Abstract
Sézary syndrome (SS), a leukemic variant of cutaneous T-cell lymphoma (CTCL), is associated with a significantly shorter life expectancy compared to skin–restricted mycosis fungoides. Early diagnosis of SS is therefore key to achieving enhanced therapeutic responses. However, the lack of a biomarker (s) highly specific for malignant CD4+ T cells in SS patients has been a serious obstacle in making an early diagnosis. We recently demonstrated the high expression of CD164 on CD4+ T cells from Sézary syndrome patients with a wide range of circulating tumor burdens. To further characterize CD164 as a potential biomarker for malignant CD4+T cells, CD164+ and CD164− CD4+ T cells isolated from patients with high circulating tumor burden, B2 stage, and medium/low tumor burden, B1-B0 stage, were assessed for the expression of genes reported to differentiate SS from normal controls, and associated with malignancy and poor prognosis. The expression of Sézary signature genes: T plastin, GATA-3, along with FCRL3, Tox and miR -214 was significantly higher, whereas STAT-4 was lower, in CD164+ compared with CD164−CD4+ T cells. While Tox was highly expressed in both B2 and B1-B0 patients, the expression of Sézary signature genes, FCRL3 and miR-214 was associated predominantly with advanced B2 disease. High expression of CD164 mRNA and protein was also detected in skin from CTCL patients. CD164 was co-expressed with KIR3DL2 on circulating CD4+ T cells from high tumor burden SS patients, further providing strong support for CD164 as a disease relevant surface biomarker.
Keywords: Sézary syndrome, CD164, miR-214, FCRL3, Tox, KIR3DL2
Introduction
Cutaneous T-cell lymphoma (CTCL) is a malignancy of skin-trafficking CD4+ T cells most commonly manifested as mycosis fungoides (MF) and Sézary syndrome (SS). Whereas mycosis fungoides is primarily an indolent disease restricted to the skin, with the presence of patches or plaques, Sezary syndrome represents a leukemic variant with erythroderma, circulating malignant CD4+ T cells and a significantly shorter life expectancy. Early diagnosis of SS is therefore key to achieving enhanced therapeutic responses [23]. The lack of a biomarker (s) highly specific for malignant CD4+ T cells in SS patients has been a serious obstacle in making an early diagnosis and in evaluating the extent of the disease, thereby prompting continuous attempts to find a CTCL specific marker.
Currently, it is accepted that malignant CD4+ T cells in Sézary syndrome are a single TCRVβ clonal population with central memory phenotype, high expression of CCR4 and lack of CD26 [7, 12, 39, 44]. At the transcriptional level, expression of GATA-3, Twist 1 and Tox is increased while the expression of STAT-4 is decreased in patients’ CD4+ T cells compared to healthy controls [13, 18, 21, 38, 41]. Additionally, the intracellular protein T plastin was found in CD4+ T cells from SS patients but not in normal controls [21]. GATA-3, T plastin, STAT-4 and CD26 were named “Sézary signature” genes and differences between patients’ and healthy individuals’ in their expression in CD4+ T cells has aided in diagnosis of Sézary syndrome [33]. Recently, small, non-coding RNA molecules, microRNAs (miR-) were found to have potential diagnostic value in CTCL. Overexpression of several microRNAs including miR-214 and miR-21 made it possible to distinguish CTCL and SS patients with a poor prognosis from benign disorders [3, 32]. The identification and isolation of malignant cells expressing the aforementioned genes critically depends on finding a cell surface marker unique to malignant cells in CTCL. The presence of such biomarker (s) will aid in diagnosis and monitoring of the disease as well as in designing effective treatments targeting malignant cells and sparing of normal immunocompetent cells. Several surface molecules, namely NKp46, syndycan 4 (SD-4), ganglioside GD3 (CD60), mucin 1 (Muc 1) and CD158k/KIR3DL2 have been found to be highly expressed on CD4+ T cells from some SS patients compared with healthy controls [5, 9, 19, 36, 37]. SD-4, CD60 and Muc-1 molecules are also upregulated on activated normal CD4+ T cells [1, 8, 27]. Prior to its detection on CD4+ T cells from SS patients, KIR3DL2, a killer immunoglobulin-like receptor was mainly associated with a population of CD8+ T cells and NK cells in healthy controls. The report by Poszepszynska-Guine et al., which demonstrated high expression of KIR3DL2 on CD4+ T cells representing the malignant clonal cell population, provided an opportunity to utilize KIR3DL2 in diagnosis of SS [36].
Our studies focus on finding cell surface marker (-s) unique to CTCL patients with varying tumor burdens in the circulation. We recently reported high expression of CD164 and FCRL3, on CD4+ T cells in Sézary syndrome patients [43]. CD164 is a sialomucin adhesion receptor that was originally demonstrated on a population of CD34+ hematopoietic progenitor cells, but recently has been shown on some human cancer cells where it has been implicated to play a role in the development as well as metastasis of cancer [16, 26, 42]. Fc-receptor-like 3 (FCRL3) expression, originally associated with naturally occurring human T regulatory cells, was found by our group to correlate with SS progression [31, 43] The expression of CD164 on CD4+ T cells correlated positively with a wide range of circulating tumor burdens, whereas FCRL3 expression was noted mainly in high tumor burden patients. The potential for CD164 to serve as a marker for malignant CD4+ T cells was underscored by the following findings: CD164 acquisition correlated with loss of CD26 expression and it was co-expressed with a dominant TCRVβ; CD164+CD4+ T cells demonstrated the morphology of Sézary cells and were diminished or no longer detectable in patients who experienced clinical remission.
In the present studies, we further addressed the specificity of CD164 as a marker of malignant cells. We used the level of expression of Sézary signature genes, along with FCRL3, Tox, miR-214 and miR-21 expression as an indication of malignancy in populations of CD164+ and CD164− circulating CD4+ T cells. The results demonstrate that the malignancy genes are predominantly associated with CD164+CD4+ T cells and that CD164 is also expressed in skin–residing CD4+ T cells of CTCL patients. Additionally, our results show the expression of both, CD164 and KIR3DL2 on CD4+ T cells in 82% of SS patients. These results provide support for CD164 as a cell surface biomarker for CD4+ T cells with a malignant phenotype in CTCL.
Material and Methods
Patients
Patients were diagnosed on the basis of clinical, histopathologic and immunohistologic criteria as previously defined [30]. Patients with erythroderma and circulating malignant T-cells were diagnosed as B0, B1 or B2, according to criteria defined by Olsen et al; all B2 patients were stage IVA1, B0-B1 patients were stage III A-B [34]. Mycosis fungoides patients, with clinically and histologically defined skin lesions of patches, plaques, tumors or erythroderma, but without blood involvement were diagnosed as stages IA through IIIA. Specimens were obtained from CTCL patients seen at the Cutaneous T Cell Lymphoma Clinic and psoriasis patients seen at Psoriasis Clinic of the University of Pennsylvania. Atopic Dermatitis patients were seen in Division of Dermatology in the Department of Medicine at Washington University School of Medicine. Healthy control skin samples were obtained as residual healthy marginal skin from patients undergoing routine dermatologic surgery at Department of Dermatology, University of Pennsylvania Health System.
All samples were collected in accordance to protocols approved by the University of Pennsylvania and Washington University Institutional Review Board and studies were conducted according to the Declaration of Helsinki Principles.
Flow cytometry
To exclude the effect of treatment on the expression of different genes, peripheral blood mononuclear cells (PBMC) isolated from patients with no prior treatments were used in the studies. The cells were stained with fluorochrome- conjugated antibodies allowing the identification of live CD164+ and CD164− CD4+/CD3+ T cells. Antibodies were purchased from BD Biosciences (San Jose, CA), and the Live/Dead Fixable Aqua Dead Cell Stain Kit was purchased from Invitrogen (Grand Island, NY). Stained PBMC were sorted using BD FACS Aria II cell sorter.
To assess the expression of CD158k/KIR3DL2 (clone MOG1-M-K322-13E4c, provided by Innate Pharma) 100μl of patient’s blood sample was incubated with antibodies against CD3, CD4, CD26 and KIR3DL2 in room temperature (RT) for 45min. To assess the co-expression of CD164 and KIR3DL2, patient’s PBMC (1×10^6) were stained with anti-CD164 antibody plus antibodies indicated above followed by 45 min incubation in RT. In addition, KIR3DL2 expression on patients’ CD4+ T cells was tested using antibody DX31. DX31 antibody was the gift of Dr. Lewis Lanier, UCSF and was purified from the hybridoma by the UCSF Hybridoma Core Facility. PBMC were stained with biotinylated antibody DX31 and with anti-CD3, CD4, CD26 for 30 min at 4°C followed by 15 min incubation at 4°C with Streptavidin-APC. Both antibodies against KIR3DL2 generated similar results.
Skin lymphocytes were isolated using a modified method described by Kim et al [22]. Skin biopsies were stored in 0.08% trypsin, overnight at 4C followed by two hours incubation in a 37C/5%CO2 incubator with 0.125mg/ml Libertase TL (Roche). Isolated cells were stained with fluorochrome-conjugated antibodies to identify CD3/CD4 T cells expressing CD164. Cells were analyzed on a LSRII flow cytometer (Becton Dickenson, San Jose, CA) at the Flow Cytometry and Cell Sorting Core, Abramson Cancer Center, University of Pennsylvania. FlowJo software was used to analyze data (Tree Star, Ashland, OR).
RNA isolation and quantitative real time PCR
To assess the expression of T-plastin, Gata-3, STAT-4, FCRL3, and Tox on sorted CD164+/CD164− CD4+ T cells, total RNA was extracted from sorted and normal CD4+ T cells using the Qiagen RNeasy Mini Kit (Qiagen) according to the manufacturers’ instructions. For skin samples, total RNA was extracted from formalin-fixed paraffin embedded (FFPE) samples and normal tissue using the Qiagen RNeasy FFPE Kit and Fibrous Tissue Kit (Qiagen) following the manufacturers’ instructions. Single-stranded cDNA was synthesized by reverse transcription of 40 nanograms of RNA from CD164+/CD164− CD4+ T cells and skin biopsies using the High Capacity RNA to cDNA Kit (Applied Biosystems) for further analysis. QRT-PCR was performed using Taqman gene expression assays (Applied Biosystems) with Taqman Gene Expression Master Mix (Applied Biosystems) according to the manufacturers’ instructions. Expression levels were normalized to β-actin and analyzed using the ΔΔCT method.
To evaluate expression of miR-214 and miR-21 in CD4+ T cells and skin biopsies, 10 nanograms of total RNA was reverse transcribed to cDNA using the Taqman microRNA Reverse Transcription Kit (Applied Biosystems) following their custom RT and preamplification protocols. QRT-PCR was performed with Taqman microRNA primers (Applied Biosystems) according to the manufacturers’ instructions. Samples always were run in triplicate on the Applied Biosystem 7500 Ral Time PCR System. Expression levels were normalized to U6 snRNA and analyzed using the ΔΔCT method.
Results
The expression of Sézary signature genes, FCRL3, Tox and miR-214 is mainly associated with circulating CD164+CD4+ T cells in Sézary syndrome patients
We assessed the expression of Sézary signature genes in sorted CD164+/CD164−CD4+T cells. Additionally, we evaluated expression of FCRL3 and Tox (Fig. 1). [31,43]. Tox, a nuclear factor critically involved in CD4+ development in the thymus but downregulated on normal peripheral CD4+ T cells, was demonstrated in CD4+ T cells from CTCL patients and its high expression correlated with increased mortality [2, 6, 11, 29].
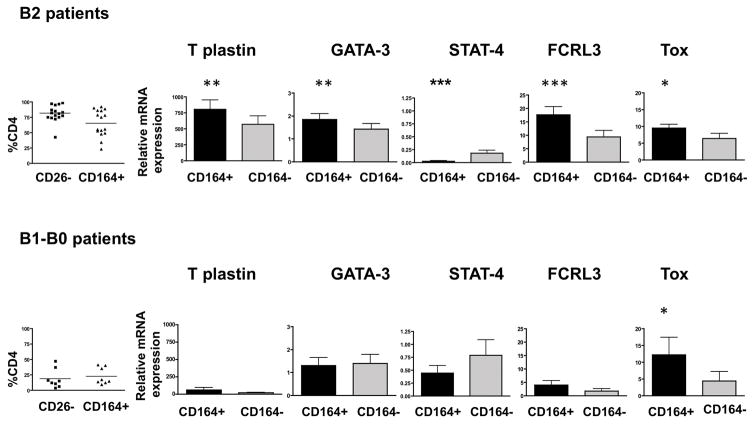
Fig. 1. CD164+CD4+ T cells from Sézary syndrome patients are the predominant population expressing the Sézary signature phenotype and significantly upregulated expression of FCRL3 and Tox.
PBMC from high tumor burden SS patients diagnosed as B2, upper panel (with ≥50% of CD26−CD164+CD4+ T cells, n=15, left graph), and PBMC from medium-to low tumor burden patients diagnosed as B1-B0, lower panel (with ≤50% CD26−CD164+CD4+ T cells, n=9, left graph), were sorted into CD164+/CD164− CD4 + T cells. Each population was assessed for the expression of mRNA for T plastin, GATA-3, STAT-4, FCRL3 and Tox by qRT-PCR. CD4+T-cells isolated from PBMC of healthy donors (n=9, pooled) were used as controls. Graphs show relative expression of given mRNA in CD164+ and in CD164− CD4+ T cells. Error bars represent mean +/− SEM. The nonparametric Wilcoxon signed-rank test was used to assess differences between groups. P values: *** <0.0005; **<0.005; * <0.05.
Patients’ PBMC were sorted into CD164+CD4+ and CD164−CD4+ T cells and the expression of Sézary signature genes as well as FCRL3 and Tox were assessed by quantitative real time PCR (qRT-PCR). In patients defined as B2; stage IVA1, experiencing advanced disease and ≥50% of CD26−CD4+ T cells highly expressing CD164, the population of CD164+CD4+ T cells demonstrated a significant increase in mRNA expression of T plastin (1.4 fold), GATA-3 (1.3 fold), but 6.3 fold decrease in STAT-4 mRNA expression compared with CD164−CD4+ T cells. Similarly, FCRL3 and Tox mRNA expression was significantly higher in CD164+CD4+ T cells from B2 patients compared with CD164−CD4+ T cells (1.9 and 1.5 fold higher, respectively) (Fig. 1, upper panel). In patients with low tumor burden, B1-B0; stages IIIB-A, and ≤50% CD26−CD164+CD4+ T cells, CD164+ CD4+ T cells demonstrated a 4.4 fold higher expression of T plastin and 1.8 fold lower expression of STAT-4 compared with CD164− CD4+ T cells (Fig. 1 lower panel). However, the differences between these two populations lacked statistical significance due to the variability between patients. An overall expression of T plastin in B1-B0 patients was several folds lower, whereas STAT-4 expression was higher compared with levels in B2 patients. The levels of GATA-3 expression in both CD164+ and CD164−CD4+ T cells were comparable to those in healthy controls. B1-B0 patients also demonstrated low levels of FCRL3 expression. Although a 2.2 fold increase in FCRL3 expression was observed in CD164+ compared with CD164−CD4+ T cells, it was not significant. Interestingly, the expression of Tox was significantly increased (2.9 fold) in CD164+CD4+ T cells compared with CD164−CD4+ T cells from B1-B0, patients pointing to the potential association between CD164 and Tox expression in patients with a low circulating burden of malignant cells (Fig. 1, lower panel).
CD164 expression is increased in skin from CTCL patients
To establish CD164 expression in skin-residing malignant cells, skin biopsies from patients diagnosed with SS, MF, atopic dermatitis (AD), psoriasis (PS), and healthy marginal skin from patients undergoing routine dermatologic surgery (HC), were assessed for CD164 mRNA expression. As shown in Fig. 2, the expression of CD164 mRNA in skin from SS and MF patients was significantly several fold increased (19.5 and 12.2 fold respectively) compared with its level in skin from AD patients or control skin (left panel). A 5.3-fold increase in CD164 mRNA was noted in skin from psoriasis patients. T plastin mRNA was also increased in skin lesions of CTCL patients, as previously reported by other authors (data not shown) [25].
Fig. 2. CD164 mRNA is expressed in skin from Sézary syndrome and Mycosis Fungoides patients.
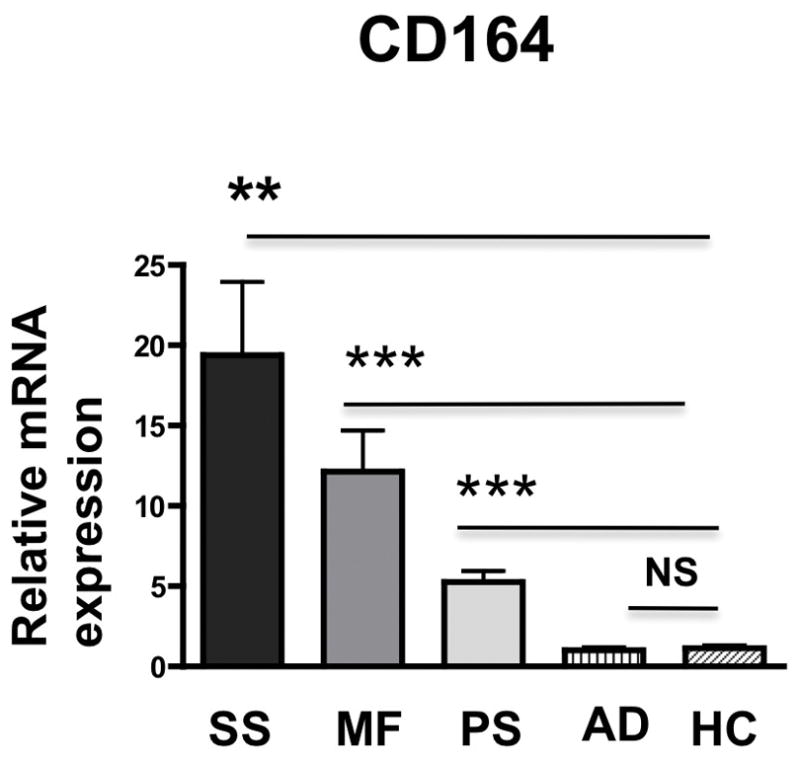
Skin samples from 7 Sézary syndrome patients (SS), 9 mycosis fungoides patients (MF), 4 atopic dermatitis patients (AD), 3 psoriasis patients (PS) and 9 healthy controls (HC) were examined for the expression of the genes by qRT-PCR. Fold difference for CD164 was calculated versus expression levels in skin from healthy controls. Error bars represent mean +/− SEM. Unpaired t- test used to assess differences. P values: *** <0.0005; **<0.005; * <0.05.
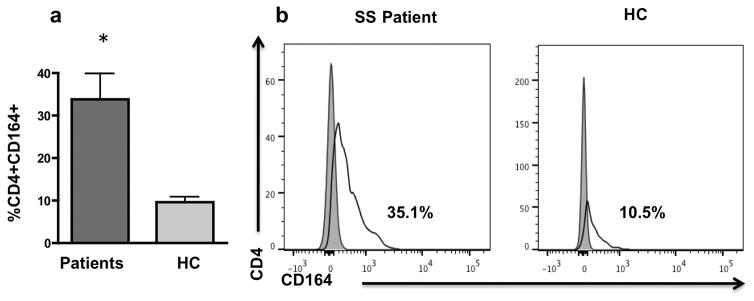
To determine if CD164 protein is expressed in skin CD4+ T cells, lymphocytes were isolated from skin biopsies of SS patients and healthy controls using the modified method described by Kim et al. (2013) [22]. Cells were stained with fluorochrome-conjugated antibodies and analyzed by flow cytometry to identify CD4+ T cells expressing CD164. As shown in Fig. 3a, 33.7% of the CD4+ T cells from the skin of patients expressed CD164 compared with 9.7% CD4+ T cells from normal skin. In Fig. 3b, a flow cytometry histogram shows CD164 expression on CD4+ T cells of a representative SS patient and control.
Fig. 3. CD164 is expressed on the cell surface of skin-resident CD4+ T cells from Sézary syndrome patients.
Fig. 3a demonstrates percentage of CD164+ CD4+ T cells isolated from skin of 5 Sézary syndrome patients (SS) and 11 healthy controls (HC) analyzed by flow cytometry. Error bars represent mean +/− SEM. P value: * <0.05. Fig. 3b shows a representative histogram illustrating CD164 expression on CD4+ T cells isolated from skin of SS patient (left panel) and healthy control (right panel).
MiR-214 expression is significantly upregulated in circulating CD164+CD4+ T cells
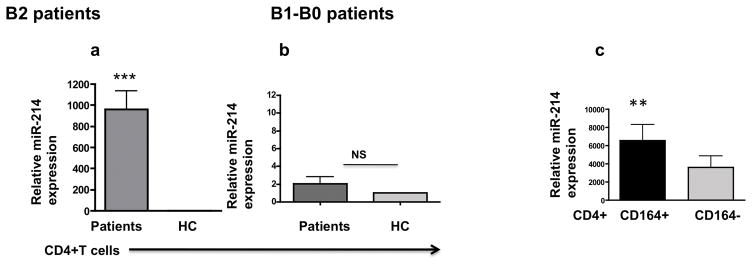
It has been reported that miR-214 and miR-21 are overexpressed in CD4+ T cells of SS patients with a poor prognosis [32]. We first determined that miR-214 was expressed in circulating CD4+ T cells of 89% of patients, whereas miR-21 (data not shown) was detected only in 17% of patients. This result prompted us to focus on miR-214. MiR-214 expression was highly upregulated in CD4+ T cells from B2 patients, but only marginally upregulated in B1-B0 patients (Fig. 4a,b). Next we determined the expression of miR-214 in sorted CD164+ and CD164−CD4+ T cells from B2 patients. As shown in Fig. 4c, miR-214 expression was significantly, 1.8 fold, upregulated in CD164+CD4+ T cells compared with CD164−CD4+ T cells.
Fig. 4. MicroRNA-214 is mainly expressed in CD164+CD4+ T cells from Sézary syndrome patients.
CD4+T cells from B2 patients (Fig. 4a, n=17), and from B1-B0 patients (Fig. 4b, n=8) were assessed for the expression of miR-214 by qRT-PCR. The expression of miR-214 was subsequently assessed in sorted CD164+/CD164−CD4+ T cells from B2 patients (Fig. 4c, n=15). Error bars represent mean +/− SEM. P values: **<0.005;
In contrast to circulating CD4+ T cells, miR-21 but not miR-214 expression was demonstrated in skin biopsies from both SS and MF, suggesting that the skin environment may promote the expression of different microRNAs (data not shown).
CD164 and KIR3DL2 are co-expressed on CD4+ T cells from SS patients
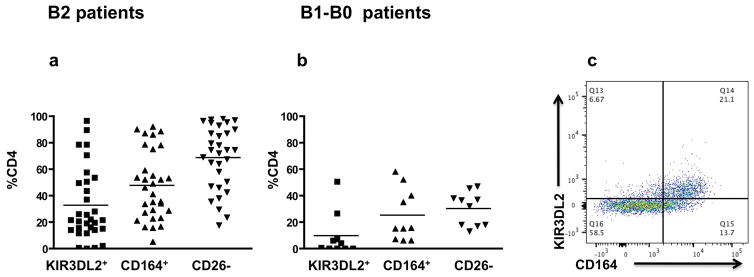
Since KIR3DL2 was demonstrated as a reliable cell surface marker for Sézary cells, we examined the association between KIR3DL2 and CD164 expression on CD4+ T cells from SS patients [28, 36]. As shown in Fig. 5 CD164 and KIR3DL2 are expressed on patients’ CD4+ T cells and the level of expression correlates with the severity of disease. In B2 patients, (Fig. 5a), with an average of 68.7% of CD4+T cells lacking CD26 expression, KIR3DL2 expression was demonstrated on 32.7% of CD4+ T cells whereas CD164 was present on 47.7% of CD4+ T cells. Only 4 out of 31 B2 patients (13%) showed no or ≤ 2% of KIR3DL2 expression on their CD4+ T cells that were positive for CD164. In contrast, 5 out of 10 B1-B0 patients (Fig. 5b), showed no expression of KIR3DL2. In the remaining 5 patients, KIR3DL2 expression on CD4+ T cells varied from 4.2 to 50.6 percent. On average, 30.2% of CD4+ T cells from B1-B0 patients showed no expression of CD26, 25% of CD4+ T cells expressed CD164, but only 9.5% expressed KIR3DL2. Our results indicate that KIR3DL2 expression is mainly evident in patients with advanced B2 disease. Importantly, in patients expressing both molecules, CD164 and KIR3DL2 were predominantly co-expressed on the same CD4+ T cells (Fig. 5c).
Fig. 5. CD164 and KIR3DL2 are co-expressed on CD4+ T cells from Sézary syndrome patients.
PBMC from patients diagnosed as B2 (Fig. 5a, n=31) and B1-B0 (Fig. 5b, n=10) were gated on CD3+/CD4+ T cells and analyzed for the expression of KIR3DL2, CD164 and CD26 by flow cytometry. Graphs show percentage of CD4+ T cells from each patient expressing these molecules. Fig. 5c shows that CD164 and KIR3DL2 are co-expressed on CD4+T cells from SS patient diagnosed with advanced B2 disease. Error bars represent mean +/− SEM.
Discussion
In our previous report we showed that CD164 was co-expressed with a single dominant TCRVβ on CD26− CD4+T cells and its expression correlated positively with a wide range of circulating tumor burdens in SS patients [43]. Our results were subsequently supported by studies of Guenova et al. confirming that CD164 is expressed on clonal malignant CD4+T cells [14]. Based on this data, we have postulated that CD164 may serve as a positive cell surface marker that distinguishes between normal and malignant CD4+ T cells in SS patients, particularly among patients, whose TCRVβ could not be defined by antibodies. Our current studies were designed to further examine the malignant status of CD164+CD4+ T cells by assessing the expression of genes reported to differentiate SS patients from healthy individuals and by examining the pattern of expression of CD164 and KIR3DL2 on patients’ CD4+T cells.
We show that predominantly CD164+CD4+ T cells express genes associated with the Sézary signature phenotype. In accordance with this phenotype, the expression of T-plastin and the Th2–associated transcription factor GATA-3, is significantly higher in CD164+CD4+ T cells compared with CD164−CD4+ T cells. As expected, the expression of STAT-4 is significantly lower in CD164+ compared to CD164−CD4+ T cells. Furthermore, the expression of FCRL3, Tox and miR-214 is also higher in CD164+CD4+T cells in comparison to CD164−CD4+ T cells. We demonstrate the presence of CD164 mRNA and protein in skin-residing CD4+ T cells from SS patients. Levels of CD164 mRNA are significantly increased in skin lesions from SS and MF, compared to AD, PS, and normal skin. Interestingly, low levels of CD164 expression was demonstrated in skin biopsies from patients with psoriasis, however significantly lower, compared with SS or MF patients (3.6 fold, p=0.006 and 2.4 fold, p=0.02, respectively). Considering that CD164 is expressed in skin residing CD4+ T cells, this result is different from pattern of CD164 expression in circulating CD4+ T cells where CD164 expression is restricted to Sézary sybdrome, a leukemic variant of CTCL but not MF, atopic dermatitis or psoriasis patients [14, 43]. The presence of CD164 expression in skin of psoriatic patients is suggestive of some common pathways operational in the skin manifestations of these diseases, but this remains to be established.
Our results also suggest the likely existence of an association between T plastin and CD164 in patients’ skin-residing CD4+ T cells, similar to circulating malignant CD4+T cells, as mRNAs for both CD164 and T plastin are present in skin lesions from SS and MF patients.
Our data show that B1-B0 patients do not demonstrate a fully developed malignant status identified by the presence of Sézary signature phenotype, high expression of FCRL3, and miR-214, that is apparent in B2 patients. In B1-B0 patients, T plastin, FCRL3 and miR-214 are expressed in some patients, predominantly in CD164+CD4+ T cells. However, the levels are low and the differences between CD164+ and CD164− populations are not significant due to high variability in expression of these genes between the patients. Similarly, STAT-4 and Gata-3 expression in CD164+ CD4+ T cells is not significantly different from that in CD164−CD4+T cells. It is noticeable that overall, levels of STAT-4 expression in B1-B0 patients are higher than those in B2 patients whereas Gata-3 expression is comparable to the levels observed in normal controls. However, Tox, which is highly expressed in B2 patients, is also highly expressed in B1-B0 patients and its expression is significantly higher (2.9 fold) in CD164+CD4+ T cells compared with CD164−CD4+ T cells. Our results point to an important observation; the significant expression of Tox predominantly in CD164+CD4+T cells from B1-B0 patients strongly suggests that Tox may be one of the early transcription factors associated with malignant transformation in CD164+CD4+ T cells in SS patients. Its high expression in CD4+T cells and in the skin from SS patients, in conjunction with the expression of CD164 and other clinical data, may aid in specific diagnosis of SS [11].
Our data demonstrate that CD164+ CD4+T cells are the population that predominantly expresses genes linked with malignancy and poor prognosis. However, particularly in B2 patients, CD164−CD4+T cells also express the Sézary signature phenotype as well as FCRL3, Tox and miR-214, but at significantly lower levels compared to CD164+CD4+ T cells. The level of gene expression in the CD164− population appears to depend upon circulating tumor burden; the highest expression is found in patients with the highest percentage of circulating CD4+ T cells bearing a single TCRVβ. The study of Guenova et al, (2013) showed that in the presence of malignant cells, benign CD4+ T cells from SS patients acquire a Th2 biased phenotype including elevated GATA-3 expression that could be reversed by separating malignant and benign CD4+ T cells [15]. This study implies that the phenotype of CD164−CD4+ T cells in patients is influenced by the presence of pathogenic CD164+CD4+ T cells and factor (s) released by these cells. A high percentage of malignant cells may correspond to a high concentration of factor (s) influencing the phenotype of benign cells. This may explain the highly reduced STAT-4 expression in CD164−CD4+ T cells from B2 patients compared with close to normal expression of STAT-4 in CD164−CD4+ T cells from B1 patients. It is also possible that some of CD164−CD4+ T cells are normal T cells activated by malignant cells and as such, they may express various molecules including T plastin or miR-214, that have been shown to be upregulated in activated T cells [4, 20]. Whether the expression of malignancy associated genes in CD164−CD4+ T cells results from the direct effect of factor (s) triggering malignant transformation or results from factor (s) released by CD164+CD4+ malignant T cells, remains to be established
In support of our results indicating CD164 as an important marker for malignant cells in CTCL, elevated CD164 expression has been associated with several human cancers including prostate, ovarian, colon and breast carcinoma. It has been also identified as a new marker for acute lymphoblastic leukemia [10, 16, 17, 24, 40].
In summary, our results show that CD164 is expressed predominantly on CD4+ T cells expressing genes linked to malignancy and poor prognosis in Sézary syndrome patients. A well-defined Sézary signature phenotype and the expression of FCRL-3, Tox and miR-214 is significantly associated with CD164+ rather than with CD164−CD4+ T cells. The high expression of miR-214 in CD164+CD4+ T cells deserves further study as miR-214 contributes to cancer formation and progression by affecting several signaling pathways and is controlled by transcription factor Twist 1, reported to be highly expressed in SS patients’ CD4+T cells [35, 45]. Our results also show that CD164 is co-expressed with KIR3DL2 on CD4+T cells from a majority of SS patients with advanced B2 disease. Assessing the expression of both markers can be useful in the diagnosis of Sézary syndrome and monitoring of the disease, particularly in patients whose TCRVβ cannot be defined by the existing panel of antibodies. However, as our data show, B1-B0 patients do not consistently express KIR3DL2, therefore, the increased expression of CD164, along with the expression of Tox, could be the first indication of malignant transformation.
Our studies represent one of the first attempts to use a surface biomarker, such as CD164, to isolate malignant cells and characterize their phenotype in detail. Given the heterogeneous nature of the disease and variability among patients, potentially more markers will be discovered allowing for the precise identification of the phenotype of malignant cells, facilitating earlier diagnosis and the monitoring of disease progression, but may also provide a means for discovering new therapeutic targets.
Acknowledgments
This work was supported by the National Institute of Cancer, R21CA178424 to M.W&A.H.R., Translational Research Grant from the Leukemia and Lymphoma Society to A.H.R, National Institute of Arthritis and Musculoskeletal and Skin Diseases, K23-AR68433 to J.T., and K08-AR065577-03 to B.S.K., American Skin Association Research Grant to B.S.K., and in part by the Intramural Research Program of the National Cancer Institute to D.McV.
Footnotes
Conflict of interests
Dr. Helene Sicard is employed by Innate Pharma, Marseille, France. The authors state no conflict of interest.
Compliance with ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Agrawal B, Longenecker BM. MUC1 mucin-mediated regulation of human T cells. Int Immunol. 2005;17:391–399. doi: 10.1093/intimm/dxh219. [DOI] [PubMed] [Google Scholar]
- 2.Aliahmad P, Seksenyan A, Kaye J. The many roles of TOX in the immune system. Curr Opin Immunol. 2012;24:173–177. doi: 10.1016/j.coi.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballabio E, Mitchell T, van Kester MS, Taylor S, Dunlop HM, Chi J, Tosi I, Vermeer MH, Tramonti D, Saunders NJ, et al. MicroRNA expression in Sezary syndrome: identification, function, and diagnostic potential. Blood. 2010;116:1105–1113. doi: 10.1182/blood-2009-12-256719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begue E, Jean-Louis F, Bagot M, Jauliac S, Cayuela JM, Laroche L, Parquet N, Bachelez H, Bensussan A, Courtois G, et al. Inducible expression and pathophysiologic functions of T-plastin in cutaneous T-cell lymphoma. Blood. 2012;120:143–154. doi: 10.1182/blood-2011-09-379156. [DOI] [PubMed] [Google Scholar]
- 5.Bensussan A, Remtoula N, Sivori S, Bagot M, Moretta A, Marie-Cardine A. Expression and function of the natural cytotoxicity receptor NKp46 on circulating malignant CD4+ T lymphocytes of Sezary syndrome patients. J Invest Dermatol. 2011;131:969–976. doi: 10.1038/jid.2010.404. [DOI] [PubMed] [Google Scholar]
- 6.Boonk SE, Cetinozman F, Vermeer MH, Jansen PM, Willemze R. Differential expression of TOX by skin-infiltrating T cells in Sezary syndrome and erythrodermic dermatitis. J Cutan Pathol. 2015;42:604–609. doi: 10.1111/cup.12490. [DOI] [PubMed] [Google Scholar]
- 7.Campbell JJ, Clark RA, Watanabe R, Kupper TS. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. 2010;116:767–771. doi: 10.1182/blood-2009-11-251926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung JS, Dougherty I, Cruz PD, Jr, Ariizumi K. Syndecan-4 mediates the coinhibitory function of DC-HIL on T cell activation. J Immunol. 2007;179:5778–5784. doi: 10.4049/jimmunol.179.9.5778. [DOI] [PubMed] [Google Scholar]
- 9.Chung JS, Shiue LH, Duvic M, Pandya A, Cruz PD, Jr, Ariizumi K. Sezary syndrome cells overexpress syndecan-4 bearing distinct heparan sulfate moieties that suppress T-cell activation by binding DC-HIL and trapping TGF-beta on the cell surface. Blood. 2011;117:3382–3390. doi: 10.1182/blood-2010-08-302034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coustan-Smith E, Song G, Clark C, Key L, Liu P, Mehrpooya M, Stow P, Su X, Shurtleff S, Pui CH, et al. New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2011;117:6267–6276. doi: 10.1182/blood-2010-12-324004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dulmage BO, Akilov O, Vu JR, Falo LD, Geskin LJ. Dysregulation of the TOX-RUNX3 pathway in cutaneous T-cell lymphoma. Oncotarget. 2015 doi: 10.18632/oncotarget.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferenczi K, Fuhlbrigge RC, Pinkus J, Pinkus GS, Kupper TS. Increased CCR4 expression in cutaneous T cell lymphoma. J Invest Dermatol. 2002;119:1405–1410. doi: 10.1046/j.1523-1747.2002.19610.x. [DOI] [PubMed] [Google Scholar]
- 13.Goswami M, Duvic M, Dougherty A, Ni X. Increased Twist expression in advanced stage of mycosis fungoides and Sezary syndrome. J Cutan Pathol. 2012;39:500–507. doi: 10.1111/j.1600-0560.2012.01883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guenova E, Ignatova D, Chang YT, Contassot E, Mehra T, Saulite I, Navarini AA, Mitev V, Dummer R, Kazakov DV, et al. Expression of CD164 on Malignant T Cells in Sezary Syndrome. Acta Derm Venereol. 2015;96:464–467. doi: 10.2340/00015555-2264. [DOI] [PubMed] [Google Scholar]
- 15.Guenova E, Watanabe R, Teague JE, Desimone JA, Jiang Y, Dowlatshahi M, Schlapbach C, Schaekel K, Rook AH, Tawa M, et al. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin Cancer Res. 2013;19:3755–3763. doi: 10.1158/1078-0432.CCR-12-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havens AM, Jung Y, Sun YX, Wang J, Shah RB, Buhring HJ, Pienta KJ, Taichman RS. The role of sialomucin CD164 (MGC-24v or endolyn) in prostate cancer metastasis. BMC Cancer. 2006;6:195–207. doi: 10.1186/1471-2407-6-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang AF, Chen MW, Huang SM, Kao CL, Lai HC, Chan JY. CD164 regulates the tumorigenesis of ovarian surface epithelial cells through the SDF-1alpha/CXCR4 axis. Mol Cancer. 2013;12:115–127. doi: 10.1186/1476-4598-12-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y, Su MW, Jiang X, Zhou Y. Evidence of an oncogenic role of aberrant TOX activation in cutaneous T-cell lymphoma. Blood. 2015;125:1435–1443. doi: 10.1182/blood-2014-05-571778. [DOI] [PubMed] [Google Scholar]
- 19.Jain S, Stroopinsky D, Yin L, Rosenblatt J, Alam M, Bhargava P, Clark RA, Kupper TS, Palmer K, Coll MD, et al. Mucin 1 is a potential therapeutic target in cutaneous T-cell lymphoma. Blood. 2015;126:354–362. doi: 10.1182/blood-2015-02-628149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jindra PT, Bagley J, Godwin JG, Iacomini J. Costimulation-dependent expression of microRNA-214 increases the ability of T cells to proliferate by targeting Pten. J Immunol. 2010;185:990–997. doi: 10.4049/jimmunol.1000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kari L, Loboda A, Nebozhyn M, Rook AH, Vonderheid EC, Nichols C, Virok D, Chang C, Horng WH, Johnston J, et al. Classification and prediction of survival in patients with the leukemic phase of cutaneous T cell lymphoma. J Exp Med. 2003;197:1477–1488. doi: 10.1084/jem.20021726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Van Voorhees AS, Comeau MR, Artis D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra116. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim EJ, Hess S, Richardson SK, Newton S, Showe LC, Benoit BM, Ubriani R, Vittorio CC, Junkins-Hopkins JM, Wysocka M, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115:798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leccia F, Nardone A, Corvigno S, Vecchio LD, De Placido S, Salvatore F, Veneziani BM. Cytometric and biochemical characterization of human breast cancer cells reveals heterogeneous myoepithelial phenotypes. Cytometry A. 2012;81:960–972. doi: 10.1002/cyto.a.22095. [DOI] [PubMed] [Google Scholar]
- 25.Litvinov IV, Netchiporouk E, Cordeiro B, Zargham H, Pehr K, Gilbert M, Zhou Y, Moreau L, Woetmann A, Odum N, et al. Ectopic expression of embryonic stem cell and other developmental genes in cutaneous T-cell lymphoma. Oncoimmunology. 2014;3:e970025-970021-970028. doi: 10.4161/21624011.2014.970025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsui T, Kurosawa N, Hibi K, Akiyama S, Kasai Y, Sakamoto J, Ito K, Nakao A, Muramatsu T. The ratio of splicing variants of MGC-24/CD164, a sialomucin, correlates with the metastatic potential of colorectal carcinomas. J Biochem. 2000;127:1103–1107. doi: 10.1093/oxfordjournals.jbchem.a022704. [DOI] [PubMed] [Google Scholar]
- 27.Merritt WD, Taylor BJ, Der-Minassian V, Reaman GH. Coexpression of GD3 ganglioside with CD45RO in resting and activated human T lymphocytes. Cell Immunol. 1996;173:131–148. doi: 10.1006/cimm.1996.0259. [DOI] [PubMed] [Google Scholar]
- 28.Moins-Teisserenc H, Daubord M, Clave E, Douay C, Felix J, Marie-Cardine A, Ram-Wolff C, Maki G, Beldjord K, Homyrda L, et al. CD158k is a reliable marker for diagnosis of Sezary syndrome and reveals an unprecedented heterogeneity of circulating malignant cells. J Invest Dermatol. 2015;135:247–257. doi: 10.1038/jid.2014.356. [DOI] [PubMed] [Google Scholar]
- 29.Morimura S, Sugaya M, Suga H, Miyagaki T, Ohmatsu H, Fujita H, Asano Y, Tada Y, Kadono T, Sato S. TOX expression in different subtypes of cutaneous lymphoma. Arch Dermatol Res. 2014;306:843–849. doi: 10.1007/s00403-014-1501-7. [DOI] [PubMed] [Google Scholar]
- 30.Murphy GF. Cutaneous T-cell lymphoma. Adv Pathol. 1988;1:131–156. [Google Scholar]
- 31.Nagata S, Ise T, Pastan I. Fc receptor-like 3 protein expressed on IL-2 nonresponsive subset of human regulatory T cells. J Immunol. 2009;182:7518–7526. doi: 10.4049/jimmunol.0802230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narducci MG, Arcelli D, Picchio MC, Lazzeri C, Pagani E, Sampogna F, Scala E, Fadda P, Cristofoletti C, Facchiano A, et al. MicroRNA profiling reveals that miR-21, miR486 and miR-214 are upregulated and involved in cell survival in Sezary syndrome. Cell Death Dis. 2011;2:e151. doi: 10.1038/cddis.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nebozhyn M, Loboda A, Kari L, Rook AH, Vonderheid EC, Lessin S, Berger C, Edelson R, Nichols C, Yousef M, et al. Quantitative PCR on 5 genes reliably identifies CTCL patients with 5% to 99% circulating tumor cells with 90% accuracy. Blood. 2006;107:3189–3196. doi: 10.1182/blood-2005-07-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen EA, Whittaker S, Kim YH, Duvic M, Prince HM, Lessin SR, Wood GS, Willemze R, Demierre MF, Pimpinelli N, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598–2607. doi: 10.1200/JCO.2010.32.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penna E, Orso F, Taverna D. miR-214 as a Key Hub that Controls Cancer Networks: Small Player, Multiple Functions. J Invest Dermatol. 2014;135:960–969. doi: 10.1038/jid.2014.479. [DOI] [PubMed] [Google Scholar]
- 36.Poszepczynska-Guigne E, Schiavon V, D’Incan M, Echchakir H, Musette P, Ortonne N, Boumsell L, Moretta A, Bensussan A, Bagot M. CD158k/KIR3DL2 is a new phenotypic marker of Sezary cells: relevance for the diagnosis and follow-up of Sezary syndrome. J Invest Dermatol. 2004;122:820–823. doi: 10.1111/j.0022-202X.2004.22326.x. [DOI] [PubMed] [Google Scholar]
- 37.Scala E, Abeni D, Pomponi D, Narducci MG, Lombardo GA, Mari A, Frontani M, Picchio MC, Pilla MA, Caprini E, et al. The role of 9-O-acetylated ganglioside D3 (CD60) and {alpha}4{beta}1 (CD49d) expression in predicting the survival of patients with Sezary syndrome. Haematologica. 2010;95:1905–1912. doi: 10.3324/haematol.2010.026260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Showe LC, Fox FE, Williams D, Au K, Niu Z, Rook AH. Depressed IL-12-mediated signal transduction in T cells from patients with Sezary syndrome is associated with the absence of IL-12 receptor beta 2 mRNA and highly reduced levels of STAT4. J Immunol. 1999;163:4073–4079. [PubMed] [Google Scholar]
- 39.Sokolowska-Wojdylo M, Wenzel J, Gaffal E, Steitz J, Roszkiewicz J, Bieber T, Tuting T. Absence of CD26 expression on skin-homing CLA+ CD4+ T lymphocytes in peripheral blood is a highly sensitive marker for early diagnosis and therapeutic monitoring of patients with Sezary syndrome. Clin Exp Dermatol. 2005;30:702–706. doi: 10.1111/j.1365-2230.2005.01904.x. [DOI] [PubMed] [Google Scholar]
- 40.Tang J, Zhang L, She X, Zhou G, Yu F, Xiang J, Li G. Inhibiting CD164 expression in colon cancer cell line HCT116 leads to reduced cancer cell proliferation, mobility, and metastasis in vitro and in vivo. Cancer Invest. 2012;30:380–389. doi: 10.3109/07357907.2012.666692. [DOI] [PubMed] [Google Scholar]
- 41.van Doorn R, Dijkman R, Vermeer MH, Out-Luiting JJ, van der Raaij-Helmer EM, Willemze R, Tensen CP. Aberrant expression of the tyrosine kinase receptor EphA4 and the transcription factor twist in Sezary syndrome identified by gene expression analysis. Cancer Res. 2004;64:5578–5586. doi: 10.1158/0008-5472.CAN-04-1253. [DOI] [PubMed] [Google Scholar]
- 42.Watt SM, Butler LH, Tavian M, Buhring HJ, Rappold I, Simmons PJ, Zannettino AC, Buck D, Fuchs A, Doyonnas R, et al. Functionally defined CD164 epitopes are expressed on CD34(+) cells throughout ontogeny but display distinct distribution patterns in adult hematopoietic and nonhematopoietic tissues. Blood. 2000;95:3113–3124. [PubMed] [Google Scholar]
- 43.Wysocka M, Kossenkov AV, Benoit BM, Troxel AB, Singer E, Schaffer A, Kim B, Dentchev T, Nagata S, Ise T, et al. CD164 and FCRL3 are highly expressed on CD4+CD26− T cells in Sezary syndrome patients. J Invest Dermatol. 2014;134:229–236. doi: 10.1038/jid.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yawalkar N, Ferenczi K, Jones DA, Yamanaka K, Suh KY, Sadat S, Kupper TS. Profound loss of T-cell receptor repertoire complexity in cutaneous T-cell lymphoma. Blood. 2003;102:4059–4066. doi: 10.1182/blood-2003-04-1044. [DOI] [PubMed] [Google Scholar]
- 45.Yin G, Chen R, Alvero AB, Fu HH, Holmberg J, Glackin C, Rutherford T, Mor G. TWISTing stemness, inflammation and proliferation of epithelial ovarian cancer cells through MIR199A2/214. Oncogene. 2010;29:3545–3553. doi: 10.1038/onc.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]