Abstract
The metabolism of mannose by human erythrocytes has been investigated. Phosphorylation of mannose is achieved by an enzyme with electrophoretic mobility on starch gel indistinguishable from the glucose-phosphorylating enzyme. Mannose phosphorylation is competitively inhibited by glucose; glucose phosphorylation is competitively inhibited by mannose. The Ki values of inhibition are similar to the Km values for uninhibited phosphorylation. The normal average mannose-phosphorylating activity was found to be 0.69 U/g of Hb; the normal average glucose-phosphorylating activity was found to be 0.64 U/g of Hb. The ratio of mannose-phosphorylating activity to glucose-phosphorylating activity of a hemolysate prepared from the red cells of a subject with hexokinase deficiency was found to be within the normal range.
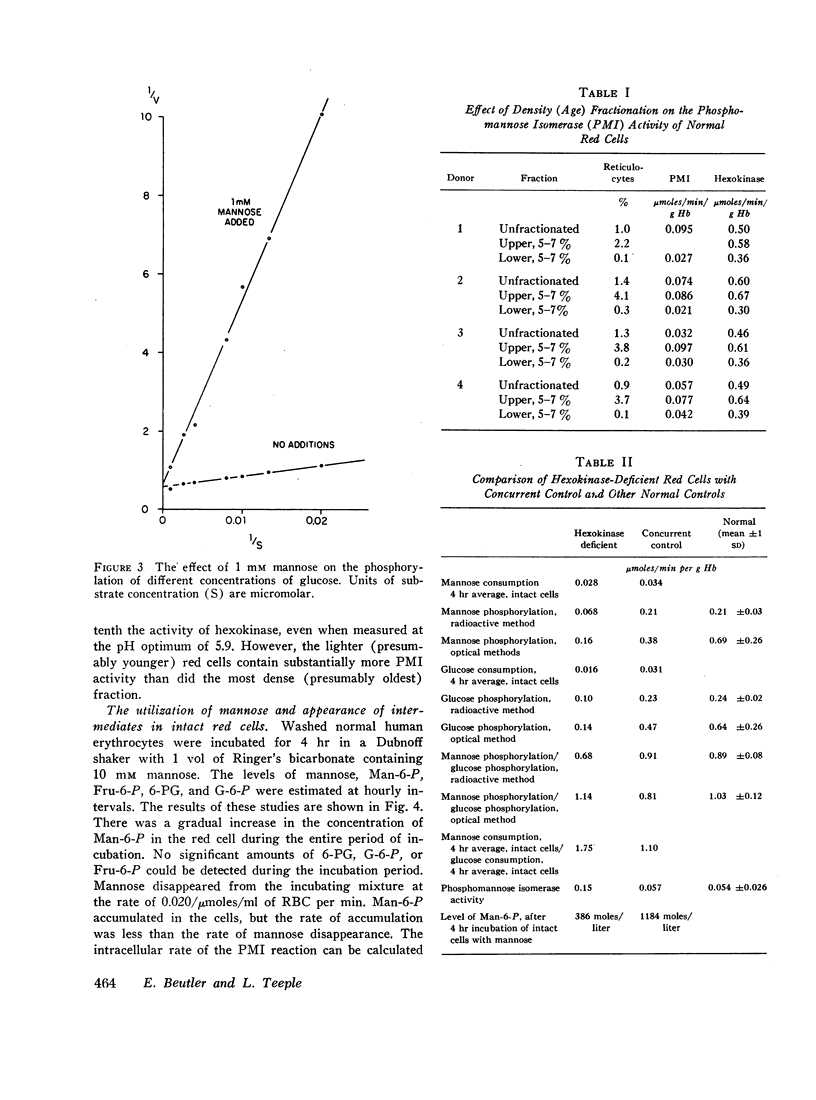
Phosphomannose isomerase (PMI) activity of the red cells was found to average 0.064 U/g of Hb at its pH optimum of 5.9 with a mannose-6-phosphate (Man-6-P) concentration of 5 mmoles/liter. The enzyme activity in young cells was greater than activity in old cells.
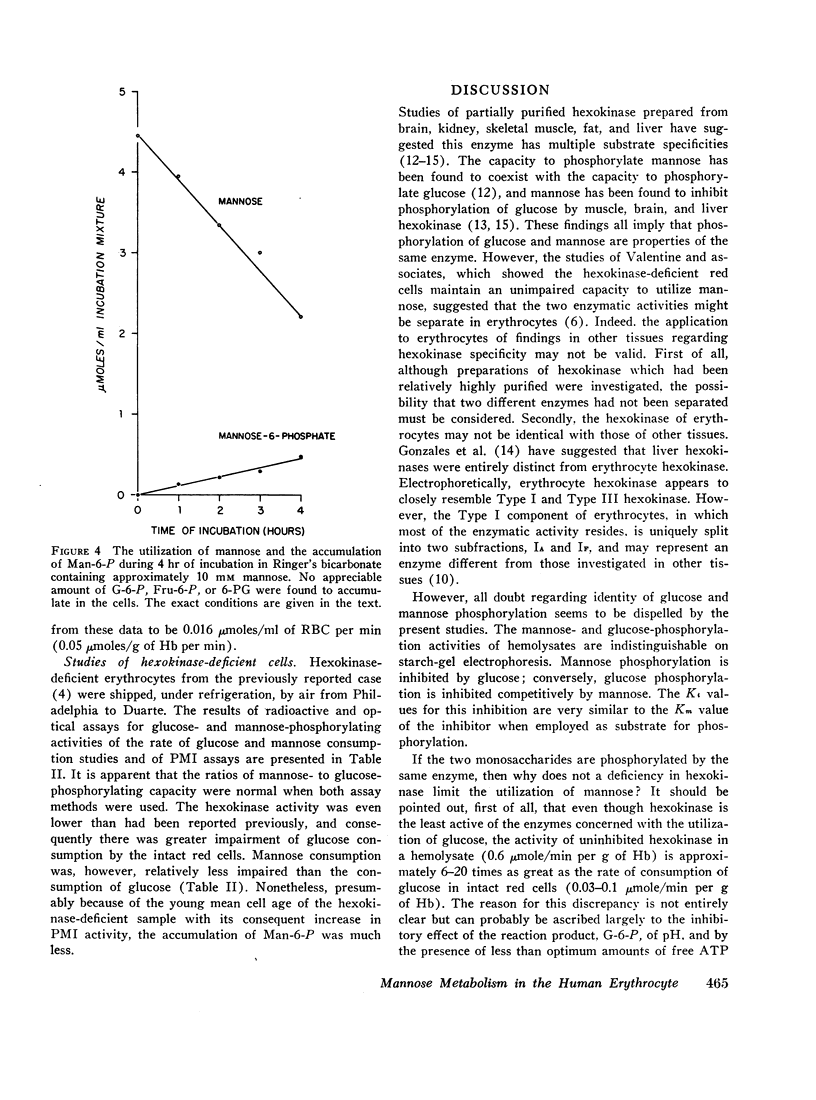
When human erythrocytes are incubated with mannose rapid accumulation of Man-6-P occurs, a finding indicating that PMI and not hexokinase is the limiting enzyme in the over-all conversion of mannose to fructose by the red cell. The ratio of mannose utilization to glucose utilization in hexokinase-deficient cells was greater than normal, as has been reported previously. These cells were found to have greatly increased PMI activity, presumably because of their young mean cell age. Consequently, Man-6-P accumulated only approximately one-third as rapidly as normal in hexokinase-deficient cells incubated with mannose. It is believed that the more rapid utilization of mannose relative to glucose by intact hexokinase-deficient cells may be explained on the basis of the regulatory effect of the PMI reaction on the rate of mannose utilization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUNS F. H., NOLTMANN E. Phosphomannoisomerase, an SH-dependent metalenzyme complex. Nature. 1958 May 24;181(4621):1467–1468. doi: 10.1038/1811467a0. [DOI] [PubMed] [Google Scholar]
- Beutler E., Duron O. Studies on blood preservation. The relative capacities of hexoses, hexitols, and ethanol to maintain red cell ATP levels during storage. Transfusion. 1966 Nov-Dec;6(6):537–542. doi: 10.1111/j.1537-2995.1966.tb04776.x. [DOI] [PubMed] [Google Scholar]
- DANON D., MARIKOVSKY V. DETERMINATION OF DENSITY DISTRIBUTION OF RED CELL POPULATION. J Lab Clin Med. 1964 Oct;64:668–674. [PubMed] [Google Scholar]
- González C., Ureta T., Babul J., Rabajille E., Niemeyer H. Characterization of isoenzymes of adenosine triphosphate: D-hexose 6-phosphotransferase from rat liver. Biochemistry. 1967 Feb;6(2):460–468. doi: 10.1021/bi00854a014. [DOI] [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- Hanson T. L., Fromm H. J. Rat skeletal muscle hexokinase. II. Kinetic evidence for a second hexokinase in muscle tissue. J Biol Chem. 1967 Feb 10;242(3):501–508. [PubMed] [Google Scholar]
- Kaplan J. C., Beutler E. Hexokinase isoenzymes in human erythrocytes. Science. 1968 Jan 12;159(3811):215–216. doi: 10.1126/science.159.3811.215. [DOI] [PubMed] [Google Scholar]
- SHERMAN J. R., ADLER J. Galactokinse from Escherichia coli. J Biol Chem. 1963 Mar;238:873–878. [PubMed] [Google Scholar]
- SOLS A., CRANE R. K. Substrate specificity of brain hexokinase. J Biol Chem. 1954 Oct;210(2):581–595. [PubMed] [Google Scholar]
- Sapico V., Anderson R. L. An adenosine 5'-triphosphate:hexose 6-phosphotransferase specific for D-mannose and D-fructose from Leuconostoc mesenteroides. Purification, properties, and evidence for a single enzyme. J Biol Chem. 1967 Nov 10;242(21):5086–5092. [PubMed] [Google Scholar]
- Valentine W. N., Oski F. A., Paglia D. E., Baughan M. A., Schneider A. S., Naiman J. L. Hereditary hemolytic anemia with hexokinase deficiency. Role of hexokinase in erythrocyte aging. N Engl J Med. 1967 Jan 5;276(1):1–11. doi: 10.1056/NEJM196701052760101. [DOI] [PubMed] [Google Scholar]



