Abstract
Fluoxetine is an antidepressant, a selective serotonin reuptake inhibitor (SSRI) used primarily in the treatment of major depression, panic disorder and obsessive compulsive disorder. Chiral separation of racemic fluoxetine is necessary due to its enantioselective metabolism. In order to develop a suitable method for chiral separation of fluoxetine, cyclodextrin (CD) modified capillary electrophoresis (CE) was employed. A large number of native and derivatized, neutral and ionized CD derivatives were screened to find the optimal chiral selector. As a result of this process, heptakis(2,3,6-tri-O-methyl)-β-CD (TRIMEB) was selected for enantiomeric discrimination. A factorial analysis study was performed by orthogonal experimental design in which several factors are varied at the same time to optimize the separation method. The optimized method (50 mM phosphate buffer, pH = 5.0, 10 mM TRIMEB, 15 °C, + 20 kV, 50 mbar/1 s, detection at 230 nm) was successful for baseline separation of fluoxetine enantiomers within 5 min. Our method was validated according to ICH guidelines and proved to be sensitive, linear, accurate and precise for the chiral separation of fluoxetine.
Keywords: Fluoxetine, Selective serotonin reuptake inhibitor, Capillary electrophoresis, Chiral separation, Cyclodextrines
1. Introduction
Fluoxetine ((±)-N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine) (Fig. 1) is a widely marketed selective serotonin (5-hydroxytryptamine) reuptake inhibitor (SSRI) used in the treatment major depressive disorder, obsessive-compulsive disorder, panic disorder, bulimia nervosa and premenstrual dysphoric disorder (Cheer and Goa, 2001).
Figure 1.

Fluoxetine chemical structure (* denotes the chiral center).
Fluoxetine has a chiral center in its structure resulting into the existence of two enantiomers, R-fluoxetine and S-fluoxetine.
The two enantiomers of fluoxetine are similarly effective in blocking serotonin reuptake. However these enantiomers are metabolized differently. Fluoxetine is extensively metabolized by cytochrome P450 enzyme system through demethylation into the active chiral metabolite norfluoxetine, allowing a more prolonged biological action of the drug. R-fluoxetine and S-fluoxetine have different metabolic rates, as the clearance of R-fluoxetine is about four times greater than the one of S-fluoxetine; these differences translate into differences in half-life, the half-life of S-fluoxetine being one quarter than that of R-fluoxetine. In the case of norfluoxetine only the S-enantiomer has similar potency as the parent drug (Brosen, 1998, Sproule et al., 1997).
The use of R-enantiomer was expected to result in less variable plasma levels of fluoxetine and its active metabolites compared to those observed with racemic fluoxetine, but the clinical development of R-fluoxetine for the treatment of depression was stopped because of a small but statistically significant prolongation of the QT interval with high doses (McConathy and Owens, 2003).
Taking into consideration the aspects presented above the elaboration of new methods for the enantioseparation of fluoxetine represents a necessity and also a challenge.
Capillary electrophoresis (CE) has become an interesting alternative, but also complementary to the more frequently used high performance liquid chromatographic (HPLC) methods, with advantages related to the low solvent and analyte consumption, short analysis time, rapid method development and high selectivity. Enantioseparations by CE are achieved by direct addition of chiral selectors, into the background electrolyte (BGE); as the enantioseparation takes place due to the different non-covalent molecular interaction of the enantiomers with a chiral selector, whose electrophoretic mobility is different to that of the enantiomers (Amini, 2011, Chankvetadze, 2007).
The most frequently additives used are cyclodextrins (CDs) because they are commercially available, UV-transparent and relatively low cost. CDs can form complexes with molecules based on their inclusion into the hydrophobic cavity; secondary interactions may include hydrogen bonding or dipole–dipole interactions with the hydroxyl groups on the CDs, or with other polar substituents of the CDs (Rezanka et al., 2014).
In chiral CE the host-guest complexation between the CD and the enantiomers is responsible for the enantioresolution and the electrophoresis and electroosmosis permit differential migrations of the host-guest complexes (Dubsky et al., 2010).
The analytical methods used for the determination of fluoxetine are mainly HPLC methods with UV (Gatti et al., 2003), fluorescence (Guo et al., 2003) or mass spectrometry (Sutherland et al., 2001) detection; enantioseparation being done using indirect methods such as derivatization (Guo et al., 2003) and direct methods using CDs (Yu et al., 2002) or chiral stationary phases (Yu et al., 2006).
Few reports on the application of CE methods for separation of fluoxetine enantiomers were found in the literature. These methods present usually the enantioseparation in acidic condition using negatively charged CDs or combined neutral and negatively charged CDs. A systematic approach to enantiomeric separations in CE and HPLC with chiral mobile phase additives or a chiral stationary phase was described in a study of fluoxetine and norfluoxetine with CDs as chiral selectors (Piperaki et al., 1995). High detection sensitivity CE was used for the stereoselective analysis of fluoxetine and norfluoxetine in plasma and serum samples using a CD-modified phosphate buffer at pH 2.5 and a dual CD system containing dimethylated-β and phosphated-γ-CDs (Desiderio et al., 1999). A complex screening including 11 neutral and charged CDs was carried out for the enantioseparation of fluoxetine and four of its structural analogs; several negatively charged CDs showed enantioresolution abilities at pH 2.5 due to the high electrophoretic mobility of these CDs in the opposite direction to fluoxetine as well as due to the enhanced binding with positively charged fluoxetine (Inoue and Chang, 2003). In order to prevent the absorption of the basic compound fluoxetine on the negatively charged capillary wall in low pH buffer and to improve enantioresolution, guanidine as cationic additive can be added to a phosphate buffer containing sulfobuthylether-β-CD at pH 2.5 (Javid et al., 2013). An electrokinetic chromatography–counter current procedure for the separation of fluoxetine enantiomers using a phosphate buffer at pH 8.0 and highly sulfated β-CD was developed and applied to the determination of the enantiomers in pharmaceutical formulations (Asensi-Bernardi et al., 2013). Sulfated maltodextrin as a novel anionic chiral selector was used as an alternative to CDs for the separation of several basic drugs including fluoxetine (Tabani et al., 2015).
The aim of the study was the development of a new, simple and rapid alternative method for the chiral separation of fluoxetine enantiomers using a CZE method and CD as chiral selectors; and also the optimization of analytical conditions and validation of the newly developed method according to ICH guidelines. For the optimization process we used an experimental design approach, a methodology of experimental research in which the variables under study are simultaneously changed inside an experiment; these strategy being aimed to guide the researcher in selecting regions of interest inside a large experimental region, with a minimum number of experiments (Orlandini et al., 2014).
2. Materials and methods
2.1. Chemicals and reagents
R,S-fluoxetine and S-fluoxetine of pharmaceutical grade were acquired from Solmag (Mulazzano, Italy). Phosphoric acid (85%), disodium hydrogenophosphate, and sodium dihydrogenophosphate were purchased from Merck (Darmstadt, Germany), and sodium hydroxide was from Lach Ner (Neratovice, Czech Republic). Deionized water was produced by a Milli-Q system (Millipore, USA). All reagents were of analytical grade.
The following CDs were used as chiral additives: native neutral CDs (β-CD, γ-CD), derivatized neutral CDs (hydroxypropyl-β-CD – HP-β-CD, randomly methylated β-CD – RAMEB, heptakis (2,6-di-O-methyl)-β-CD - DIMEB, heptakis(2,3,6-tri-O-methyl)- β-CD - TRIMEB), derivatized ionizable CDs (carboxymethyl-β-CD - CM-β-CD, sulfobuthylether-β-CD SBE-β-CD). All CDs were obtained from Cyclolab (Budapest, Hungary) with the exception of SBE-β-CD – (Capsitol®) which was obtained from Cydex Corp. (USA).
For the determination from pharmaceutical products, Prozac (Eli Lilly, USA) and Fluoxin (VimSpectrum, Romania) capsules containing 20 mg fluoxetine were used. The pharmaceutical preparations were purchased from a local pharmacy.
2.2. Instrumentation
All experiments were carried out on an Agilent 1600 CE system (Agilent Technologies, Waldbronn, Germany) equipped with a diode array UV detector. Separations were performed on an uncoated fused-silica capillary with a total length of 48 cm (40 cm effective length), having an internal diameter of 50 um (Agilent, Germany). The electropherograms were recorded and processed by Chemstation 7.01 software (Agilent, Germany). Buffer pH was determined using a Terminal 740 pH–meter (Inolab, Germany).
2.3. Electrophoretic conditions
The capillary was conditioned with 0.1 M NaOH for 30 min, purified water for 15 min and BGE for 15 min. Between runs, the capillary was preconditioned with 0.1 M NaOH, purified water and buffer electrolyte, each for 2 min.
BGE solutions were prepared dissolving the appropriate amount of buffer constituents in ultrapure water and adjusting the pH if necessary with 1 M phosphoric acid or 1 M NaOH.
Stock solutions containing 1 mg mL−1 of the racemic fluoxetine were prepared in methanol, and diluted prior to use with the same solvent to the appropriate concentration.
Both BGE and sample solutions were filtered through a 0.45 μm pore size membrane filter and degassed in an ultrasonic bath for 5 min prior to use. All solutions were kept in the refrigerator when not in use.
Samples and standards were injected by hydrodynamic injection (50 mbar for 1 s) at the anodic end of the capillary. The initial electrophoretic conditions were the following: voltage +20 kV, capillary temperature 25 °C, detector wavelength 230 nm, sample concentration 25 μg mL−1.
2.4. Pharmaceutical sample preparation
The content of ten capsules was weighed, ground and mixed in a mortar. Appropriate amount of the powder (equivalent to 20 mg of racemic fluoxetine) was taken and dissolved in 10 mL methanol, sonicated in a ultrasonic water bath for 5 min and then diluted to 100 mL with methanol in a volumetric flask. The solutions were further diluted with methanol to the appropriate concentration before being introduced in the CE system for the separation.
3. Results and discussions
3.1. Preliminary analysis
Fluoxetine is a basic analyte containing tertiary amine nitrogen, with a pKa value of 9.80, and consequently is positively charged over a relatively large range of pH (acidic and neutral pHs).
In order to establish the electrophoretic behavior of the analyte in an achiral system, different phosphate BGEs in a pH range between 2.5 and 11.0 were used, in a 25 mM concentration. Fluoxetine can be detected over a pH range between 2.5 and 7.0.
Initial electrophoretic separations were performed at three pH levels: 2.5, 5.0 and 7.0. During the CD screening process, initial concentration of 10 mM neutral CDs was added to the buffer solution, while for charged CDs we added a concentration of 5 mM in order to limit the increase in ionic strength which generated high currents and subsequent peak broadening.
The only CD that exhibited chiral interactions with fluoxetine was TRIMEB, a derivatized neutral CD. The best results were obtained when using a phosphate buffer at pH 5.0.
The results were evaluated in terms of separation factors (α) calculated as the ratio of the migration times of the optical isomers, and resolution (Rs) obtained by the Rs = 2(t2 − t1)/(w1 + w2) equation, where the migration times (t1 and t2) and the peak-widths (w1 and w2) were marked for the slow and fast migrating enantiomers, respectively.
3.2. Optimization of the analytical conditions
In order to improve the separation resolution of the enantiomers, an optimization of the developed method is crucial.
Conventional method optimization is based on changing one variable at a time, while keeping the other variables constant; this meticulous approach is the so-called “one-factor-at-a-time” approach, and involves a large number of individual experiments and consumes a long time; therefore, the simultaneous variation of several factors has recently become widespread (Hanrahan et al., 2008).
The multivariate approach usually leads to optimal conditions in a relatively reduced number of experimental runs and offers more comprehensive information on the separation system, making possible to detect also possible interactions between the studied factors.
Orthogonal experimental designs provide a simple and efficient way to screen a large number of factors in a reduced number of experimental runs in order to differentiate between significant and less important experimental variables (Orlandini et al., 2014).
In order to optimize the chiral separation of fluoxetine, an L18 (63) orthogonal array table was employed, where 18 is the total number of experiments, which results from the variation of 6 experimental factors at 3 different levels. The factors selected for optimization were the following: BGE concentration (25, 50, 100 mM), BGE pH (4.5, 5.0, 5.5), CD concentration (5, 10, 15 mM), applied voltage (15, 20, 25 kV), temperature (15, 20, 25 °C), injection parameters (50 mbar × 1 s, 50 mbar × 3 s, 30 mbar × 5 s). As response factor, resolution values were recorded in each experiment. The rank order of the studied variables was as follows: BGE pH, capillary temperature, CD concentration, BGE concentration, applied voltage, injection parameters (Table 1).
Table 1.
Orthogonal experimental design table for chiral method optimization with corresponding resolution values.
| Experiment | BGE conc. [mM] | BGE pH | CD conc. [mM] | Voltage [kV] | Temperature [°C] | Injection parameters | Rs |
|---|---|---|---|---|---|---|---|
| 1 | 25 | 4.5 | 5 | 15 | 15 | 50 mbar × 1 s | 1.05 |
| 2 | 50 | 5.0 | 10 | 20 | 15 | 30 mbar × 5 s | 1.80 |
| 3 | 100 | 5.5 | 15 | 25 | 15 | 50 mbar × 3 s | 1.62 |
| 4 | 50 | 5.5 | 5 | 15 | 20 | 30 mbar × 5 s | 1.24 |
| 5 | 100 | 4.5 | 10 | 20 | 20 | 50 mbar × 3 s | 1.20 |
| 6 | 25 | 5.0 | 15 | 25 | 20 | 50 mbar × 1 s | 1.48 |
| 7 | 100 | 5.0 | 10 | 15 | 25 | 50 mbar × 1 s | 1.28 |
| 8 | 25 | 5.5 | 15 | 20 | 25 | 30 mbar × 5 s | 1.25 |
| 9 | 50 | 4.5 | 5 | 25 | 25 | 50 mbar × 3 s | 1.01 |
| 10 | 50 | 5.0 | 15 | 15 | 15 | 50 mbar × 3 s | 1.77 |
| 11 | 100 | 5.5 | 5 | 20 | 15 | 50 mbar × 1 s | 1.30 |
| 12 | 25 | 4.5 | 10 | 25 | 15 | 30 mbar × 5 s | 1.23 |
| 13 | 25 | 5.5 | 10 | 15 | 20 | 50 mbar × 3 s | 1.20 |
| 14 | 50 | 4.5 | 15 | 20 | 20 | 50 mbar × 1 s | 1.37 |
| 15 | 100 | 5.0 | 5 | 25 | 20 | 30 mbar × 5 s | 1.54 |
| 16 | 100 | 4.5 | 15 | 15 | 25 | 30 mbar × 5 s | 1.09 |
| 17 | 25 | 5.0 | 5 | 20 | 25 | 50 mbar × 3 s | 1.32 |
| 18 | 50 | 5.5 | 10 | 25 | 25 | 50 mbar × 1 s | 1.30 |
| Q1 | 1.26 | 1.15 | 1.24 | 1.27 | 1.46 | 1.29 | |
| Q2 | 1.41 | 1.51 | 1.43 | 1.37 | 1.33 | 1.35 | |
| Q3 | 1.33 | 1.31 | 1.33 | 1.36 | 1.20 | 1.35 | |
| R | 0.15 | 0.36 | 0.19 | 0.09 | 0.26 | 0.06 |
Q1–Q3: the average resolution value under every level of the variable (Q1 – low level; Q2 – medium level; Q3 – high level).
R: range value, the difference between the maximal and minimal value of the three levels for each parameter.
Since baseline resolution was achieved and variance analysis (ANOVA) of the experimental results revealed that none of the investigated parameters had individually significant effect on resolution values in the investigated range, further optimization was not necessary.
The pH of the BGE plays an important role in determining the extent of ionization of the analyte in the separation process, affecting the electrophoretic mobility of the substance and also the magnitude of EOF.
An increase in the ionic strength of the BGE will generate longer migration time due to slower EOF as well as the reduction in electrophoretic mobility of the ionic analyte. Working with high ionic strength buffers leads to better chiral resolution but on the other hand at certain concentration the heat dissipation ability of the separation system will be exceeded.
Increasing the voltage results in shorter migration times, but generation of Joule heat affects resolution and efficiency when the voltage is increased.
As the temperature increases, both analysis time and chiral resolution decrease, due to lower viscosity of the BGE.
By increasing injection time, the sensitivity increases, but the chiral resolution decreases due to peak broadening.
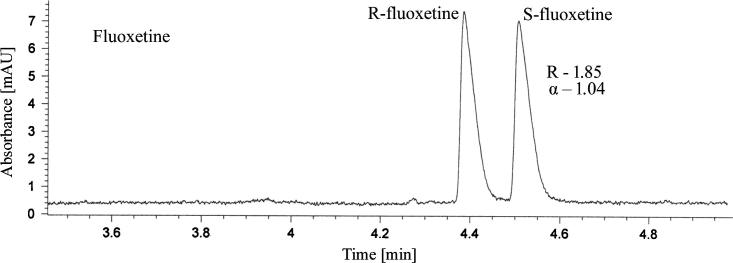
Based on the results of the experimental design, the optimal resolution would be achieved with a BGE consisting of 50 mM phosphate, pH = 5.0, 10 mM TRIMEB as chiral selector, at a temperature of 15 °C, voltage +20 kV, injection parameters 50 mbar/1 s. Adopting these optimized conditions, baseline enantiomeric separation of fluoxetine was achieved, with a Rs value of 1.90 and α of 1.04 (Fig. 2).
Figure 2.
Chiral separation of fluoxetine enantiomers in optimized conditions (experimental conditions: 50 mM phosphate, pH = 5.0, 10 mM TRIMEB, temperature 15 °C, voltage +20 kV, hydrodynamic injection 50 mbar/1 s, UV detection 230 nm).
The migration order of the two enantiomers was determined by spiking the sample solution with the stock solution of the pure S-enantiomer. The first peak to pass the detector window was determined to be R-fluoxetine followed by S-fluoxetine.
3.3. Analytical performance
The developed method was validated, in terms of repeatability, precision, linearity, sensitivity (limit of detection and limit of quantification) and accuracy.
Intra-day precision was assessed by injecting racemic fluoxetine standards at three different concentrations (10, 25, and 50 μg mL−1) six times on the same day. Moreover, the inter-day precision was verified by introducing three different concentrations of the standard (10, 25, and 50 μg mL−1) six times for three consecutive days (Table 2).
Table 2.
Intra-day and inter-day reproducibility data for repeated injections of different concentrations of racemic fluoxetine standard.
| Factor conc. (μg mL−1) | Relative standard deviation - RSD (%) |
|||
|---|---|---|---|---|
| Migration time (min) |
Peak areas |
|||
| R-fluoxetine | S-fluoxetine | R-fluoxetine | S-fluoxetine | |
| Intra-day precision (n = 6) | ||||
| 10 | 0.03 | 0.03 | 0.57 | 0.64 |
| 25 | 0.07 | 0.08 | 0.74 | 0.82 |
| 50 | 0.14 | 0.16 | 0.75 | 0.83 |
| Inter-day precision (n = 18) | ||||
| 10 | 0.40 | 0.45 | 1.27 | 1.50 |
| 25 | 0.35 | 0.40 | 1.18 | 1.40 |
| 50 | 0.42 | 0.48 | 1.20 | 1.32 |
Calibration plots were constructed by preparing standard solutions (n = 3) at six concentrations in a specific concentration range (concentration range: 2.5–50 μg mL−1) (Table 3).
Table 3.
Calibration data and LOD/LOQ values for fluoxetine chiral separation (concentration range = concentration range: 2.5–50 μg mL−1, n = 3).
| Enantiomers | Regression equation | Correlation coefficient | LOD (μg mL−1) | LOQ (μg mL−1) |
|---|---|---|---|---|
| R-fluoxetine | y = 0.6525x + 2.1230 | 0.995 | 1.69 | 5.63 |
| S-fluoxetine | y = 0.6679x + 1.9803 | 0.994 | 1.77 | 5.9 |
The limit of detection (LOD) and quantification (LOQ) were estimated as standard deviation of regression equation/slope of the regression equation multiplied by 3.3 and 10, respectively (Table 3).
The accuracy of the method was verified through the recovery test; as an appropriate amount of racemic fluoxetine tablet powder was weighed, dissolved in methanol and the solution was spiked with known amount of the standard and each was analyzed in triplicates (Table 4).
Table 4.
Recovery values obtained from the determination of fluoxetine spiked with different levels of standards.
| Racemic fluoxetine (μg mL−1) | Mean recovery (% ±SD) |
|
|---|---|---|
| R-fluoxetine | S-fluoxetine | |
| 10 | 98.62 ± 2.41 | 98.73 ± 2.63 |
| 25 | 99.31 ± 2.70 | 97.12 ± 1.91 |
| 50 | 99.23 ± 2.42 | 98,44 ± 2.34 |
3.4. Analysis from pharmaceutical preparations
The optimized method was applied for the determination of fluoxetine enantiomers in original (Prozac) and generic (Fluoxin) commercial pharmaceutical preparation. Good agreement between the value claimed by the manufacturer and that determined by the CE method was obtained (Table 5). No interference from the drug formulation excipients could be observed on the electropherogram. Fig. 3 shows typical electropherograms of the pharmaceutical preparations.
Table 5.
Determination of fluoxetine enantiomers from pharmaceutical preparations.
| Pharmaceutical product | Declared enantiomer quantity (mg) |
Found enantiomer quantity (mg) ± SD (n = 3) |
||
|---|---|---|---|---|
| R-fluoxetine | S-fluoxetine | R-fluoxetine | S-fluoxetine | |
| Prozac (20 mg fluoxetine) | 10 | 10 | 10.1 ± 0.35 | 9.9 ± 0.32 |
| Fluoxin (20 mg fluoxetine) | 10 | 10 | 10.15 ± 0.31 | 9.85 ± 0.24 |
Figure 3.
Chiral separation of fluoxetine enantiomers from pharmaceutical preparations Prozac and Fluoxin (experimental conditions: 50 mM phosphate, pH = 5.0, 10 mM TRIMEB, temperature 15 °C, voltage +20 kV, hydrodynamic injection 50 mbar/1 s, UV detection 230 nm).
4. Conclusions
A simple, rapid and cost effective CZE method has been developed for the enantioselective determination of fluoxetine enantiomers.
The developed separation system was optimized by employing an orthogonal experimental design; the main advantages of using multivariate approaches being the reduction in number of experiments and statistical data processing in order to find optimal conditions.
In comparison with the HPLC chiral separation methods described before (Guo et al., 2003, Yu et al., 2002) our method has the advantages that it does not require derivatization, expensive chiral columns or large amounts of solvents as mobile phase.
Additionally if we compare our method with the CE methods previously published in the literature, our method uses neutral derivatized CD (TRIMEB) instead of derivatized anionic CD (Inoue and Chang, 2003, Asensi-Bernardi et al., 2013, Javid et al., 2013) or dual CD systems (combination within a neutral and a negatively charged CD) (Desiderio et al., 1999), simple basic electrophoretic conditions offering also a short analysis time (less than 5 min).
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Amini A. Recent developments in chiral capillary electrophoresis and applications of this technique to pharmaceutical and biomedical analysis. Electrophoresis. 2011;22:3107–3130. doi: 10.1002/1522-2683(200109)22:15<3107::AID-ELPS3107>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Asensi-Bernardi L., Martin-Biosca Y., Fornet-Herrero E., Sagrado S., Medina Hernández M.J. Determination of fluoxetine enantiomers in pharmaceutical formulations by electrokinetic chromatography–counter current technique. Biomed. Chromatogr. 2013;27:377–381. doi: 10.1002/bmc.2802. [DOI] [PubMed] [Google Scholar]
- Brosen K. Differences in interactions of SSRIs. Int. Clin. Psychopharmacol. 1998;13:S45–47. doi: 10.1097/00004850-199809005-00009. [DOI] [PubMed] [Google Scholar]
- Chankvetadze B. Enantioseparations by using capillary electrophoretic techniques: the story of 20 and a few more years. J. Chromatogr. A. 2007;1168:45–70. doi: 10.1016/j.chroma.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Cheer S.M., Goa K.L. Fluoxetine: a review of its therapeutic potential in the treatment of depression associated with physical illness. Drugs. 2001;6:81–110. doi: 10.2165/00003495-200161010-00010. [DOI] [PubMed] [Google Scholar]
- Desiderio C., Rudaz S., Raggi M.A., Fanali S. Enantiomeric separation of fluoxetine and norfluoxetine in plasma and serum samples with high detection sensitivity capillary electrophoresis. Electrophoresis. 1999;20:3432–3438. doi: 10.1002/(SICI)1522-2683(19991101)20:17<3432::AID-ELPS3432>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Dubsky P., Svobodova J., Tesarova E., Gas B. Enhanced selectivity in CZE multi-chiral selector enantioseparation systems: proposed separation mechanism. Electrophoresis. 2010;31:1435–1441. doi: 10.1002/elps.200900742. [DOI] [PubMed] [Google Scholar]
- Gatti G., Bonomi I., Marchiselli R., Fattore C., Spina E., Scordo G., Pacifici R., Perucca E. Improved enantioselective assay for the determination of fluoxetine and norfluoxetine enantiomers in human plasma by liquid chromatography. J. Chromatogr. B. 2003;784:375–383. doi: 10.1016/s1570-0232(02)00820-6. [DOI] [PubMed] [Google Scholar]
- Guo X., Fukushima T., Li F., Imai K. Determination of fluoxetine and norfluoxetine in rat plasma by HPLC with pre-column derivatization and fluorescence detection. Biomed. Chromatogr. 2003;17:1–5. doi: 10.1002/bmc.198. [DOI] [PubMed] [Google Scholar]
- Hanrahan G., Montes R., Gomez F. Chemometric experimental design based optimization techniques in capillary electrophoresis. Anal. Bioanal. Chem. 2008;390:169–179. doi: 10.1007/s00216-007-1619-y. [DOI] [PubMed] [Google Scholar]
- Inoue T., Chang J.P. Chiral separation of fluoxetine and its analogs with charged cyclodextrins by capillary electrophoresis. J Liq Chromatogr Rel Technol. 2003;26:2351–2367. [Google Scholar]
- Javid F.S., Shafaati A., Zarghi A. Improvement of capillary electrophoretic enantioseparation of fluoxetine by a cationic additive. Iran J Pharm Res. 2013;12:71–76. [PMC free article] [PubMed] [Google Scholar]
- McConathy J., Owens M.J. Stereochemistry in drug action primary care companion. J. Clin. Psychiatry. 2003;5:70–73. doi: 10.4088/pcc.v05n0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandini S., Gotti R., Furlanetto S. Multivariate optimization of capillary electrophoresis methods: a critical review. J. Pharm. Biomed. Anal. 2014;87:290–307. doi: 10.1016/j.jpba.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Piperaki S., Penn S.G., Goodall D.M. Systematic approach to treatment of enantiomeric separations in capillary electrophoresis and liquid chromatography II. A study of the enantiomeric separation of fluoxetine and norfluoxetine. J. Chromatogr. A. 1995;700:59–67. [Google Scholar]
- Rezanka P., Navratilova K., Rezanka M., Kral V., Sykora D. Application of cyclodextrins in chiral capillary electrophoresis. Electrophoresis. 2014;35:2701–2721. doi: 10.1002/elps.201400145. [DOI] [PubMed] [Google Scholar]
- Sproule B.A., Naranjo C.A., Brenmer K.E., Hassan P.C. Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence. Clin. Pharmacokinet. 1997;33:454–471. doi: 10.2165/00003088-199733060-00004. [DOI] [PubMed] [Google Scholar]
- Sutherland F.C., Badenhorst D., de Jager A.D., Scanes T., Hundt H.K., Swart K.J., Hundt A.F. Sensitive liquid chromatographic-tandem mass spectrometric method for the determination of fluoxetine and its primary active metabolite norfluoxetine in human plasma. J. Chromatogr. A. 2001;914:45–51. doi: 10.1016/s0021-9673(00)01213-9. [DOI] [PubMed] [Google Scholar]
- Tabani H., Mahyari M., Sahragard A., Fakhari A.R., Shaabani A. Evaluation of sulfated maltodextrin as a novel anionic chiral selector for the enantioseparation of basic chiral drugs by capillary electrophoresis. Electrophoresis. 2015;36:305–311. doi: 10.1002/elps.201400370. [DOI] [PubMed] [Google Scholar]
- Yu H.W., Ching C.B., Fu P., Ng S.C. Enantioseparation of fluoxetine on a new β-cyclodextrin bonded phase column by HPLC. Sep. Sci. Technol. 2002;37:1401–1415. [Google Scholar]
- Yu L., Mei-Li F., Jie-Guo X. Enantiomeric separation of fluoxetine derivatives on polysaccharide-based chiral columns. Arch. Pharm. 2006;339:461–465. doi: 10.1002/ardp.200500261. [DOI] [PubMed] [Google Scholar]