Abstract
Various methods have been used to enhance production of chemically diverse phytochemicals especially medicinal natural products. With the advancement in nanotechnology, nanoparticles have been reported to have varying impact in plant growth and inducibility of phytochemical composition. Major objective of the study was to study the secondary metabolite modulatory effect of silver nanoparticles. In the current study, treatment of fenugreek seedlings with biosynthesized silver nanoparticles (Ag-NPs) was found to have significant impact on its growth parameters such as leaf number, root length, shoot length and wet weight. On HPLC based analysis, Ag-NPs treated seedlings showed an enhancement in the production of major phytochemical diosgenin to a level of 214.06 ± 17.07 μg/mL. An untreated control gave an yield of only 164.44 ± 7.67 μg/mL of diosgenin, and the observed phytochemical enhancement effect induced by Ag-NP was very significant. Most remarkably, the Ag-NP used in the study was found to play dual role of enhancement of both plant growth and diosgenin synthesis. Hence the study is of immense application as it opens up development of new methods based on nanoelicitors to enhance the biosynthesis of medicinal natural products in plants.
Keywords: Silver nanoparticles (Ag-NPs), Plant growth promotion (PGP), Fenugreek, Diosgenin induction
1. Introduction
Enhanced biosynthesis of phytochemicals is highly attractive because of their broad bioactivity properties. Because of this, various methods have been used to enhance the phytochemical composition of medicinal plants. This ranges from tissue culture based methods to the development of transgenic plants. But with the advancement in nanotechnology and various bio-interaction properties of nanoparticles, there lies much opportunities to explore nano-elicitor potential of NPs. The nano-elicitive function of nanoparticle may likely to be determined by nature of nanoparticle and method used for synthesis. Various methods have been used for nanoparticles synthesis and among these, microbial synthesis is highly attractive as it is eco-friendly and the process occurs at normal temperature and pressure (Das et al., 2014). Among the various nanoparticles, one of the most widely studied one is silver nanoparticles (Ag-NP) because of its diverse antimicrobial applications, use as coating material for stainless steel, and also in treatment for water purification (Durán et al., 2007). Their remarkable physical and chemical features and the resulting activity are considered to be due to its small size which usually ranges from 1 to 100 nm (Nowack and Bucheli, 2007).
Nanoparticles have been reported to have both positive and negative effects on growth and development of various plant species. Ag-NPs have been shown to have its effect on activating the aminocyclopropane-1-carboxylic acid (ACC)-derived inhibition of root elongation in Arabidopsis seedlings. Enhanced seed germination and seedling growth of tree Boswellia ovaliofoliolata have also been reported upon treatment with Ag-NPs (Savithramma et al., 2012). Effect of Ag-NPs on plant growth enhancement parameters such as shoot and root length, leaf area and biochemical attributes such as chlorophyll, carbohydrate and protein contents and antioxidant enzymes were also reported on Brassica juncea (Sharma et al., 2012, Zea and Salama, 2012). However, the influence of NPs towards secondary metabolite enhancement in medicinal plants are least studied. This is very important as the positive modulatory effect of Ag-NPs on phytochemical composition can be exploited for the enhanced production of medicinally important natural products from plants. So we have selected Fenugreek as a model system to study the effect of Ag-NPs on both plant growth and secondary metabolite modulation.
Fenugreek (Trigonella foenum-graecum L.) is a well-known spice which is used commonly in most parts of the world. Its significance is due to the presence of sapogenins such as diosgenin which has both pharmaceutical and nutraceutical applications (Acharya et al., 2008, McAnuff et al., 2002). In addition, diosgenin has significant effect in plant physiology to control both biotic and abiotic stress responses as the elevated level of phytochemicals playing significant role in plant stress management. So the study used changes in diosgenin concentration as a metabolite marker to know nanoelicitor role of Ag-NPs. As diosgenin is used as medicines and is also a precursor for the production of steroidal drugs and hormones such as testosterone, glucocorticoids and progesterone, its enhanced production is of significant applications. So the effect of biosynthesized Ag-NPs was analyzed on the plant growth and diosgenin variation in Trigonella foenum-graecum L.
2. Materials and methods
2.1. Biosynthesis of silver nanoparticles
Bacillus sp. (SJ 14), which was found to have highly efficient Ag-NPs synthesizing properties as confirmed by our previous study using UV–Vis spectroscopy, FTIR, SEM, HR-TEM and EDS was selected for the Ag-NPs synthesis (Thomas et al., 2015). For this, the bacterial isolate was inoculated into 100 mL nutrient broth and incubated at room temperature for 24 h in a rotating shaker (200 rpm). After incubation, the biomass was collected by centrifugation and about 2 g of bacterial wet biomass was resuspended in 100 mL aqueous solution of 1 mM AgNO3 in a 250 mL Erlenmeyer flask. The mixture was then kept on a rotating shaker set at 200 rpm for a period of 24 h at room temperature under visible light. The formed nanoparticles were purified as per previous report (Thomas et al., 2014). Briefly, the whole bacterial mixture with AgNPs was centrifuged at 15,000 rpm for 15 min and the collected pellets were washed and resuspended in 50 mM Tris buffer (pH 7). The cells were disrupted by ultrasonication and the resulting solution was filtered through a 0.22 μM filter (Millipore) and the purified AgNPs thus obtained were further characterized by UV–Vis spectroscopy and HR-TEM.
2.2. Effect of Ag-NPs on plant growth promotion
For the study, Fenugreek (Trigonella foenum) seeds were surface sterilized using 2% sodium hypochlorite and 70% ethanol (Aravind et al., 2009) and were soaked in sterile distilled water for 24 h. After this, sprouting seedlings were placed on 2% agar in conical flasks and 200 μL of Ag-NPs (1 μg/mL (w/v)) was added to each seedling and were kept for 5 days along with control. The concentration of Ag-NPs for treatment was selected based on previous report on effect of Ag-NPs on plants (Harris and Bali, 2008). After 5 days of incubation on agar, the seedlings were taken and planted on conical flask containing 100 g sterile soil. Ag-NPs addition to the planted seedlings was repeated by adding the same concentration used earlier (200 μL of 1 μg/mL (w/v)) to each seedlings. The planted flasks were kept under regulated conditions for 5 more days. The experiment was conducted in triplicate in which each set contained 10 seedlings. After incubation the seedlings were collected and the root length, shoot length, number of leaves, and wet weight were measured.
2.3. Diosgenin quantification
For this, the seedlings were dried in a hot air oven at 65 °C for 5 h and powdered and equal quantity (0.8 g) from each treatment was extracted with 50 mL of hexane. The container with the mixture was sealed and was then incubated for 10 days at 50 °C under shaking condition. Finally, the extract was filtered using Whatman No. 1 filter paper and was evaporated. The residual powder was reconstituted in 1 mL of methanol. Diosgenin present in the methanolic extract of the sample was quantified by C18 reverse-phase column of analytical HPLC (Thermo Scientific) with elution using an isocratic binary system of acetonitrile/water (90:10), at flow rate of 1 mL min−1 (Jasim et al., 2015). Quantification of the diosgenin produced was calculated from the area of the peak using the equation.
2.4. Statistical analysis
The results were analyzed using statistical program IBM SPSS Statistics 20. One-way analysis of variance was used for comparison among two treatments. Post hoc multiple comparison test was used to determine the significant difference among groups (Jasim et al., 2013).
3. Results and discussion
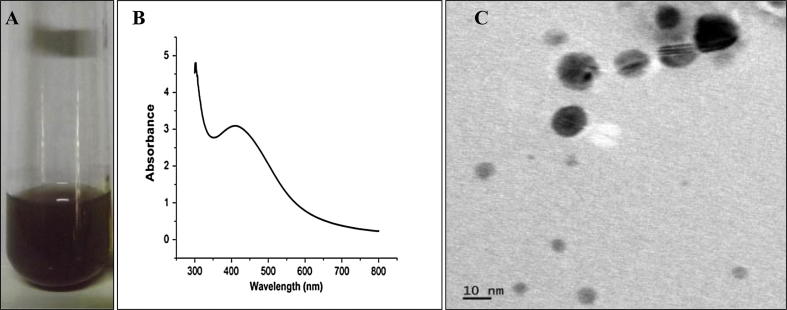
The formation of Ag-NPs by bacteria was indicated by change of color of treated solution to brown within six hours of incubation at room temperature and the UV–visible spectrum purified from Ag-NPs showed an absorption peak at 420 nm, corresponding to the surface Plasmon resonance band of silver nanoparticles. Further, HR-TEM analysis confirmed the presence of spherical shaped Ag-NPs with size range of 8–21 nm (Fig. 1). These showed the consistent ability of the selected organism to form Ag-NPs as the same features were observed in our previous report on Ag-NPs synthesis using the same organism (Thomas et al., 2014).
Figure 1.
Biosynthesis and confirmation of Ag-NPs. A – Visual observation; B – UV–Vis absorption spectrum; C – TEM images of biosynthesized AgNPs.
3.1. Effect of the Ag-NPs on plant growth
For studying the effect of the Ag-NPs on the growth, Fenugreek (Trigonella foenum) seedlings were treated with Ag-NPs at a final concentration of 0.2 mg/seedling for each treatment. After treatment, the growth parameters such as leaf number, root length, shoot length and wet weight were studied. The seedling treated with the Ag-NPs showed the significantly high values in most of the growth parameters (Fig. 2). When treated with the Ag-NPs the plantlet showed the highest mean leaf number of 1.36 ± 0.23 whereas in the case of control was 1.03 ± 0.06. The root length of the plants after treatment was 1.73 ± 0.08 cm while the same for control was 0.86 ± 0.18 cm. In the case of shoot length, it was 6.03 ± 0.45 cm for Ag-NPs treated seedlings but that of control was 3.09 ± 0.37 cm. This proved the efficiency of Ag-NPs to promote the growth of the seedlings selected for the study. The wet weight of the treated plants was also analyzed which revealed the Ag-NP treated seedlings with a biomass value of 0.38 ± 0.089 g while that of control with 0.12 ± 0.072 g. All parameters of the treatment and control were subjected to statistical analysis for testing the significance in the variance with control using SPSS Software and found to be significant at 0.05 level.
Figure 2.
Plantlets showing plant growth enhancement when treated with the Ag-NPs along with control.
Most reports focus on the adverse effect of the nanoparticle treatment at higher concentrations. However there is significantly positive response of the plants on the presence of lower concentration of the nanoparticles. Studies by Syu et al. (2014) have reported the effect of Ag-NPs with different morphology and size on root growth promotion in Arabidopsis. There is also report on cells treated with Ag-NPs to have up-regulation in the expression of genes involved in ABA signaling pathway, IAA biosynthesis, etc. (Arase et al., 2012). Treatment of AgNP in Eruca sativa suggests the promotion of root growth and induced expression of Jacalin-related lectin (JAL) gene which is involved in the release of precursor of auxin synthesis (nitrile) (Vannini et al., 2013). The effect of silver metal nanoparticles on the growth and antioxidant status of Brassica juncea seedlings has also showed enhanced fresh weight, root and shoot length, and vigor index of seedlings. The Ag-NPs have also been found to be capable of inducing the seedling growth with increase up to 326% in root length and 133% in vigor index (Sharma et al., 2012). All these results suggest role of Ag-NP to interfere with multiple pathways in plant cells to influence plant growth. As role of Ag-NPs on secondary metabolite modulation is least investigated, the results of the current study open up new avenues to enhance medicinal natural product biosynthesis and hence is novel in its approach.
3.2. HPLC based quantification of diosgenin
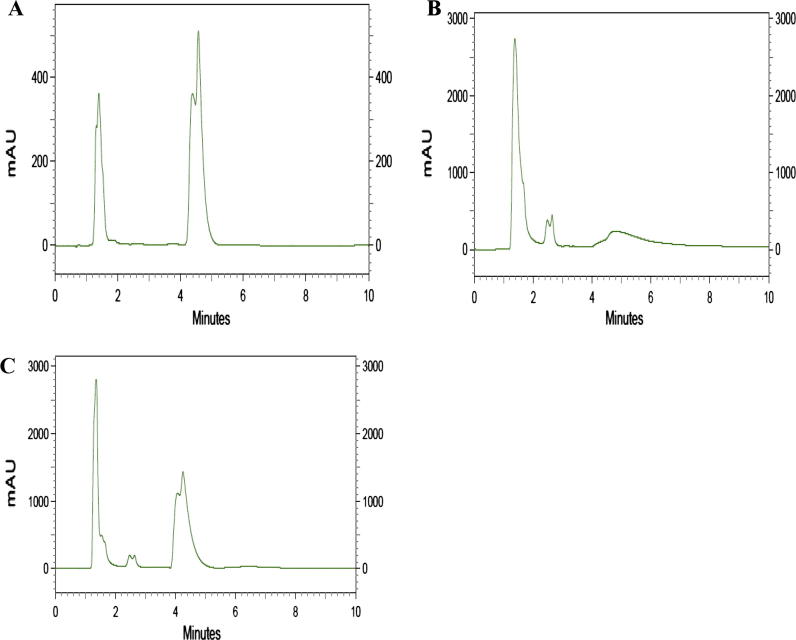
The diosgenin content of the treated as well as the control seedlings was analyzed for studying the effect of Ag-NPs on diosgenin biosynthesis. For this, the seedlings used were dried and used for extraction of diosgenin using hexane. The extract obtained was dried, and dissolved in methanol and was then used for quantification of diosgenin using HPLC. The HPLC chromatogram of standard diosgenin and the Ag-NP treated sample showed peak at RT 4.8 (Fig. 3). The concentration of diosgenin present in the treated seedlings was 214.06 ± 17.07 μg/mL and in the case of untreated (control) it was 164.49 ± 7.67 μg/mL. Elevation of biosynthesis of diosgenin in cell suspension cultures of Dioscorea zingiberensis when treated with the oligosaccharides from Fusarium oxysporum Dzf17 has already been reported (Li et al., 2011). Diosgenin is a sapogenin of the steroidal saponins with diverse biological activities and it plays important role in disease resistance and other growth properties of plant. The observed result is also in agreement with the report on the inducing effect of Ag-NPs on anthocyanin content of Arabidopsis seedlings (Syu et al., 2014). Priyadarshini et al. (2012) have also suggested a dose dependent induction of plant growth, antioxidant property as well as photosynthesis in Brassica juncea on treatment with Ag-NPs. As the role of Ag-NPs as nanoelicitors of medicinal products in plants is least investigated, the current result is remarkably novel and significant. The Ag-NP used in the study might has activated multiple signaling pathways in fenugreek to result in enhanced diosgenin production. The plant growth promoting effect of the Ag-NP might be due to its effect on ACC and also on production of phytohormones as stated in previous reports (Syu et al., 2014).
Figure 3.
HPLC chromatogram showing the difference in the Diosgenin biosynthesis by the plantlets of Fenugreek (Trigonella foenum) when treated with Ag-NPs. A – Diosgenin standard; B – Control (untreated plantlet); C – Ag-NPs treated plant.
In conclusion, the current study focused on the effect of the biologically synthesized nanoparticles on plant growth and diosgenin content of Fenugreek (Trigonella foenum). The results observed were highly significant as the seedlings treated with the nanoparticles were found to have enhanced growth as well as diosgenin concentration. Enhancement in diosgenin content as observed in the study in the nanoparticle treated plants can be a mechanism to counteract the stress produced by the treatment. But this is of advantage as it results in enhanced diosgenin content. Also this suggests the use of Ag-NPs as elicitors of secondary metabolite in medicinal plants which will be of immense pharmaceutical application. Additionally the effects of nanoparticles on plant growth promoting properties are also very significant.
Acknowledgment
This study was supported by Kerala State Council for Science, Technology and Environment under KSCSTE – SARD Programme support and Department of Biotechnology (DBT), Government of India under DBT-MSUB support scheme (BT/PR4800/INF/22/152/2012 Dtd 23.03.2012). The authors also acknowledge Dr. Jayachandran K, Associate Professor, School of Biosciences, Mahatma Gandhi University, PD Hills PO, Kottayam, India, and Principal Investigator, Kerala Biotechnology Commission – Young Investigators Programme in Biotechnology (YIPB) programme for the help and support in performing the HPLC analysis.
Footnotes
Peer review under responsibility of King Saud University.
References
- Acharya S.N., Thomas J.E., Basu S.K. Fenugreek, an alternative crop for semiarid regions of North America. Crop Sci. 2008;48:841–853. [Google Scholar]
- Arase F., Nishitani H., Egusa M., Nishimoto N., Sakurai S., Sakamoto N., Kaminaka H. IAA8 involved in lateral root formation interacts with the TIR1 auxin receptor and ARF transcription factors in Arabidopsis. PLoS ONE. 2012;7:e43414. doi: 10.1371/journal.pone.0043414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind R., Kumar A., Eapen S.J., Ramana K.V. Endophytic bacterial flora in root and stem tissues of black pepper (Piper nigrum L.) genotype: isolation, identification and evaluation against Phytophthora capsici. Lett. Appl. Microbiol. 2009;48:58–64. doi: 10.1111/j.1472-765X.2008.02486.x. [DOI] [PubMed] [Google Scholar]
- Das V., Thomas R., Varghese R., Soniya E.V., Mathew J., Radhakrishnan E.K. Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. 3 Biotech. 2014;4:121–126. doi: 10.1007/s13205-013-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán N., Marcato P.D., De Souza G.I.H., Alves O.L., Esposito E. Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J. Biomed. Nanotechnol. 2007;3:203–208. [Google Scholar]
- Harris A., Bali R. On the formation and extent of uptake of silver nanoparticles by live plants. J. Nanoparticle Res. 2008;10:691–695. [Google Scholar]
- Jasim B., Geethu P.R., Mathew J., Radhakrishnan E.K. Effect of endophytic Bacillus sp. from selected medicinal plants on growth promotion and diosgenin production in Trigonella foenum-graecum. Plant Cell, Tissue Organ Cult. 2015;122:565–572. [Google Scholar]
- Jasim B., John Jimtha C., Jyothis M., Radhakrishnan E.K., Jimtha C.J. Plant growth promoting potential of endophytic bacteria isolated from Piper nigrum. Plant Growth Regul. 2013;71:1–11. [Google Scholar]
- Li P., Mao Z., Lou J., Li Y., Mou Y., Lu S., Peng Y., Zhou L. Enhancement of diosgenin production in Dioscorea zingiberensis cell cultures by oligosaccharides from its endophytic fungus Fusarium oxysporum Dzf17. Molecules. 2011;16:10631–10644. doi: 10.3390/molecules161210631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnuff M.A., Omoruyi F.O., Morrison E.Y.S.t., Asemota H.N. Plasma and liver lipid distributions in streptozotocin-induced diabetic rats fed sapogenin extract of the Jamaican bitter yam (Dioscorea polygonoides) Nutr. Res. 2002;22:1427–1434. [Google Scholar]
- Nowack B., Bucheli T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007;150:5–22. doi: 10.1016/j.envpol.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Priyadarshini K.A., Murugan K., Panneerselvam C., Ponarulselvam S., Hwang J.S., Nicoletti M. Biolarvicidal and pupicidal potential of silver nanoparticles synthesized using Euphorbia hirta against Anopheles stephensi Liston (Diptera: Culicidae) Parasitol. Res. 2012;111:997–1006. doi: 10.1007/s00436-012-2924-8. [DOI] [PubMed] [Google Scholar]
- Savithramma, N., Ankanna, S., Bhumi, G., 2012. Effect of nanoparticles on seed germination and seedling growth of Boswellia ovalifoliolata – an endemic and endangered medicinal tree taxon. Nano Vision 2 2, 61–68.
- Sharma P., Bhatt D., Zaidi M.G.H., Saradhi P.P., Khanna P.K., Arora S. Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotechnol. 2012;167:2225–2233. doi: 10.1007/s12010-012-9759-8. [DOI] [PubMed] [Google Scholar]
- Syu Y., Hung J.-H., Chen J.-C., Chuang H. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol. Biochem. 2014;83:57–64. doi: 10.1016/j.plaphy.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Thomas R., Nair A., Soumya K.R., Mathew J., Radhakrishnan E.K. Antibacterial activity and synergistic effect of biosynthesized AgNPs with antibiotics against multidrug-resistant biofilm-forming coagulase-negative staphylococci isolated from clinical samples. Appl. Biochem. Biotechnol. 2014;173:449–460. doi: 10.1007/s12010-014-0852-z. [DOI] [PubMed] [Google Scholar]
- Thomas R., Soumya K.R., Mathew J., Radhakrishnan E.K. Inhibitory effect of silver nanoparticle fabricated urinary catheter on colonization efficiency of Coagulase Negative Staphylococci. J. Photochem. Photobiol. B Biol. 2015;149:68–77. doi: 10.1016/j.jphotobiol.2015.04.034. [DOI] [PubMed] [Google Scholar]
- Vannini C., Domingo G., Onelli E., Prinsi B., Marsoni M., Espen L., Bracale M. Morphological and proteomic responses of Eruca sativa exposed to silver Nanoparticles or silver nitrate. PLoS ONE. 2013;8:e68752. doi: 10.1371/journal.pone.0068752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zea L., Salama H.M.H. Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn. Int. Res. J. Biotechnol. 2012;3:190–197. [Google Scholar]