Abstract
Neuroinflammation is the commonest cause of neurodegenerative diseases such as Alzheimer’s disease. Present investigation evaluates the inhibitory effect of ethanolic root extract of Aster tataricus (AS) on inflammatory mediators production in lipopolysaccharide (LPS) stimulated C6 cells. C6 cells were treated with AS (20 and 40 mg/kg) and nimesulide (NSL, 1.5 μg/ml) for 1 day. Thereafter various parameters such as production of ROS, release of nitrite, MDA, glutathione level and NF-κB translocation were estimated in C6 cell lines. Effect of AS was estimated on the expressions of tumor necrosis factor α (TNF-α) of human monocytic leukemia cell line (THP-1). It was observed that AS (20 and 40 mg/ml) treated group shows significant (p < 0.01) decrease in production of ROS, Nitrite release and MDA level in LPS activated C6 cell lines compared to negative control group. Moreover, treatment with it decreases glutathione level and inhibits the translocation of NF-κB in LPS activated C6 cell lines compared to negative control group. There were significant (p < 0.01) decreases in expression of TNF-α in AS treated group compared to negative control group in THP-1 cell lines. Present investigation concludes the anti neuroinflammatory effect of ethanolic extract of AS root by decreasing oxidative stress and attenuates the cytokine.
Keywords: Aster tataricus, Astrocytoma, C6 cell lines, THP-1 cell lines, Lipopolysaccharide (LPS)
1. Introduction
Neuroinflammation is one of the pathogenic factors of neurodegenerative diseases as activated astrocyte enhances the release of cytokines and chemokines like inflammatory mediators. There are several neurodegenerative disorders such as Huntington’s disease, Alzheimer’s and Parkinson’s diseases (McCoy and Tansey, 2008). Events after the chronic inflammation are resulted in release of cytotoxic factors such as Interleukin (IL), TNF-α, reactive oxygen species, cyclooxygenase-2 and inducible nitric oxide synthase (Choi et al., 2010).
Lipopolysaccharide (LPS) is a endotoxin which stimulates the brain cell and thereby increases the release of TNF-α, IL (Quan et al., 1994). These releases of cytokines increase the oxidative stress and decrease the antioxidant molecules present endogenously (Tyagi et al., 2010). Reported studies also suggested that LPS increases the expression of COX and iNOS in brain cell in a dose dependent manner (Minghetti et al., 1996). In Alzheimer’s disease release of these inflammatory mediators contributes in the neurodegeneration. Antioxidant therapies are known to decrease the free radicals and prevent the neuronal damage (Thomas, 1994). Medicinal plants that are known to have strong antioxidant property alter the neuronal damage.
Aster tataricus (AS) is used for medicinal purpose traditionally in China last from 2000 years. Roots of AS contain the chemical constituents such as triterpenes and saponins (Dongliang and Yu, 1993). Other reported chemicals are isolated from roots such as epifriedelinol, caffeoylquinic acids, aster saponins, shionone and aster peptides (Yu et al., 2015). Traditionally it has been used as antibacterial, antifungal, anticancer and management of chronic bronchitis and tuberculosis (Bown, 1995). Literature suggested that root extract of AS posses anti-inflammatory, expectorant and antitussive activity (Yu et al., 2015). Caffeoylquinic acids and epifriedelinol possess strong antioxidant, anti-inflammatory and anticancer activity (Duke and Ayensu, 1985, Ma et al., 2011, Peluso et al., 1995). It protects the neuronal cells in NMDA induced neurotoxicity (Miyamae et al., 2012). Hence it is worthy to evaluate the effect of AS on inflammatory mediators in LPS induced C6 astrocytoma cell lines and THP-1 cells.
2. Materials and methods
2.1. Extraction of plant material
Root of AS was procured from local supplier and authentified from Institute of Medicinal Plant Development, Beijing. Extraction of AS roots was done by maceration. Dried root was powdered and kept with 70% ethanol for 72 h. Thereafter ethanol was evaporated from the extract at low temperature by rota vapor apparatus. Percentage yield of extract was found to be 6.3% w/w.
2.2. Chemicals
Fetal bovine serum (FBS), Pentoxifylline and TNF-α were purchased from (R and D biosciences systems, USA). 5-Diphenyltetrazolium bromide tetrazolium, thiobarbituric acid, dimethysulfoxide, napthylethylenediamide, and trichloroacetic acid were obtained from Sigma–Aldrich, USA. Other chemicals such as Medium of cell culture, streptomycin, fetal bovine serum, penicillin and 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased (St. Louis, MO, USA).
2.3. Cell culture
Rat C6 astrocytoma cell lines were cultured in DMEM cell culture medium with 100 μg/ml of streptomycin, 10% v/v of FBS and 100 U/ml of penicillin. In a 5% CO2 humidified incubator at 37 °C cell lines (5 × 103) in 96 well plate were incubated for 1 day. Test was carried out on the cell lines as per different groups such as control group treated with vehicle, Negative control group treated with LPS (10 mg/ml) and other LPS treated group that were treated with AS (20, 40 and 80 mg/ml) and nimesulide (1.5 μg/ml) for 1 day. Estimation of ROS, MDA, GSH and nitrite release was performed in this study.
2.4. MTT assay
MTT assay provides the mitochondrial integrity and activity, which is considered as % of cell viability. MTT assay was done as described by Xiao et al., with slight modification. In this assay cells (5 × 103 cells/well) were seeded in the 96 well plates and treated with AS (20 and 40 mg/ml) and nimesulide (1.5 μg/ml) for 1 day. MTT (50 μl) was added to the well plate and keep for incubation for 4 h at 37 °C. Thereafter, supernatant was washed out from the reaction mixture and subsequently dimethylsulfoxide (200 μl) was added to all the well plates at room temperature. After 10 min, the absorbance was read at 530 nm, using multi well microplate reader (Mosmann, 1983).
2.5. Estimation of nitrite release
C6 cells (5 × 103 cells/well) were placed at 96 well plate and incubated for the period of 1 day. Thereafter supernatant culture of each group was reacted with Griess reagent (1:1) and incubated for 20 min. Griess reagent was a chemical mixture of 1% p-amino-benzene sulfonamide and 0.01% napthylethylenediamide in 2.5%v/v phosphoric acid. Estimation of absorbance was carried out at wavelength of 570 nm (Niranjan et al., 2012).
2.6. Estimation of ROS generation
Estimation of ROS in C6 cell lines was done by using dichlorofluorescein-diacetate (DCF-DA) a fluorescence dye. C6 cells (1 × 104 cells/well) were placed at 24 well plate and incubated for the period of 1 day. Thereafter from all the treated groups culture medium was aspirated and DCF-DA 100 ml/well was added to each well. These plates were kept for incubation for 30 min by CO2 incubator at 37 °C. Estimation of Fluorescence was done at a excitation wavelength of 485 nm and emission wavelength of 530 nm (Niranjan et al., 2012).
2.7. Estimation of MDA
Estimation of MDA was done as per previously described method of Colado. C6 cells (4 × 105 cells/well) were placed at 6 well plate and incubated for the period of 1 day. 100 ml of sodium buffer of pH 7 was added to the well plate and by the help of sonication cells were lysed after centrifuged at 10,000 RPM for 5 min. Supernatant was collected and mixed with 60 ml of TCA and 30 ml of HCl. This mixture was incubated for 5 min and later it was treated with 30 ml of TBA. All the samples were heated and centrifuged for 10 min at 10,000 RPM. Measurement of absorbance was done at 532 nm (Niranjan et al., 2012).
2.8. Estimation of glutathione
Reduced glutathione was estimated by placing C6 cell (20,000 cells/ml) in Petri dish and the cells were incubated for 1 day to get attached. After drug treatment, in all the Petridis treatments media were aspirated and wash correct by PBS. Thereafter, cells were treated with PBS and solution was collected in test tube. Later ultrasonication (for 2 min) was done for cell lysis and cell lysate solution was centrifuged for 5 min at 10,000 RPM. Supernatant was withdrawn and mixed with 10% TCA, later incubated at 4 °C for 1 h. This solution was further centrifuged and supernatant (75 μl) was mixed with distilled water (25 μl), buffer (100 μl) and 5,5′-dithiobis-(2-nitro-benzoic acid) (DTNB; 50 μl). This mixture was incubated for 10 min and absorbance was measured at 412 nm (Niranjan et al., 2012).
2.9. Immunohistochemical estimation of NF-κB
C6 cells (2 × 105 cells/well) were placed at 6 well plate and incubated for the period of 1 day. Cells were prepared to be permeable by treating them with H2O2 (0.05%) in methanol for the period of 1 h at room temperature. After this it was treated with buffer that contains BSA (0.02%) and triton X (0.002%) for the period of 30 min. Primary antibody of NF-κB was treated with cells for 1 h and thereafter cells were treated with secondary antibody that conjugated with fluorescence for 1 h with blocking buffer. Fluorescence microscope was used to capture the images at a magnification of 100×.
2.10. Estimation of TNF-α in THP 1 cells
THP-1 cell lines were selected for the present investigation as it is having phagocytic property due to presence of Fc and C3b receptor. Moreover these cell lines produce cytokines and chemokines after LPS stimulation (Banka et al., 1991).
Cells (5 × 105 cells/well) were placed at 12 well plate containing serum free medium and incubated at 37 °C. All the cells were treated with LPS (25, 50, 100, 200 ng) for the duration of 4 h and supernatant was withdrawn and stored at 20 °C. NaOH (0.1 mol/l) was used for the cell lysis and release of cytokines is estimated as per milligram of protein. Cell lines were treated with AS (20 and 40 mg/ml) and 1 h after the treatment cells were again treated with LPS at a concentration of 100 μg/l for 4 h. TNF-α was estimated in the supernatant solution by using ELISA kit and Pentoxifylline was used as a standard in this study (Singh et al., 2005).
2.11. Statistical analysis
All the values of these experiments were articulated as mean ± SEM and the data were statistically analyzed by one-way ANOVA and thereafter applied to Dunnett’s post hoc test. p < 0.05 was considered statistically significant.
3. Result
3.1. Effect of Aster tataricus on cell viability
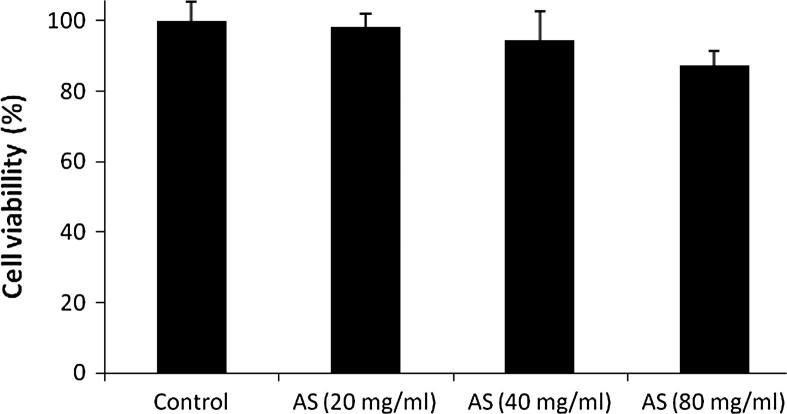
MTT assay was used for the estimation of cell viability of C6 astrocytoma cell lines. Cell viability was not altered after treatment with different concentrations of ethanolic extract of AS root such as 20, 40 and 80 mg/ml (Fig. 1).
Figure 1.
Effect of Aster tataricus on cell viability of C6 astrocytoma cell lines.
3.2. Effect of Aster tataricus on release of nitrite
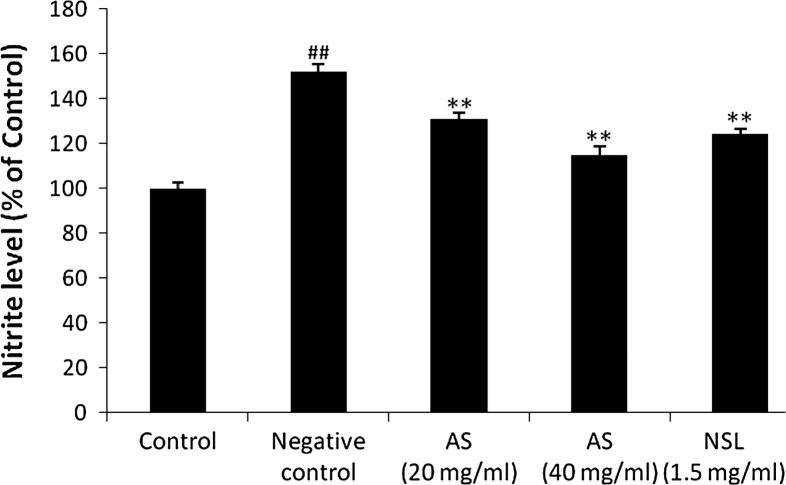
Effect of AS on release of nitrite from LPS treated C6 cells is shown in Fig. 2. There were significant increases in nitrite release from LPS treated C6 cells compared to control group. However, treatment with AS (20 and 40 mg/ml) significantly decreases (p < 0.01) the release of nitrite from LPS treated C6 cells compared to negative control group.
Figure 2.
Effect of Aster tataricus on nitrite release in C6 astrocytoma cell lines. Data of this study expressed as mean ± SEM; Vs group I, ##p < 0.01; Vs group II, **p < 0.01.
3.3. Effect of Aster tataricus on generation of ROS
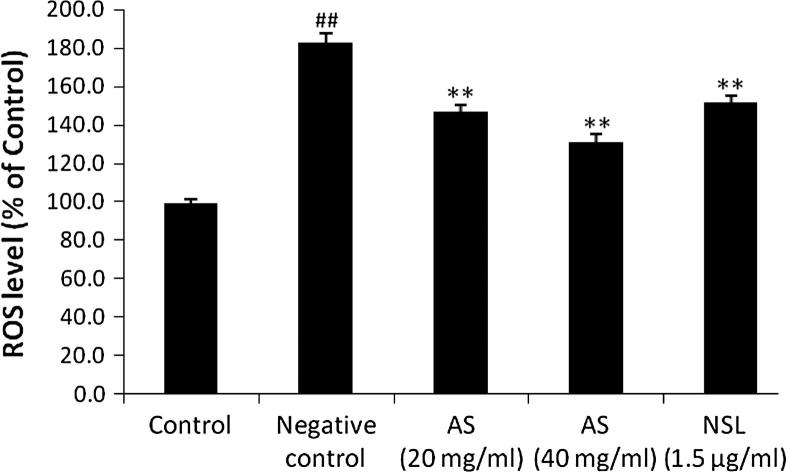
Effect of AS on generation of ROS in C6 astrocytoma cell lines is shown in Fig. 3. LPS stimulated the generation of ROS production compared to untreated control group, whereas, the level of ROS significantly (p < 0.01) decreases after treatment with AS (20 and 40 mg/ml) and NSL (1.5 μg/ml) group compared to negative control group.
Figure 3.
Effect of Aster tataricus on generation of ROS in C6 astrocytoma cell lines. Data of this study expressed as mean ± SEM; Vs group I, ##p < 0.01; Vs group II, **p < 0.01.
3.4. Effect of Aster tataricus on formation of MDA
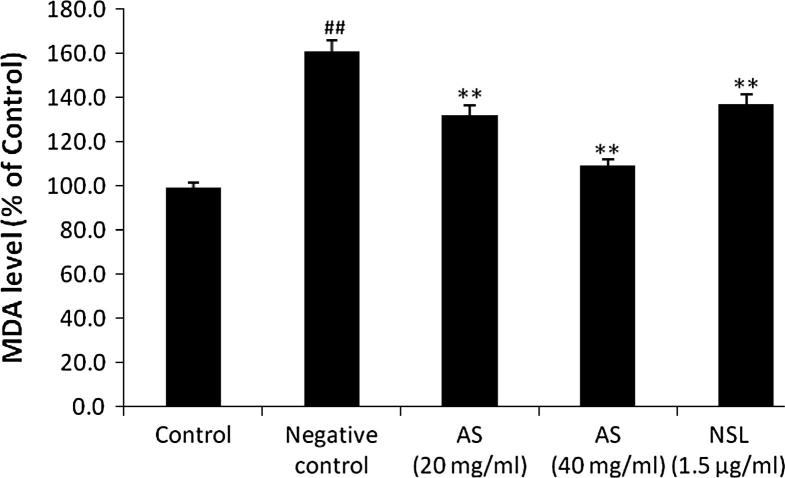
MDA formation significantly (p < 0.01) increases in LPS stimulated C6 astrocytoma cell lines. AS (20 and 40 mg/ml) and NSL (1.5 μg/ml) treated group shows significant decrease (p < 0.01) in the formation of MDA in LPS stimulated C6 cells compared to negative control group (Fig. 4).
Figure 4.
Effect of Aster tataricus on the formation of MDA in C6 astrocytoma cell lines. Data of this study expressed as mean ± SEM; Vs group I, ##p < 0.01; Vs group II, **p < 0.01.
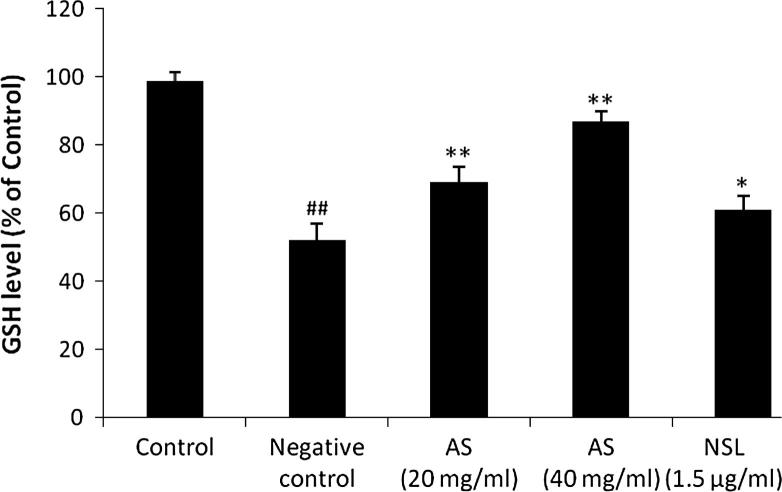
3.5. Effect of Aster tataricus on glutathione
Effect of AS on the level of reduced glutathione in C6 astrocytoma cell line is shown in Fig. 5. There were significant decreases in the level of glutathione in LPS stimulated C6 cell lines compared to untreated. Moreover, level of glutathione was found to be increased in AS 20 mg/ml and AS 40 mg/ml treated groups compared to negative control group (see Fig. 6).
Figure 5.
Effect of Aster tataricus on glutathione in C6 astrocytoma cell lines. Data of this study expressed as mean ± SEM; Vs group I, ##p < 0.01; Vs group II, **p < 0.01, *p < 0.05.
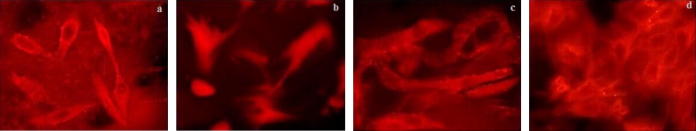
Figure 6.
Effect of Aster tataricus on NF-κB translocation in C6 astrocytoma cell lines. (a) Control, (b) Negative control, (c) AS (20 mg/ml) and (d) NSL (1 μg/ml).
3.6. Effect of Aster tataricus on NF-κB translocation
Glial cells activation involves the NF-κB translocation as it alters the expressions of various inflammatory genes. Thus to prove the possible anti-inflammatory mechanism of AS, estimation of NF-κB translocation was done in the given study. It was observed that treatment with LPS translocates the NF-κB (p65) from cytosol to nucleus, whereas, this translocation of NF-κB (p65) was inhibited by the treatment with AS 40 mg/ml and NSL (1.5 μg/ml).
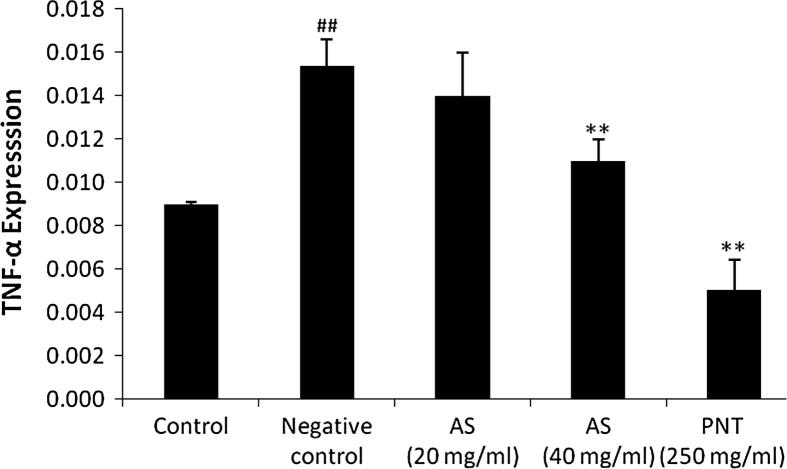
3.7. Effect of Aster tataricus on TNF-α in THP-1 cell line
It was observed LPS stimulation significantly (p < 0.01) increases the expressions of TNF-α in THP-1 cells compared control group, whereas, treatment with AS (40 mg/ml) and PNT (250 mg/ml) significantly decreases the expressions of TNF-α compared to negative control group as shown in Fig. 7.
Figure 7.
Effect of Aster tataricus on TNF-α in LPS stimulated THP-1 cell line. Data of this study expressed as mean ± SEM; Vs group I, ##p < 0.01; Vs group II, **p < 0.01, *p < 0.05.
4. Discussion
Present investigation evaluates the effect of AS on inflammatory mediators in LPS stimulated C6 cell lines. This study also postulates the possible mechanism of AS for the management of neuroinflammatory disorders. LPS is known for its effect to stimulate the neuronal cell by increasing the release of nitrite and thus produces pathological condition similar to neuroinflammation (Niranjan et al., 2010). Increased oxidative stress and neuroinflammation are important pathogenic factors that result in neuronal death and cause neurodegenerative diseases (Tyagi et al., 2010).
Elevated level of ROS stimulates the various inflammatory pathways and thereby causes neuronal cell death (Niranjan et al., 2010). Result of the given study suggested that treatment with AS (20 and 40 mg/ml) significantly decreases the level of ROS and MDA in LPS treated C6 cell lines compared to negative control. Moreover treatment with it also decreases the glutathione level in LPS treated C6 cell lines than negative control group. Thus by decreasing oxidative stress parameters AS could be used in the management of neuroinflammatory diseases such as Parkinson’s and Alzheimer’s diseases.
Literature suggested that increase in inflammatory mediators such as TNF-α, COX-2, IL and NF-κB results in neurodegenerative diseases (Quan et al., 1994). Immunohistochemical results suggested that AS and NSL treatment inhibits the translocation of NF-κB in LPS stimulated C6 cells. Moreover the expressions of TNF-α significantly decrease (p < 0.01) in AS treated group compared to negative control group in LPS stimulated THP-1 cells. Inhibition of inflammatory mediators such as TNF-α is a molecular target for the drug development in the management of neurodegenerative disorder (Tweedie et al., 2007).
5. Conclusion
Findings of this study suggest that AS produced anti-neuroinflammatory effect by preventing free radical generation, enhancement of antioxidants enzymes activity and attenuation of pro-inflammatory cytokines. In addition, it possesses analgesic effect possibly mediated through peripheral and central mechanisms involving inhibition of release and/or actions of vasoactive substances.
Footnotes
Peer review under responsibility of King Saud University.
References
- Banka C.L., Black A.S., Dyer C.A., Curtiss L.K. THP-1 cells form foam cells in response to coculture with lipoproteins but not platelets. J. Lipid Res. 1991;32:35–43. [PubMed] [Google Scholar]
- Bown D. Dorling Kindersley; London: 1995. Encyclopaedia of Herbs and their Uses. ISBN 0-7513-020-31. [Google Scholar]
- Choi D.K., Koppula S., Choi M., Suk K. Recent developments in the inhibitors of neuroinflammation and neurodegeneration: inflammatory oxidative enzymes as a drug target. Expert Opin. Ther. Pat. 2010;20:1531–1546. doi: 10.1517/13543776.2010.525220. [DOI] [PubMed] [Google Scholar]
- Dongliang C., Yu S. Terpenoid glycosides from the roots of Aster tataricus. Phytochemistry. 1993;35(1):173–176. [PubMed] [Google Scholar]
- Duke J.A., Ayensu E.S. Reference Publications, Inc.; 1985. Medicinal Plants of China. ISBN 0-917256-20-4. [Google Scholar]
- Ma C., Dastmalchi K., Whitaker B.D., Kennelly E.J. Two new antioxidant malonated caffeoylquinic acid isomers in fruits of wild eggplant relatives. J. Agric. Food Chem. 2011;59(17):9645–9651. doi: 10.1021/jf202028y. [DOI] [PubMed] [Google Scholar]
- McCoy M.K., Tansey M.G. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J. Neuroinflammation. 2008;17(5):45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti L., Polazzi E., Nicolini A., Creminon C., Levi G. Interferon-g and nitric oxide down-regulate lipopolysaccharide-induced prostanoid production in cultured rat microglial cells by inhibiting cyclooxygenase-2 expression. J. Neurochem. 1996;66:1963–1970. doi: 10.1046/j.1471-4159.1996.66051963.x. [DOI] [PubMed] [Google Scholar]
- Miyamae Y., Kurisu M., Murakami K., Han J., Isoda H., Irie K., Shigemori H. Protective effects of caffeoylquinic acids on the aggregation and neurotoxicity of the 42-residue amyloid β-protein. Bioorg. Med. Chem. 2012;20(19):5844–5849. doi: 10.1016/j.bmc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Niranjan R., Kamat P.K., Nath C., Shukla R. Evaluation of guggulipid and nimesulide on production of inflammatory mediators and GFAP expression in LPS stimulated rat astrocytoma, cell line (C6) J. Ethnopharmacol. 2010;127:625–630. doi: 10.1016/j.jep.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Niranjan R., Nath C., Shukla R. Melatonin attenuated mediators of neuroinflammation and alpha-7 nicotinic acetylcholine receptor mRNA expression in lipopolysaccharide (LPS) stimulated rat astrocytoma cells, C6. Free Radical Res. 2012;46(9):1167–1177. doi: 10.3109/10715762.2012.697626. [DOI] [PubMed] [Google Scholar]
- Peluso G., De Feo V., De Simone F., Bresciano E., Vuotto M.L. Studies on the inhibitory effects of caffeoylquinic acids on monocyte migration and superoxide ion production. J. Nat. Prod. 1995;58(5):639–646. doi: 10.1021/np50119a001. [DOI] [PubMed] [Google Scholar]
- Quan N., Sundar S.K., Weiss J.M. Induction of interleukin-1 in various brain regions after peripheral and central injections of lipopolysaccharide. J. Neuroimmunol. 1994;49:125–134. doi: 10.1016/0165-5728(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Singh U., James T., Senthil K.V., Sridevi D., Ishwarlal J. Development of an in vitro screening assay to test the anti-inflammatory properties of dietary supplements and pharmacologic agents. Clin. Chem. 2005;51:2252–2256. doi: 10.1373/clinchem.2005.056093. [DOI] [PubMed] [Google Scholar]
- Thomas J.A. In: Oxidative Stress, Oxidant Defence and Dietary Constituents. Maurice E., Shils M.E., Olson J.A., Shike M., editors. Modern Nutrition in Health and Disease; Waverly, Philadelphia: 1994. pp. 501–512. [Google Scholar]
- Tweedie D., Sambamurti K., Greig N.H. TNF-alpha inhibition as a treatment strategy for neurodegenerative disorders: new drug candidates and targets. Curr. Alzheimer Res. 2007;4:378–385. doi: 10.2174/156720507781788873. [DOI] [PubMed] [Google Scholar]
- Tyagi E., Agrawal R., Nath C., Shukla R. Cholinergic protection via a7 nicotinic acetylcholine receptors and PI3K-Akt pathway in LPS-induced neuroinflammation. Neurochem. Int. 2010;56:135–142. doi: 10.1016/j.neuint.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Yu P., Cheng S., Xiang J., Yu B., Zhang M., Zhang C., Xu X. Expectorant, antitussive, anti-inflammatory activities and compositional analysis of Aster tataricus. J. Ethnopharmacol. 2015;164:328–333. doi: 10.1016/j.jep.2015.02.036. [DOI] [PubMed] [Google Scholar]