaled manganese (54MnCl2) to the rat brain: toxicokinetic investigations in a unilateral nasal occlusion model. Toxicol Appl Pharmacol. 2000;169(3):238–48. 10.1006/taap.2000.9073 [DOI] [PubMed] [Google Scholar]
- 79.Dorman DC, Brenneman KA, McElveen AM, Lynch SE, Roberts KC, Wong BA. Olfactory transport: a direct route of delivery of inhaled manganese phosphate to the rat brain. J Toxicol Environ Health A. 2002;65(20):1493–511. 10.1080/00984100290071630 [DOI] [PubMed] [Google Scholar]
- 80.Ray TW, Deysach LJ. Storage of manganese by thyroid. Effect on oxygen consumption of the guinea pig. Proc Soc Exp Biol Med. 1942;51(2):228–9. [DOI] [PubMed] [Google Scholar]
- 81.Deysach LJ, Ray TW. Absorption of manganese by the thyroid gland of the guinea pig. Proc Soc Exp Biol Med. 1949;71(2):188–9. [DOI] [PubMed] [Google Scholar]
- 82.Kawada J, Nishida M, Yoshimura Y, Yamashita K. Manganese ion as a goitrogen in the female mouse. Endocrinol Jpn. 1985;32(5):635–43. [DOI] [PubMed] [Google Scholar]
- 83.Nishida M, Kawada J. Hormonal control of manganese transport in the mouse thyroid. Experientia. 1992;48(3):262–5. [DOI] [PubMed] [Google Scholar]
- 84.Wang D, Du X, Zheng W. Alteration of saliva and serum concentrations of manganese, copper, zinc, cadmium and lead among career welders. Toxicol Lett. 2008;176(1):40–7. PubMed Central PMCID: PMC3980858. 10.1016/j.toxlet.2007.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Graves S, Hernandez R, Valdovinos H, Barnhart T, Cai W, Nickles R. Probing the impact of isoflurane on acute pancreatic function with 52Mn-PET. J Nucl Med. 2016;57(Suppl 2):167. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
At 1 h p.i., 52Mn was observed in the digestive tract, kidneys, and likely the pancreas. At 3 d p.i., 52Mn had cleared from the digestive tract, while it is retained in the kidneys, as well as liver, pancreas, and thyroid gland. Manganese-52 was eluted from the cation-exchange column in 0.067 M ammonium oxalate solution. The ammonium oxalate product was heated to dryness, and then the heat was increased to burn away the ammonium oxalate. Manganese-52 was resuspended by adding water followed by a drop of 6 M hydrochloric acid, then heated to dryness. Evaporation and resuspension in water was repeated and then the 52Mn was finally resuspended in water, resulting in a solution with pH of ~6.5. Mice (n = 2; C57-Black-6; male) were anesthetized by isoflurane (1–2% induction), injected in the tail vein with ~41 μCi of 52Mn in 50 μL total volume, and imaged simultaneously side-by-side at 1 h and 3 d p.i. At the imaging timepoints, anatomic images were obtained by non-contrast CT using an Inveon small animal PET/CT scanner (Siemens Preclinical Solutions, Knoxville, Tennessee, United States), and PET data were acquired using either the Inveon PET/CT scanner or a microPET Focus 220 scanner (Siemens Preclinical Solutions). Static PET data were acquired for 30 minutes at the 1-hour timepoint and for 1 hour at the 3-day timepoint. Attenuation maps for each subject were obtained from either the CT scan in the Inveon scanner or by a transmission scan on the Focus 220 scanner. Images were analyzed using Inveon Research Workplace software (Siemens Preclinical Solutions).
(TIF)
This file is a workbook made using Microsoft Excel for Mac and contains multiple sheets.
(XLSX)
This file is a workbook made using Microsoft Excel for Mac and contains multiple sheets.
(XLSX)
This file is a workbook made using Microsoft Excel for Mac and contains multiple sheets. The workbook includes results from S1 File and S2 File, comparison of these results by plotting, and hypothesis testing of these results by Student’s t-test with Welch’s correction at 1 d p.i. only.
(XLSX)
Data Availability Statement
The data for Fig 1 are presented in Table 1. The data behind both Fig 1 and Table 1 are found in supplementary files as Excel workbooks.


