Abstract
The isolated active compound “F12” from the culture media of the Streptomyces sp. KH-F12 was identified using different spectroscopic techniques. Both 1D- and 2D-NMR as well as HRESIMS were utilized to characterize the structure of the isolated compound. ‘F12” was found to be the known systemic antifungal drug terbinafine marketed under the name “Lamisil”. Full analysis of the COSY, HSQC and HMBC enables the full assignment of proton and carbon atoms. Terbinafine is a synthetic allylamine and is reported here for the first time from natural source.
Keywords: Streptomyces sp., Structure elucidation, NMR, HRMS, Terbinafine
1. Introduction
Actinomycetes are the source of approximately two-thirds of the discovered microbial bioactive substances (Berdy, 2005, Demain and Sanchez, 2009). The discovery of new antibiotics from Actinomycetes decreased dramatically in the recent years as a result of frequent rediscovery rate of known compounds (Stach, 2010, Subramani and Aalbersberg, 2013) as well as the exhaustion of the common resources (Demain and Sanchez, 2009). However, the American Academy of Microbiology estimated that less than 1% of bacterial species are discovered, and millions of microbes in the environment are yet to be discovered (Cragg and Newman, 2005).
The need for new, more effective and safe antifungal compounds is a major challenge to the pharmaceutical industry (Dharumaduari et al., 2008). Many antifungal agents were discovered from Actinomycetes. Pyrrolomycin A produced from the culture of Actinosporangium vitaminophilum SF-2080 showed broad spectrum antimicrobial activity including antifungal effect (Parry et al., 2011). The monocyclic nitrated antifungal agent ayamycin was isolated from Nocardia sp. (El-Gendy et al., 2008, Parry et al., 2011). The nitrophenyl pyrone aureothin was discovered among other members from Streptomyces spp. (Parry et al., 2011). Another antifungal antimicrobial and antiparasitic compounds bafilomycins were found in Streptomyces spp. (Yu et al., 2011). The antifungal caerulomycin A was reported from both Streptomyces caeruleus and Actinoalloateichus cyanogriseus (Ambavane et al., 2014).
Previously, we reported on the isolation compound designated as F12 from Streptomyces sp. recovered from a soil sample of Adilamm, Riyadh Province (KSA), with promising antifungal activity (Muharram and Abdel-Kader, 2014). In the present work we report on the full characterization of F12.
2. Materials and methods
2.1. General experimental procedures
Ultraviolet absorption spectra were obtained in methanol on a Unicum Heyios α UV-Visible spectrophotometer. Optical rotations were recorded on a Jasco P-2000 Polarimeter. 1H, 13C NMR spectra as well as 2D-NMR experiments (COSY, HSQC and HMBC) were obtained using standard Bruker program on a UltraShield Plus 500 MHz (Bruker) (NMR Unite at the College of Pharmacy, Salman Bin Abdulaziz University) spectrometer operating at 500 MHz for proton and 125 MHz for carbon, respectively. The chemical shift values are reported in δ (ppm) relative to the residual solvent peak, and the coupling constants (J) are reported in Hertz (Hz). HRESIMS was obtained using Agilent 6220 Accurate Mass TOF (Time of Flight) LC/MS Spectrometer (Analytical Services Unite at Department of Chemistry, Virginia Polytechnic Institute and State University). The accurate mass was measured through ESI in the positive mode.
2.2. Sample collection and isolation of actinomycetes
Soil samples were collected from Adilamm (KSA) as previously described (Muharram et al., 2013). The isolate KH-F12 showed promising antifungal activity. Fermentation was carried out in ISP2 broth medium (International Streptomyces Project medium No. 2) also known as Yeast Malt Agar (YM Agar) for 10 days following the previously reported procedures with little modifications (Muharram and Abdel-Kader, 2014). The medium was inoculated with 3 ml/100 ml medium of a pre-culture broth of isolate KH-F12 prepared with the same medium and incubated at 30 °C for 2 days. The cultures were incubated on a rotary shaker (200 rpm) at 30 °C for 10 days. Fermentations were repeated to obtain a total of 5.0 L of culture broth. At the end of the fermentation cycle the culture was filtered and the supernatant was separated by centrifuging at 8000 rpm for 15 min. The supernatant was extracted with CHCl3 (6× 500 ml). The CHCl3 extract was concentrated using rotary vacuum evaporator and the semi solid residue (1.2 g) was subjected to Vacuum Liquid Chromatography (VLC) over RP18 230–400 mesh (80 g, 7 cm i.d.) and elution was started with 100% water, and then water/MeOH mixtures with gradual increase of MeOH content in a gradient elution mode of analysis. All fractions were subjected to antifungal assay. Activity was traced to fractions 11 eluted with 90% MeOH (17 mg) and 12 eluted with 100% MeOH (44 mg). The two fractions were combined after TLC screening and subjected to centrifugal preparative TLC (CPTLC) using Chromatotron (Harrison Research Inc. model 7924), 2 mm silica gel P254 disc and 10% EtOAc in hexane as eluting system. Pure UV active zone was collected and dried to afford 15 mg of pure semisolid compound F-12 with Rf value = 0.47 (Silica gel, 20% EtOAc inn-hexane) (see Fig. 1).
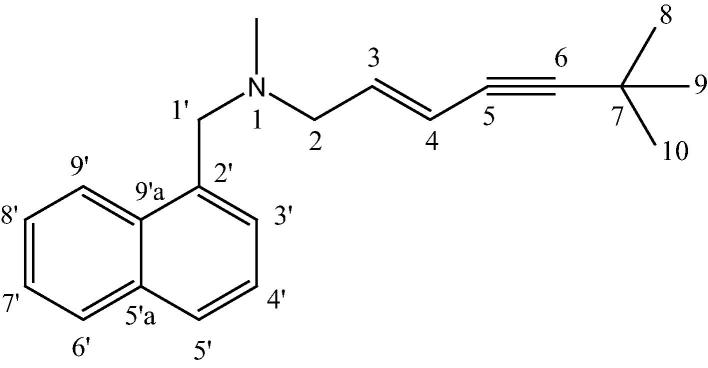
Figure 1.
Structure of “F12”.
2.3. The physical and spectral data of compounds F-12
C21H25N; white semi solid; [α]D = 16.8 (c = 0.0004, CHCl3). UV (CHCl3) 295 (sh), 284, 274, 240 nm. 1H and 13C NMR (CDCl3 and C6D6): Table 1. HRESIMS m/z 292.2065 [M++1] (calc. for [C21H25N + H] 292.2065).
Table 1.
1H NMR and 13C NMR data of F-12 in ppm (multiplicity, J in Hz).a
| Pos. | CDCl3 |
Pos. | C6D6 |
||
|---|---|---|---|---|---|
| 1H NMR | 13C NMR | 1H NMR | 13C NMR | ||
| 2 | 2.97 (d, J = 6.8) | 60.7 | 2 | 2.83 (d, J = 6.5) | 59.2 |
| 3 | 5.90 (dt, J = 6.8, 15.8) | 139.4 | 3 | 5.90 (dt, J = 6.6, 16.0) | 139.4 |
| 4 | 5.59 (d, J = 15.8) | 114.9 | 4 | 5.59 (d, J = 16.0) | 112.9 |
| 5 | – | 78.5 | 5 | – | 77.8 |
| 6 | – | 99.4 | 6 | – | 98.5 |
| 7 | – | 29.0 | 7 | – | 27.8 |
| 8–10 | 1.07 (s, 9H) | 31.7 | 8–10 | 1.20 (s, 9H) | 30.8 |
| 1′ | 3.62 (s, 2H) | 60.5 | 1′ | 3.64 (s, 2H) | 60.1 |
| 2′ | – | 135.4 | 2′ | – | 134.9 |
| 3′ | 7.18 (m) | 129.0 | 3′ | 7.28 (m) | 127.1 |
| 4′ | 7.29 (m) | 127.0 | 4′ | 7.34 (t, J = 7.0) | 125.6 |
| 5′ | 8.32 (d, J = 8.3) | 126.7 | 5′ | 8.32 (d, J = 8.5) | 125.0 |
| 5′a | – | 135.3 | 5′a | – | 134.1 |
| 6′ | 7.64 (d, J = 7.6) | 129.6 | 6′ | 7.66 (d, J = 8.0) | 128.4 |
| 7′ | 7.18 (m) | 125.7 | 7′ | 7.20 (t, J = 7.3) | 125.0 |
| 8′ | 7.29 (m) | 126.8 | 8′ | 7.28 (m) | 125.5 |
| 9′ | 7.56 (d, J = 8.5) | 129.4 | 9′ | 7.59 (d, J = 8.0) | 128.0 |
| 9′a | – | 133.8 | 9′a | – | 132.7 |
| N-CH3 | 1.95 (s, 3H) | 42.6 | N-CH3 | 1.96 (s, 3H) | 41.6 |
Assignments were done based on DEPT, COSY, HSQC and HMBC experiments.
3. Results and discussion
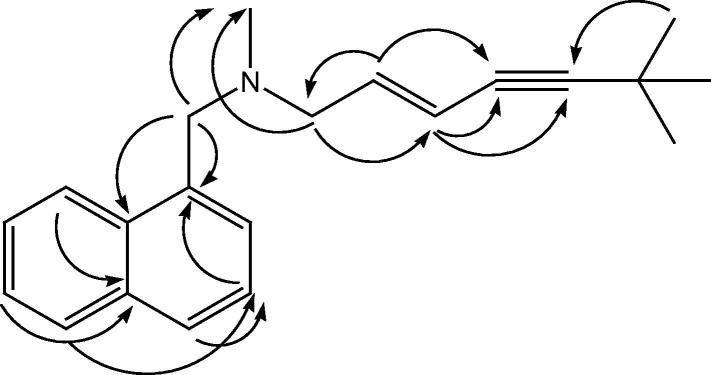
HRESIMS showed an [M++1] at m/z 292.2065 for the molecular formula C21H25N. The 13C NMR spectrum of F-12 in CDCl3 (Table 1) showed 19 signals indicating that 3 carbon atoms were magnetically equivalent. The carbon signal at δC 31.7 ppm correlated with the 1H NMR singlet at δH 1.07 integrated for 9 protons as well as the quaternary carbon signal at δC 29.0 was assigned for a tert-butyl moiety. The singlet at δH 1.07 showed 3 bond correlations with the acetylenic quaternary carbon signal at δC 99.4 ppm in an HMBC experiment (Fig. 2). Two vinylic carbons at δC 139.4 and 114.9 ppm correlated with the proton signals at δH 5.90 (dt)(H3) and 5.59 (d)(H4) respectively. The J value = 15.8 Hz was indicative for the trans orientation between the two protons. The upfield doublet showed HMBC correlation with two acetylenic carbons (Fig. 2). COSY experiment clarified the coupling between the vinylic double triplet with the N-CH2 protons doublet at δH 2.97 ppm. In turn HMBC correlation was observed between the N-CH2 doublet at δH 2.97 ppm and both N-CH3 (δH 1.95 (s), δC 42.64) and N-CH2 (δH 3.62 (s), δC 60.5). The rest of proton and carbon signals in the spectra of F-12 was assigned for mono substituted naphthalene nucleus by careful analysis of the 1H, 13C NMR, COSY, HSQC and HMBC in both CDCl3 and C6D6 (Table 1).
Figure 2.
HMBC correlations of “F-12”.
From the above discussion the structure of “F12” is identical with that of terbinafine. Terbinafine is the first oral antimycotic from the allylamine family discovered in 1983 and approved in 1996 in the US (Abdel-Rahman and Nahata, 1997) and act by inhibiting the synthesis of ergosterol (Ryder and Dupont, 1985). This compound was synthetically developed by Novartis under the trade name Lamisil® (Abdel-Rahman and Nahata, 1997, Lamisil Description – U.S.). However, this is the first report for the discovery of terbinafine from microbial culture medium and may enable the production of this active compound for industry from natural sources.
4. Conclusion
The systemic antifungal allylamine terbinafine developed synthetically by Novartis under the name Lamisil® is now reported for the first time from natural source. The compound was isolated from the culture media of Streptomyces sp. KH-F12 isolated from soil sample collected from Adilamm, KSA. The structure was elucidated utilizing spectroscopic data such as UV, 1D- and 2D-NMR and HRESIMS. Isolation from natural source could share in providing the compound for Pharmaceutical industry.
Acknowledgment
We would like to thank Mr. William Bebout at the Analytical Services Unite at Department of Chemistry, Virginia Polytechnic Institute and State University, for carrying out the HRESIMS and Mr. Younos Nour for his help in preparing different culture media.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Rahman S.M., Nahata M.C. Oral terbinafine: a new antifungal agent. Ann. Pharmacother. 1997;31(4):445–456. doi: 10.1177/106002809703100412. [DOI] [PubMed] [Google Scholar]
- Ambavane V., Tokdar P., Parab R., Sreekumar E.S., Mahajan G., Mishra P.D., D’Souza L., Ranadive P. Caerulomycin A an antifungal compound isolated from Marine Actinomycetes. Adv. Microbiol. 2014;4:567–578. [Google Scholar]
- Berdy J. Bioactive microbial metabolites: a personal view. J. Antibiot. 2005;58(1):1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- Cragg G.M., Newman D.J. Biodiversity: a continuing source of novel drug leads. Pure Appl. Chem. 2005;77:7–24. [Google Scholar]
- Demain A.L., Sanchez S. Microbial drug discovery: 80 years of progress. J. Antibiot. 2009;62:5–16. doi: 10.1038/ja.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharumaduari D., Thajuddin N., Panneerselvam A. An antifungal compound: 4′ phenyl-1-napthyl–phenyl acetamide from Streptomyces spp. DPTB16. F. U. Med. Biol. 2008;15:7–12. doi: 10.1016/j.compbiomed.2012.01.007. [DOI] [PubMed] [Google Scholar]
- El-Gendy M.M., Hawas U.W., Jaspars M. Novel bioactive metabolites from a marine derived bacterium Nocardia sp. ALAA 2000. J. Antibiot. 2008;61(6):379–386. doi: 10.1038/ja.2008.53. [DOI] [PubMed] [Google Scholar]
- Lamisil Description – U.S. Food and Drug Administration. <http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm052213.pdf> (T2001-29 89006802 2295-25-01A) (accessed 20.05.16).
- Muharram M.M., Abdel-Kader M.S., Alqasoumi S.I. Antimicrobial activity of soil actinomycetes Isolated from Alkharj, KSA. Int. Res. J. Microbiol. 2013;4(1):12–20. [Google Scholar]
- Muharram M.M., Abdel-Kader M.S. Taxonomic characterization and chemical study of the antifungal constituents of Streptomyces sp. KH-F12. J. Biol. Sci. 2014;14(6):403–413. [Google Scholar]
- Parry R., Nishino S., Spain J. Naturally-occurring nitro compounds. Nat. Prod. Rep. 2011;28:152–167. doi: 10.1039/c0np00024h. [DOI] [PubMed] [Google Scholar]
- Ryder N.S., Dupont M.-C. Inhibition of squalene epoxidase by allylamine antimycotic compounds: a comparative study of the fungal and mammalian enzymes. Biochem. J. 1985;230:765–770. doi: 10.1042/bj2300765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stach J. Antimicrobials: treasures from the oceans. Microbiol. Today. 2010;105:1–3. [Google Scholar]
- Subramani R., Aalbersberg W. Culturable rare Actinomycetes: diversity, isolation and marine natural product discovery. Appl. Microbiol. Biotechnol. 2013;97:9291–9321. doi: 10.1007/s00253-013-5229-7. [DOI] [PubMed] [Google Scholar]
- Yu Z., Zhao L.X., Jiang C.L., Duan Y., Wong L., Carver K.C., Schuler L.A., Shen B. Bafilomycins produced by an endophytic actinomycete Streptomyces sp. YIM56209. J. Antibiot. 2011;64(1):159–162. doi: 10.1038/ja.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]