Graphical abstract
Keywords: Egg phospholipids, Liposomes, Encapsulation, Black seed oil (Nigella sativa)
Abstract
This study aimed to formulate black seed oil (Nigella sativa) loaded liposomes using the ethanol injection method to enhance oral bioavailability and improve therapeutic activity in small animal studies of analgesia. The impact of formulation attributes and process parameters on the liposomal system was evaluated with key quality attributes being particle size, morphology, and entrapment efficiency. The particle size and entrapment efficiency of the liposome preparation were found to be between the range of 50–900 nm and 34–87% respectively. Particle size distribution data suggested that increasing the percentage of oil, up to a certain concentration, reduced the size of the liposomes significantly from 520 ± 81.2 nm to 51.48 ± 1.31 nm. Stirring and injection rate were shown to have marked impact on the average particle size of liposome. It was observed that entrapment efficiency of liposomes was greatly influenced by the amount of cholesterol and type of cryoprotectant used during formulation. The stability study indicated that the liposomal preparation was stable at ambient conditions for one month. In vivo studies showed that the liposomal preparation demonstrated significant analgesic activity in mice.
1. Introduction
In recent years, considerable interest has been focused on the use of liposomes as efficient and promising carriers for drugs, proteins and even Deoxyribose Nucleic Acid (DNA). Liposomes are artificially prepared spherical lipid bilayer vesicles with an aqueous core which can encapsulate both water-soluble and lipid-soluble drugs. Hydrophilic drugs can be entrapped in their interior aqueous compartments, whereas lipophilic drugs are mainly entrapped within the lipid bilayers. Encapsulation of drugs in liposomes has become an emerging platform to provide targeted and controlled delivery, enhance bioavailability, reduce toxicity of drugs, and provide better patient compliance. Liposomes can deliver the drug to target tissues and minimize distribution of the drug to non-target tissues which could result in better utilization of the active agent (Schnyder and Huwyler, 2005). They have been used to improve the therapeutic index of new or established drugs by modifying drug absorption, reducing metabolism, and prolonging biological half-life or reducing toxicity (Li et al., 2012).
Liposomes were first described by British haematologist Dr. Alec D Bangham in 1961 (Bangham and Horne, 1964). Since these early reports, researchers have made a number of important technical advances such as long-circulating (PEGylated) liposomes, triggered release liposomes, liposomes containing nucleic acids, ligand-targeted liposomes and liposomes containing combinations of drugs. More recently, liposomes have been used in immunology, dermatology, eye disorders, brain targeting, infectious diseases, as vaccine adjuvants and in cancer therapy (Allen and Cullis, 2013).
Low solubility and insufficient absorption of hydrophobic drugs are major problems of the oral route of administration. Due to smaller particle size and larger surface area of nanosized liposomes, they can be used to increase absorption rate and bioavailability of poorly soluble drugs, by maintaining drug in a molecular dispersed form in the upper GI tract.
During the 1970s, liposomal drug carriers were developed for the oral route of administration. The use of liposomes as a carrier was shown to increase the uptake of some drugs, while also decreasing degradation rates. When used by the oral route of administration, liposomes have been used to protect drug molecules (both hydrophilic and hydrophobic) from the effects of low pH, while increasing transport of the drug into the intestinal lymph which has led to increased systemic bioavailability (Kalepu et al., 2013).
Black seed oil, isolated from the seeds of Nigella sativa, has been widely used for centuries in the treatment of various ailments. It consists of approximately 24.9% carbohydrate, 26.7% protein and 28.5% fats (Ahmad et al., 2013). The main component in the essential oil of black seed is thymoquinone (30%-48%), whilst crystalline nigellone (dithymoquinone), arginine, carotene, pinene, cymene, carbony, nigelline and trace amounts of other species are also present (Alijabre et al., 2015). The oil is believed to demonstrate antioxidant, chemotherapeutic, hepatoprotective, antinephrotoxic, antidiabetic, antimicrobial, analgesic and anti-inflammatory activities (Al-Awadi et al., 1991, Al-Ghamdi, 2001, Daba and Abdel-Rahman, 1998, El Daly, 1998, Farah and Begum, 2003, Gali-Muhtasib et al., 2004, Toppozada et al., 1965). However, the oil being hydrophobic demonstrates low oral bioavailability of its components and therefore demonstrates limited therapeutic benefit when administered by this route. This study aimed to incorporate black seed oil into liposomes with the intention to enhance its bioavailability and improve its therapeutic activity in small animal models of analgesia.
To date, there have been very few publications which have highlighted the benefits of using nanosized dosage forms for the enhanced oral delivery of black seed oil. In a study, Ravindran et al. (2010) loaded thymoquinone into polylactide-co-glycolide nanoparticles, while Odeh et al. (2012) encapsulated thymoquinone into liposomes. In these studies it was shown that thymoquinone-loaded liposomes were effective in suppressing the proliferation of breast cancer cells.
The study reported here is focused on the preparation of black seed oil encapsulated liposomes using the ethanol injection method. The effect of several process parameters and formulation components on the average particle size, size distribution and entrapment efficiency of liposomes was studied. Influence of the amount of cholesterol and type of cryoprotectant used on entrapment efficiency of liposomes was also investigated. Stability studies of liposomes were performed under a range of different storage conditions for the duration of one month. The analgesic effects of the optimized black seed oil loaded liposomes were evaluated using a small animal in vivo model of analgesia.
2. Materials and methods
2.1. Materials
2.1.1. Chemical
Phospholipid (phosphatidylcholine) was extracted from chicken egg yolk in the laboratory. Egg phosphatidylcholine (PC, purity > 96.5%) was obtained from Lipoid (Germany) to be used as a reference standard in the UHPLC analysis of extracted egg phospholipid (EP). Acetonitrile and HPLC grade methanol were purchased from BDH Chemicals Ltd. (U.K.).
Black seed oil was bought from local market (Bangladesh). For quantification of amount of thymoquinone present in black seed oil, thymoquinone and Potassium dihydrogen phosphate were procured from Sigma-Aldrich (USA) and Winlab Ltd (UK) respectively. Cholesterol (Ch) was purchased from LobaChemie (India). Methanol, ethanol, dicholoromethane, acetone, BHT (Butylatedhydroxytoluene), sodium dihydrogen phosphate, and poly-ethylene glycol 6000 (PEG600) were obtained from Merck (Germany). Sodium chloride (NaCl) was obtained from Sigma-Aldrich (USA). All other reagents were of analytical grade and used without further purification.
2.2. Methods
2.2.1. Extraction of phospholipids from egg yolk
Egg yolk was mixed in a solvent mixture containing dichloromethane and methanol in the ratio of 2:1 and kept for 10 min to separate out the lipids. It was then filtered, mixed with 1%NaCl and transferred into a separator funnel. The bottom NaCl layer containing phosvitin, was runoff in order to separate the lipid portion. Butylatedhydroxytoluene (BHT) was then added as an antioxidant. The solid fraction was dried in a water bath at 45 °C. Acetone was added to the yellow precipitate and cooled in an ice bath for 15 min. The portions not containing the phospholipids were dissolved in acetone and removed by filtration (de Koning, 1974). UHPLC analysis was then performed to confirm the presence of egg phospholipid by comparing the result with that for the reference standard of egg phosphatidylcholine.
2.2.2. Ultra High Performance Liquid Chromatography (UHPLC) analysis of phospholipids
Sample was prepared by dissolving egg phospholipid in methanol. 1.0 μl of the solution was injected in UHPLC system (Ultimate 3000® binary solvent manager) equipped with automatic sampler and a Photodiode Array (PDA) eλ detector obtained from Thermo scientific, USA. The separation was achieved by reverse-phase isocratic elution using a mobile phase consisting of methanol and acetonitrile (90/10%V/V). It was freshly prepared, filtered using a 0.22 μm filter paper and degassed in the UHPLC system continuously by an online degasser. Acquity® UPLC column BEH C18 (2.1 × 50 mm, 1.7 μm) was maintained at 30 °C and eluted with the mobile phase at a flow rate of 0.2 ml/min. The detector wavelength was set at 200 nm. The total run time was 4 min. The chromatogram of the sample obtained was then compared with that of the reference standard.
2.2.3. Identification of thymoquinone in black seed oil by HPLC
A known amount of black seed oil was dissolved in 10 ml methanol within an amber volumetric flask. 20 μL of the solution was injected and examined in triplicate and chromatographed using HPLC system (Shimadzu, Japan) with model SPD 20A dual UV absorbance detector. The mobile phase consisted of methanol and potassium dihydrogen phosphate buffer (10 mM), in the ratio of 90:10 v/v, which was delivered isocratically at a flow rate of 0.9 ml/min. Waters 5 m Atlantis® dC-18 Bond pack column (10 μm, 150 × 3.9 mm) was utilized to elute the compound thymoquinone at a λmax of 254 nm. The column temperature was maintained at room temperature (23 ± 2 °C). Amount of thymoquinone was then determined using calibration curve, which was obtained by plotting peak area against standard drug concentration.
2.2.4. Preparation of black seed oil loaded liposomes by ethanol injection method
Liposomes containing black seed oil were prepared using the ethanol injection method. Egg phospholipids and cholesterol, in different ratios (3:1 and 3:2) were dissolved in ethanol which was followed by addition of black seed oil at a range of different injection and stirring rates (Table 1). For selected formulations, this process was assisted by heat to observe its effect on encapsulation efficiency. The resulting solutions were injected into excess phosphate buffer solution (PBS) at pH 5.7 with magnetic stirring. Liposomes were formed spontaneously as soon as the ethanolic solution came in contact with the aqueous phase. The liposomal dispersion was stirred on a magnetic stirrer for an additional 60 min at room temperature (25 ± 2 °C) to remove the traces of solvent and ensure proper mixing (Samad et al., 2007).
Table 1.
Preparation of liposomes.
| Batch no. | Oil (%v/v) | EP:Ch | Injection rate (ml/min) | Stirring rate (rpm) |
|---|---|---|---|---|
| 1 | Blank | 3:1 | 1 | 2200 |
| 2 | 0.67 | 3:1 | 1 | 2200 |
| 3 | 0.67 | 3:1 | 0.5 | 2200 |
| 4 | 0.67 | 3:1 | 1 | 1500 |
| 5 | 0.67 | 3:2 | 1 | 2200 |
EP = egg phospholipid; Ch = cholesterol.
2.2.5. Freeze-drying and Reconstitution
The cryoprotectants (lactose or sucrose) were dissolved in phosphate buffer saline at a concentration of 3 g/g of dry phospholipids. Liposomal suspensions were then frozen to −30 °C and subsequently freeze-dried for 24 h under vacuum at −10 °C. The lyophilized cakes were stored in desiccators over silica gel. Lyophilized products were reconstituted with phosphate buffer (pH 5.7) during analysis (Abdelwahed et al., 2006).
2.2.6. Particle morphology and size distribution analysis
2.2.6.1. Inverse phase microscopy
Inverse phase microscopy (Olympus, model no.AxioCam ERc 5s, Japan) was initially used to characterize the size and shape of blank and oil encapsulated liposomes. Each sample was analyzed in triplicate.
2.2.6.2. Field Emission Scanning Electron Microscopy (FESEM)
High-resolution images of liposomes were produced using a Jeol JSM.7600F field emission scanning electron microscope. Samples were analyzed at a variety of magnifications with direct data capture of the images onto a personal computer. The freeze-dried liposomes were scattered on double-side adhesive carbon tapes, which were attached to FESEM specimen mounts. The specimens were sputter-coated for 2 min to obtain uniform coating on the sample by Jeol JFC-1600 Auto fine coater (Maheshkuma et al., 2013).
2.2.6.3. Zetasizer
The particle size and size distribution of the liposomes were determined using the Malvern Zetasizer Nano-ZS dynamic light scattering instrument (Malvern Instruments). Each sample was diluted in 1/1000 (% v/v with distilled water) and freshly prepared before measurement. The mean particle size was determined from three measurements.
2.2.7. Separation and determination of free drug
The supernatant containing free drug was separated from liposomal suspension by centrifugation at 9677 rcf for 60 min at 4 °C in an Eppendorf. Phosphate buffer (pH5.7) was added to the pellets and centrifuged again under same conditions. The second supernatant was also collected and both supernatants were diluted 100 times separately with buffer and the concentration of free drug was determined at 255 nm using a UV–visible spectrophotometer (model UV-1800; Shimadzu, Kyoto, Japan) in triplicate (Panwar et al., 2010).
2.2.8. Determination of entrapment efficiency
Entrapment efficiency of liposomes was determined after lysis of liposomes with ethanol followed by sonication for 10 minutes (Ramana et al., 2010). The resulting solution was then diluted with buffer and absorbance of encapsulated drug in the diluted solution was determined at 255 nm using a UV–visible spectrophotometer in triplicate. The concentration of the encapsulated and free drug was determined using the calibration curve.
The encapsulation efficiency expressed as entrapment percentage was calculated using the following formula:
2.2.9. Stability testing
Stability of liposomal batches was studied under a range of different storage conditions (10 °C/45% RH; 25 °C/65% RH and 40 °C/75% RH). After 1 month, entrapment efficiency and particle size distribution were determined.
2.2.10. In vivo study
2.2.10.1. Experimental animals
Male Swiss albino mice (age: 4 weeks, weight: 20–25 g) were divided into 4 groups. Control group was provided only with distilled water orally, whereas experimental group was given a dose of 300 μl of the formulation. The rest of the male mice were further divided into two more groups: one was a standard group which was given standard drug (diclofenac) of dose 300 μl and the other group was given 0.25 ml of black seed oil orally. The hot plate (Eddy and Leimbach, 1953, Turner, 1971) method was used for the evaluation of centrally acting analgesics (Woolfe and MacDonald, 1994). The experimental procedure was reviewed and approved by the institutional ethical committee at North South University, Dhaka, Bangladesh.
2.2.10.2. Hot plate test
Hot plate test was performed according to the method described earlier (Ezeja et al., 2011). The test was performed in order to evaluate the central sensation of pain in mice. The procedure is based on the observation that morphine-like drugs selectively prolong the reaction time of the typical withdrawal reflex in mice (Toma et al., 2003). The hot plate test (Hot/Cold Plate Model-35100-001, UGO Basile, Italy) was carried out at a fixed temperature of 55 ± 1 °C. The animals were placed on the hot plate and the latency period determined with a stop watch was recorded which represents the time taken for the mice to react to the pain stimulus. The response to pain stimulus was jumping and licking of hind feet. The latency to respond (reaction time) by each mouse was recorded 5 times. The cutoff time was 25 s.
3. Results and discussion
3.1. UHPLC analysis of egg phospholipid
The representative chromatographic results of the blank sample (methanol only, Fig.1a), standard PC (Fig.1b) and Egg yolk PC sample (Fig.1c) are shown in Fig. 1. The chromatographic results from the current UHPLC analysis showed that the separation of the PC peak and its detection were adequate without any interference of the other components present in the egg yolk (Gong et al., 2006). The PC analyte was well separated at the retention time of 2.71 min without having any interference from degradation products (Fig. 1). The total run time was as short as 4 min, where the peaks were of good shape and completely resolved. PC sample chromatogram also exhibited additional peaks at retention times of 2.18 and 2.34 when compared to the PC standard which is probably related to the presence of some other components in the sample. The presence of these additional components has the potential to affect liposome formation with potential consequences for the use of this system for drug delivery. Further studies are therefore required in order to understand the impact of impurities present in PC on the formation and characteristics of liposomes.
Figure 1.
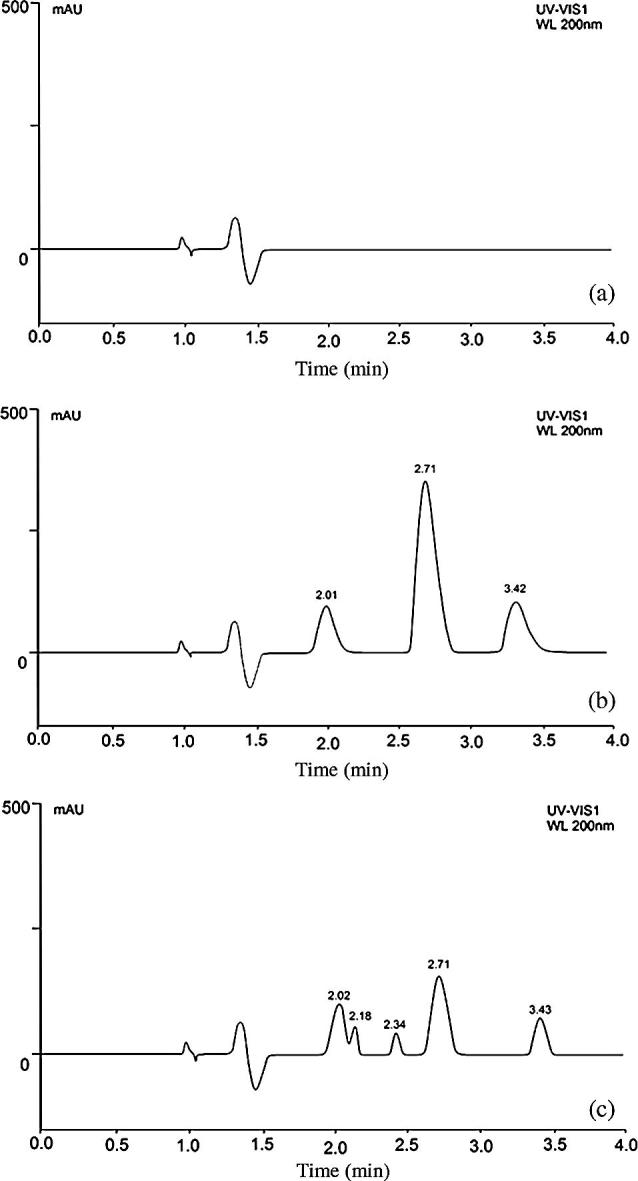
UPLC chromatograms of (a) blank sample, (b) egg yolk PC standard and (c) egg yolk PC sample at 200 nm.
3.2. HPLC analysis of black seed oil
According to the HPLC data (Fig. 1), the concentration of thymoquinone was found to be 2.28 ± 0.68 mg/g of black seed oil. In large liposomal batches (105 ml), 522.2 mg (0.7 ml) of oil equivalent to 1.19 mg of thymoquinone was used during formulation. Approximately 1.04 mg of thymoquinone was entrapped within the liposomes. The in vivo results showed significant analgesic effects of black seed oil loaded liposomes in mice when compared with the control group. However the potential exists to increase the amount of oil or thymoquinone in the formulation to achieve an improved therapeutic effect.
3.3. Particle size and morphology analysis
3.3.1. Inverse phase microscopy image
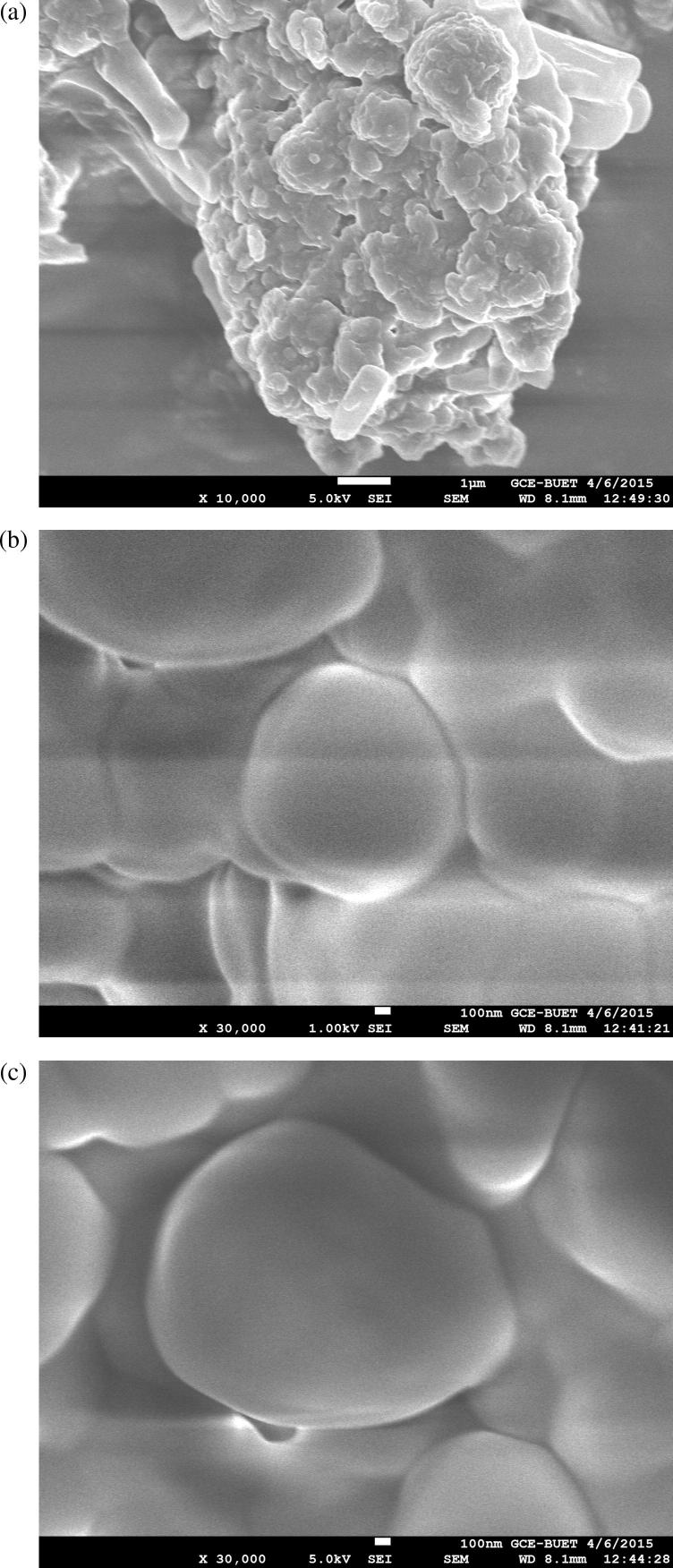
The inverse phase micrograph (Fig. 2) shows that liposomes were formed in the range of 330–450 nm. This finding was supported by field emission scanning electron microscopic (FESEM) for data observed on freeze-dried liposomes (Fig. 3). Twins and aggregates of these liposomes were also in the submicron range (Fig.3a).
Figure 2.
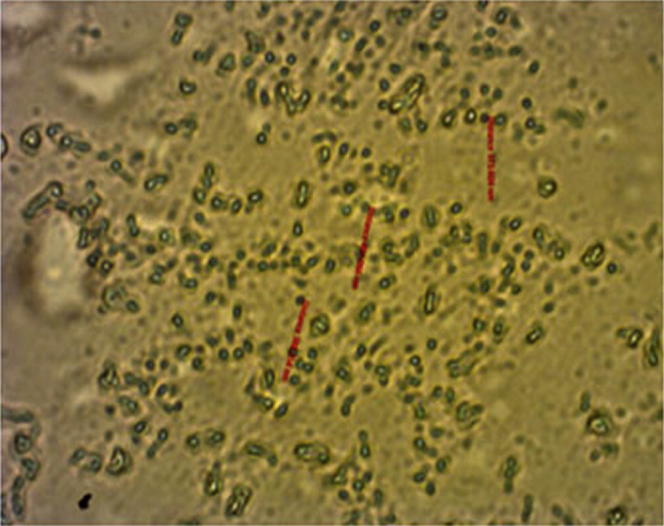
Inverse phase micrograph of liposomes (scale bar represents 1000×).
Figure 3.
FESEM micrographs of liposomes [Magnification (a) 10,000×; (b & c) 30,000×].
3.3.2. Field Emission Scanning Electron Microscopy (FESEM)
The FESEM image of the liposomes containing black seed oil also showed spherical like shapes in the submicron size range as shown in Fig.3b. Any aggregation that was observed during the study assumed to be related to the freeze drying of the liposomal suspensions. SEM data (Fig.3b) showed liposomes (30000×) aggregated into polygonal shapes, while inverse phase micrographs (Fig. 2) showed small disk like structures at low magnification level (1000×). Polygonal shaped liposomes were previously observed by Johnsson and Edwards (2003) using transmission electron microscopy (TEM).
3.3.3. Effect of formulation and processing parameters on average particle size
3.3.3.1. Effect of oil concentration on average liposomal particle size
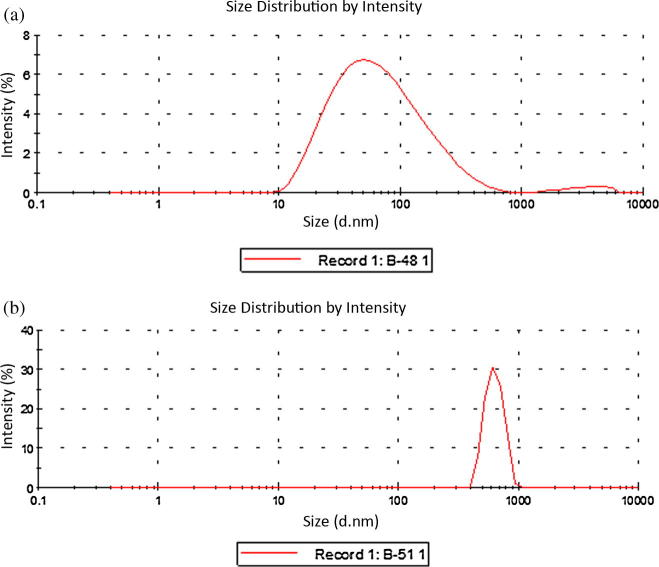
Particle size distribution data obtained from the Zetasizer showed that liposomes prepared in this study, and both blank liposomes and those containing oil were in the size range 50–900 nm. Liposomes containing oil were smaller in average size (51.48 ± 1.31 nm) compared to blank liposomes (520.70 ± 81.17 nm) as shown in Fig.4a (batch 1) and 4b (batch 2). This phenomenon may be linked to the antioxidant properties of black seed oil, which reduces the oxidation of phospholipids and the aggregation of liposomes leading to smaller sized liposomes (Kumar et al., 2011).
Figure 4.
Effect of oil on average particle size of liposomes, (a) blank liposome and (b) oil encapsulated liposome.
For a range of formulations (Table 2), increasing the percentage of oil, up to a certain concentration (from 0.17 to 0.67% v/v), reduced the size of liposomes significantly. This might be due to antioxidant activity of the oil reducing oxidation of phospholipid bilayer, preventing growth of liposomes.
Table 2.
Effect of oil on average size of liposome.
| Batch no. | Oil (%v/v) | EP:Ch | Injection rate (ml/min) | Stirring rate (rpm) | Ave. particle size (nm) | Standard deviation (nm) |
|---|---|---|---|---|---|---|
| 6 | Blank | 3:2 | 1 | 2000 | 913 | 184 |
| 7 | 0.17 | 3:2 | 1 | 2000 | 640 | 38 |
| 8 | 0.40 | 3:2 | 1 | 2000 | 509 | 43 |
| 9 | 0.67 | 3:2 | 1 | 2000 | 219 | 22 |
| 10 | 1.33 | 3:2 | 1 | 2000 | 238 | 8 |
Upon further increasing the volume of oil (0.67% v/v), there is no marked effect on the average size of liposomes (Fig. 5). The oil concentration of 0.67% v/v was found to be optimum for its antioxidant effect probably due to liposomes becoming saturated with oil at this point and beyond this concentration, liposomes did not incorporate extra oil.
Figure 5.
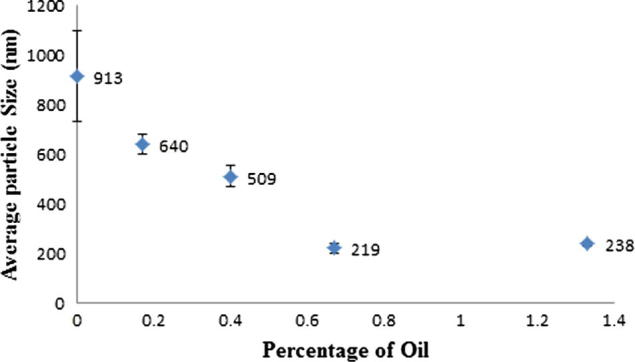
Percentage of oil influencing average particle size of liposome.
3.3.3.2. Effect of injection rate and stirring rate on average particle size
In this study it was observed that, the optimum injection and stirring rates were 1 ml/min and 2200 rpm respectively (Fig.6a and b). Under these conditions, small sized liposomes were formed. Injection rate was varied from 0.5 to 2 ml/min and found that injection rate has significant effect on the liposome particle size (Fig.6a). The optimum injection rate was found to be 1 ml/min, where low injection rate (0.5 ml/min) probably has accelerated lipid oxidation and high injection rate (2 ml/min) led to a polydisperse distribution of liposomes (Song et al., 2011), which might be related to the secondary nucleation of liposomes.
Figure 6.
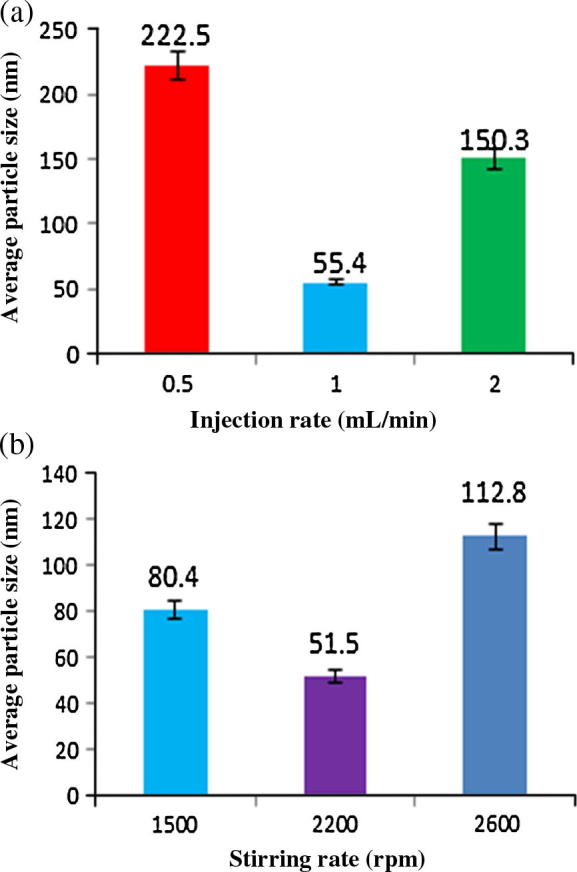
Effect of (a) injection rate and (b) stirring rate on average particle size.
Stirring rate of the aqueous phase varied from 1500 to 2600 rpm. It was observed that smallest liposomes were formed at 2200 rpm (Fig.6b). The variation of liposome particle size due to stirring rate can be explained by the intensification of micromixing between two phases, which suggest that 2200 rpm is optimum for micromixing of two phases (Laouini et al., 2013).
3.4. Entrapment efficiency
3.4.1. Effect of cryoprotectant
It was observed that sucrose enhanced the encapsulation efficiency of liposomes from 34.8 ± 1.8% to 66.6 ± 2.0% (Table 3) which was probably due to reduced interactions between water and phospholipids, resulting in associated stabilization of the liposomal bilayer. This effectively reduced drug leakage and led to enhanced entrapment efficiency (Lopes et al., 2013). It was also observed that sucrose enhanced entrapment efficiency of liposomes compared to lactose when used as a cryoprotectant (Table 3). Sucrose might have reduced the interaction between water and phospholipid to a greater extent compared to lactose.
Table 3.
Effect of cryoprotectant on entrapment efficiency.
| Oi l(%v/v) | EP:Ch | Injection rate (ml/min) | Stirring rate (rpm) | Cryoprotectant (3g/g of phospholipid) | Entrapment efficiency (%) |
|---|---|---|---|---|---|
| 0.67 | 3:1 | 1 | 2200 | Without cryoprotectant | 34.8 ± 1.7 |
| 0.67 | 3:1 | 1 | 2200 | Lactose | 40.8 ± 2.5 |
| 0.67 | 3:1 | 1 | 2200 | Sucrose | 66.6 ± 1.8 |
3.4.2. Effect of cholesterol
Increasing the ratio of egg extract to cholesterol from 3:1 to 3:2 enhanced the encapsulation efficiency from 39.8% ± 2.0% to 49.9 ± 5.6% (Table 4). This observation may be linked to the increased amounts of cholesterol filling the free spaces within the lipid chains, which reduces their flexibility and attenuates molecular mobility, diffused with concomitant loss of flexibility of the oil constituents (Shivhare et al., 2009).
Table 4.
Effect of cholesterol on entrapment efficiency.
| Batch no. | Oil (%v/v) | EP:Ch | Ave. particle size (nm) ± SD | Entrapment efficiency (%) |
|---|---|---|---|---|
| 2 | 0.67% | 3:1 | 55.0 ± 2.0 | 39.8 ± 2.3 |
| 5 | 0.67% | 3:2 | 89.0 ± 5.6 | 49.9 ± 1.9 |
3.5. Stability study at different conditions
No significant changes in drug entrapment efficiency and mean particle size were observed for the formulation stored at 25 °C/65%RH. Low entrapment efficiency and increased mean particle size of liposomes were observed when stored at 10 °C/45% RH and 40 °C/75% RH (Table 5, Figure 7, Figure 8). At accelerated conditions, liposomes became unstable due to oxidation and become aggregated which led to leakage of drug (Torchilin and Weissing, 2003).
Table 5.
Stability study of liposomes at different conditions.
| Test condition | Temperature (°C) | Relative humidity (%) | % Encapsulated efficiency | Particle size (nm) |
|---|---|---|---|---|
| 0 Month | 25 | 65 | 87% ± 1.6 | 173 ± 15 |
| 1 Month | 25 | 65 | 85% ± 0.6 | 222 ± 8 |
| 1 Month | 10 | 45 | 63% ± 1.5 | 382 ± 14 |
| 1 Month | 40 | 75 | 72% ± 1.1 | 255 ± 19 |
Figure 7.
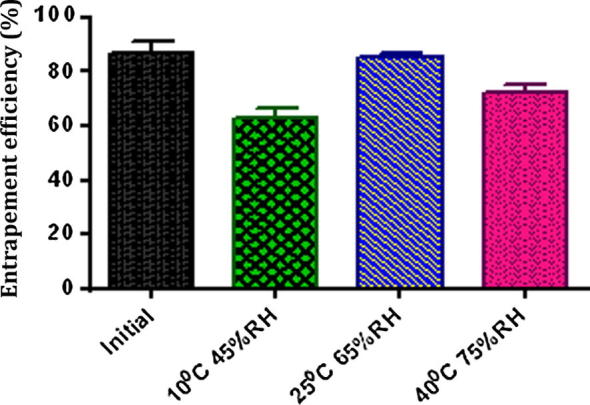
Entrapment efficiency of liposome batches after one month stored at different conditions (mean value with 95% confidence interval).
Figure 8.
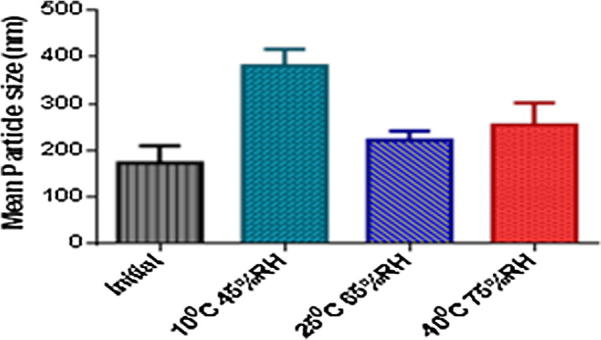
Mean particle size of liposome batches after one month stored at different conditions (mean value with 95% confidence interval).
Previous stability studies showed that the liposomal preparations were stable at 2–8 °C. But this formulation was stable at 25 °C. This is probably due to the differences in the concentration and composition of the egg phospholipid and cholesterol.
3.6. In vivo study
The control mice, which received distilled water responded within 15 s exposure to the hot plate. Significant increase in response time has been observed in the animals treated with liposomes or the standard Fig. 9. Black seed oil loaded liposomes exhibited better analgesic activity than black seed oil only, which is probably related to the greater bioavailability achieved for the liposomal formulation (Fig. 9).
Figure 9.
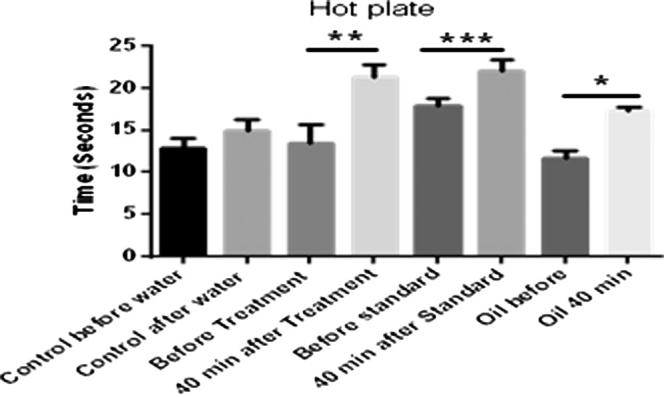
Hot plate study of liposomes loaded with black seed oil (where ‘*’ = p < 0.05, ‘**’ = p < 0.01 and ‘***’ = p < 0.001).
The exact mechanism of action of black seed oil is still deceptive. However, previous studies suggest that black seed oil possesses analgesic and anti-inflammatory activity (Al-Naggar et al., 2003, Ahmad et al., 2013). Thymoquinone, as one of the major components of black seed oil, probably accounts for this pharmacological effect, based on previous literature findings (El-Dakhakhny et al., 2002, Bashir and Quresh, 2010). It is however not clear as to whether this compound is the only component contributing to the analgesic property or other minor ingredients are influencing therapeutic response. Further studies are required to deconvolute the analgesic activities of the components of the black seed oil and their levels of entrapment in the liposomal preparations.
4. Conclusion
Process parameters and formulation attributes were shown to have marked impact on the average particle size and encapsulation efficiency of liposomes. In this study the optimum injection rate and stirring rate were found to be 1 ml/min and 2200 rpm respectively. Incorporation of the oil into liposomes was shown to have a major effect on average size of liposomes. Sucrose and amount of cholesterol were shown to improve the encapsulation efficiency of black seed oil. The stability study suggests that liposomal batches were unstable at low temperature and accelerated conditions.
The in vivo study demonstrated improved analgesic activity in mice when the black seed oil was encapsulated into liposomes, which suggests that improved oral bioavailability was achieved. In this study it is concluded that liposomes are greatly dependent on processing conditions and composition, and thus significant optimization is required to achieve maximal therapeutic response from formulations of this type.
Acknowledgment
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the research group project number (RGP#1435-017).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohsin Kazi, Email: mkazi@ksu.edu.sa.
Mohammad H. Shariare, Email: mohammad.shariare@northsouth.edu.
References
- Abdelwahed W., Degobert G., Stainmesse S., Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006;58(15):1688–1713. doi: 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Ahmad A., Husain A., Mujeeb M., Khan S.A., Najmi A.K., Siddique N.A., Damanhouri A.Z., Anwar F. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pacific J. Trop. Biomed. 2013;3(5):337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Awadi F., Fatania H., Shamte U. The effect of a plant mixture extract on liver gluconeogenesis in streptozotocin-induced diabetic rats. Diabetes Res. 1991;18(4):163–168. [PubMed] [Google Scholar]
- Al-Ghamdi M.S. Anti-inflammatory, analgesic and anti-pyretic activity of Nigella sativa. J. Ethnopharmacol. 2001;76(1):45–48. doi: 10.1016/s0378-8741(01)00216-1. [DOI] [PubMed] [Google Scholar]
- Alijabre S.H.M., Alakloby O.M., Randhawa MA. Dermatological effect of nagellasative. J. Dermatol. Dermatol. Surg. 2015;19(2):92–98. [Google Scholar]
- Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Al-Naggar T.B., Gomez-Serranillos M.P., Carretero M.E., Villar A.M. Neuropharmacological activity of Nigella sativa L. extracts. J. Ethnopharmacol. 2003;88(1):63–68. doi: 10.1016/s0378-8741(03)00157-0. [DOI] [PubMed] [Google Scholar]
- Bangham A.D., Horne R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964;8(5):660–668. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- Bashir M.U., Quresh H.J. Analgesic effect of Nigella sativa seeds extract on experimentally induced pain in albino mice. J. College Phys. Surg. Pakistan. 2010;20(7):464–467. [PubMed] [Google Scholar]
- Daba M.H., Abdel-Rahman M.S. Hepatoprotective activity of thymoquinone in isolated rat hepatocytes. Toxicol. Lett. 1998;95(1):23–29. doi: 10.1016/s0378-4274(98)00012-5. [DOI] [PubMed] [Google Scholar]
- De koning A.J. Analysis of egg lipids. A student project. J. Chem. Educ. 1974;51(1):48. doi: 10.1021/ed051p48. [DOI] [PubMed] [Google Scholar]
- Eddy N.B., Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines (PDF) J. Pharmacol. Exp. Ther. 1953;107(3):385–393. [PubMed] [Google Scholar]
- Ezeja M., Omeh Y., Ezeigbo I., Ekechukwu A. Evaluation of the analgesic activity of the methanolic stem bark extract of DialiumGuineense (Wild) Ann. Med. Health Sci. Res. 2011;1(1):55–62. [PMC free article] [PubMed] [Google Scholar]
- El Daly ES. Protective effect of cysteine and vitamin E, crocus sativus and Nagella sativa extracts on cisplatin-induced toxicity in rats. J. Pharm. Belg. 1998;53(2):87–93. Discussion 93-5. [PubMed] [Google Scholar]
- El-Dakhakhny M., Madi N.J., Lembert N., Ammon H.P. Nigella sativa oil, nigellone and derived thymoquinone inhibit synthesis of 5-lipoxygnase products in polymorphonuclear leukocytes from rats. J. Ethnopharmacol. 2002;81:161–164. doi: 10.1016/s0378-8741(02)00051-x. [DOI] [PubMed] [Google Scholar]
- Farah I.O., Begum R.A. Effect of Nigella sativa (N. sativa L.) and oxidative stress on the survival pattern of MCF-7 breast cancer cells. Biomed. Sci. Instrum. 2003;39:359–364. [PubMed] [Google Scholar]
- Gali-Muhtasib H., Diab-Assaf M., Boltze C., Al-Hmaira J., Hartiq R., Roessner A., Schneider-Stock R. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int. J. Oncol. 2004;25(4):857–866. [PubMed] [Google Scholar]
- Gong Y., Wang Q., Yang Y., Ding M. Determination of egg-yolk phosphatidylcholine by normal phase performance liquid chromatography with evaporative light scattering detection. Se Pu=Chin. J. Chromatogr. 2006;24(4):373–375. PMID17017163. [PubMed] [Google Scholar]
- Johnsson M., Edwards K. Liposomes, disks, and spherical micelles: aggregate structure in mixtures of gel phase phosphatidylcholines and poly(ethylene glycol)-phospholipids. Biophys. J . 2003;85:3839–3847. doi: 10.1016/S0006-3495(03)74798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.L.V.V.S.N.K.S., Jyothi T., 2, Nagendar S., Devipriya S., Vijaya G.K., Babu A.M.S.S. Stability of liposomes. Pharmanest. 2011;2(4) <http://pakacademicsearch.com/pdf-files/med/96/pharamanest-2%284%29-3.pdf> 2231-0541, ISSN: 0976-3090. [Google Scholar]
- Kalepu S., Manthina M., Padavala V. Oral lipid-based drug delivery system-an overview. Acta Pharmaceut. Sinica B. 2013;3(6):361–372. [Google Scholar]
- Laouini A., Charcosset C., Holdich R.G., Vladisavljevic G.T. Preparation of liposomes: a novel application of microengineered membranes-investigation of the process parameters and application to the encapsulation of vitamin E. RSC Adv. 2013;3(15):4985–4994. [Google Scholar]
- Li C., Zhang Y., Su T., Feng L., Long Y., Chen Z. Silica-coated flexible liposomes as a nanohybrid delivery system for enhanced oral bioavailability of curcumin. Int. J. Nanomed. 2012;7:5995–6002. doi: 10.2147/IJN.S38043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes S.C.A., Giuberti C.D.S., Rocha T.G.R., Ferreira D.S., Leite E., Oliveira M.C. Liposomes as carriers of anticancer drugs. In: Rangel Leticia., editor. Cancer Treatment - Conventional and Innovative Approaches. In Tech; 2013. pp. 85–124. (Chapter 4) [Google Scholar]
- Maheshkuma S.S., Reddy K.N., Goud P.P., Kiranmayi N., Arvind G. Formulation and characterization of doxorubicin hydrochloride liposomes by double emulsion method. Int. Res. J. Pharm. 2013;4(4):197–201. [Google Scholar]
- Odeh F., Ismail S.I., Abu-Dahab R., Mahmoud I.S., Al Bawab A. Thymoquinone in liposomes: a study of loading efficiency and biological activity towards breast cancer. Drug Deliv. 2012;19(8):371–377. doi: 10.3109/10717544.2012.727500. [DOI] [PubMed] [Google Scholar]
- Panwar P., Pandey B., Lakhera P.C., Singh K.P. Preparation, characterization, and in vitro release study of albendazole-encapsulated nanosize liposomes. Int. J. Nanomed. 2010;5:101–108. doi: 10.2147/ijn.s8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramana L.N., Sethuraman S., Ranga U., Krishnan U. Development of a liposome nanodelivery system for Nevirapine. J. Biomed. Sci. 2010;17:57. doi: 10.1186/1423-0127-17-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran J., Nair H.B., Sung B., Prasad S., Tekmal R.R., Aggarwal B.B. Thymoquinone poly (lactide-co-glycolide) nanoparticles exhibit enhanced anti-proliferative, anti-inflammatory, and chemosensitization potential. Biochem. Pharmacol. 2010;79(11):1640–1647. doi: 10.1016/j.bcp.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Samad A., Sultana Y., Aqil M. Liposomal drug delivery systems: an update review. Curr. Drug Deliv. 2007;4(4):297–305. doi: 10.2174/156720107782151269. [DOI] [PubMed] [Google Scholar]
- Schnyder A., Huwyler J. Drug transport to brain with targeted liposomes. NeuroRx. 2005;2(1):99–107. doi: 10.1602/neurorx.2.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivhare U.D., Ambulkar D.U., Mathur V.B., Bhusari K.P., Godbole M.D. Formulation and evaluation of pentoxifylline liposome formulation. Digest J. Nanomater. Biostruct. 2009;4(4):857–862. [Google Scholar]
- Song J., Shi F., Zhang Z., Zhu F., Xue J., Tan X., Zhang L., Jia X. Formulation and evaluation of celastrol-loaded liposomes. Molecules. 2011;16:7880–7892. doi: 10.3390/molecules16097880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma W.O., Graciosa J.S., Hiruma C.A., Andrade F.D.P., Vilegas W., Souza-Brita A.R.M. Evaluation of the analgesic and anti-edamatogenic activities of Quassiamara barks extract. J. Ethnopharmacol. 2003;85:19–23. doi: 10.1016/s0378-8741(02)00334-3. [DOI] [PubMed] [Google Scholar]
- Toppozada H.H., Mazloum H.A., el-Dakhakhny M. The anti-bacterial properties of Nigella sativa seeds: active principle with some clinical application. J. Egypt. Med. Assoc. 1965;48(Suppl):187–202. [PubMed] [Google Scholar]
- Torchilin, V., Weissing, V., 2003. Liposomes: A Practical Approach, second ed. OUP Oxford. pp. 149-164 (Chapter 5).
- Turner R.A. Academic Press; New York: 1971. Screening Methods in Pharmacology; pp. 100–113. [Google Scholar]
- Woolfe G., MacDonald A.D. The evaluation of the analgesic action of Pethidine Hydrochloride. J. Pharmacol. Exp. Ther. 1994;80:300. [Google Scholar]