Abstract
Rationale and objectives
Alcohol and nicotine are often taken together. In humans, intake of nicotine, via smoked tobacco, increases alcohol drinking, and alcohol increases smoking. Chronic nicotine treatment increases alcohol self-administration (SA) in laboratory animals; the reverse relationship is less clear. Most animal work modeling this has used passive administration, which lacks relevance to human co-abuse. Here, we describe a model based on sequential operant SA of alcohol and nicotine.
Methods
Animals are first trained on alcohol SA (0.19 ml of 12% w/v/delivery) and then receive separate alcohol (8% w/v) and nicotine (15 μg/kg/infusion) SA sessions on the same day (“daily dual access”). Animals then receive access to alcohol and then to nicotine (or in the reverse order) in alternating 5 min periods in 2h sessions (“alternating access”). We then determine if alternating access modifies the effects of naltrexone on responding for alcohol and nicotine.
Results
We found that with daily dual access, nicotine significantly increased alcohol SA when alcohol access occurred prior to nicotine access, and that nicotine SA significantly decreased when the alcohol SA session preceded it. During alternating access, nicotine also significantly increased alcohol intake. Naltrexone (0.3 or 1 mg/kg) significantly reduced alcohol SA during these alternating access sessions in animals that also received nicotine SA, but had minimal effects on animals receiving alcohol SA alone. Naltrexone did not affect nicotine SA under any condition.
Conclusions
This sequential access procedure effectively models the effects of nicotine on alcohol intake noted in humans.
Keywords: Abuse, Addiction, Alcohol, Drug abuse, Nicotine, Operant
Alcohol and nicotine are frequently taken together. It has been estimated that 70–80% of alcoholics smoke, a rate three times higher than in the general population (Falk et al. 2006). People that consume alcohol, but are not alcoholic are also more likely to smoke (Kandel et al. 1997). The liability to use either or both drugs is co-inherited, indicating a strong biological basis (Dani and Harris 2005; Funk et al. 2006). Exposure to nicotine via smoking or transdermal patch increases alcohol intake (Acheson et al. 2006; Barrett et al. 2006). Alcohol increases smoking, urges to smoke and smoking satisfaction (Glautier et al. 1996; King et al. 2010; Nil et al. 1984; Rose et al. 2002; 2004; Sayette 2002).
The effects of nicotine on alcohol intake in rodent models have been studied extensively. On the other hand, there are few studies on the effects of alcohol on nicotine self-administration (SA) in rodents. Home-cage intake and operant SA of alcohol are increased by the passive, repeated injection of nicotine (Bito-Onon et al. 2011; Le et al. 2000; Le et al. 2003; Olausson et al. 2001). Importantly, the positive effects of nicotine on alcohol SA develop with repeated daily injections, with no effects, or reductions occurring upon the first exposures to nicotine. Reduced intake of alcohol with repeated treatment with nicotine in a similar dose range was, however reported in one study (Sharpe and Samson 2002). In this study, animals lever pressed for access to a drinking spout; it is not known if this procedural difference could account for the observed negative effect of nicotine on alcohol intake.
Recently, it has been shown that the effects of nicotine injections on alcohol SA are highly dependent on the time of alcohol access relative to nicotine administration (Doyon et al. 2013; Hauser et al. 2012). When given immediately prior to alcohol SA sessions, nicotine injections mildly suppressed or had no effect on alcohol SA, but caused significant increases when given 3–4 h before (Doyon et al. 2013; Hauser et al. 2012). These results may help to explain the observation that the positive effects of nicotine on alcohol intake occur with repeated treatment. Taken together, these data suggest that the effects of nicotine on alcohol SA depend on the paradigm used, chronicity of the treatment with nicotine and the interval between nicotine administration and alcohol access.
We recently modeled voluntary alcohol and nicotine co-administration using an operant-based procedure (Le et al. 2010). Animals lever pressed for oral alcohol (dispensed into a drinking well) and IV nicotine simultaneously in daily sessions. We reported that alcohol intake remained the same, and nicotine intake decreased modestly under such conditions. To explain this, we suggest that differences in the within-session pattern of responding for the two drugs may mask any activational effects of nicotine on alcohol SA. We have observed that most alcohol reinforcements are earned early in limited access sessions (e.g. the first 20 min of a 1 h session), while nicotine reinforcements occur at a steady rate across the session. Therefore, circulating levels of nicotine would be significantly increased only in the latter part of the session, after alcohol intake has largely ceased. Together, these data argue that this procedure relying on simultaneous SA may not be sensitive to any positive effect of nicotine on alcohol consumption.
Here, we report the development of a new model of voluntary nicotine and alcohol co-use designed to overcome this problem. This model is based on sequential nicotine and alcohol SA. Briefly, animals are first trained to self-administer alcohol and nicotine in separate 1 h sessions on the same day (“Daily dual access”). After such training, rats respond for alcohol and/or nicotine on a multiple schedule in daily 2 h sessions. During these sessions, they receive access to alcohol and then to nicotine (or vice versa) in alternating 5 min periods for the duration of the sessions (Czachowski et al. 1999; Stairs et al. 2010). In either of these phases, nicotine and alcohol SA in animals receiving access to both drugs is compared to animals receiving access to either nicotine or alcohol alone. This first major goal of the present experiment is to determine if SA of alcohol and nicotine can be established under these conditions and to determine the impact of such combined access on alcohol and nicotine intake. Based on previous reports of delayed enhancements of alcohol intake following nicotine injections (Doyon et al. 2013; Hauser et al. 2012), we hypothesize that animals receiving nicotine SA sessions prior to alcohol SA sessions during daily dual access will self-administer more alcohol than animals receiving alcohol SA first, or alcohol alone. Based on work showing increased alcohol SA following nicotine administration in humans and animals, we hypothesize that alternating access to nicotine will also increase alcohol SA (Barrett et al. 2006; Bito-Onon et al. 2011; Le et al. 2003).
Co use of alcohol and nicotine has important implications for treatment. There is preliminary clinical data suggesting that co-abuse modifies the effects of drugs used to treat alcohol and nicotine addiction (Fucito et al. 2012; King et al. 2009; King et al. 2010), but to date, there is little animal work. Therefore, the second goal of this study is to close this research gap using the sequential access alcohol and nicotine SA procedure. Naltrexone is a non-selective opioid receptor antagonist most commonly used to treat alcohol dependence (Heilig and Egli 2006), but it also can reduce smoking (King and Meyer 2000). It reduces alcohol SA in laboratory rodents (Bienkowski et al. 1999; Dhaher et al. 2012; Le et al. 1999), but does not affect nicotine SA except at very high doses, calling into question the specificity of its effects on nicotine SA (Corrigall and Coen 1991; Liu et al. 2009). In heavy drinkers that smoke, naltrexone may be more effective in reducing alcohol intake (Fucito et al. 2012; King et al. 2009). There is also evidence for the converse relationship, in that naltrexone may preferentially improve smoking quit rates in heavy drinkers who smoke (King et al. 2009). Based on these data, we hypothesize that co-intake of nicotine in our sequential access model will enhance naltrexone-induced attenuation of alcohol intake.
Methods
Animals
Sixty male Long Evans rats, 200-225 g, were obtained from Charles River, Montreal, and allowed to acclimatize to the animal facility for 1 wk prior to the experiments. Animals were individually housed and were fed 25 g of standard lab chow and tap water 2–3 h after the daily experimental sessions finished. The vivarium temperature was 21°C and lights were on from 7 p.m. to 7 a.m. The experimental procedures followed the National Institutes of Health “Principles of laboratory animal care” (Eighth edition, 2011) and were approved by the local animal care committee of the Centre for Addiction and Mental Health.
Apparatus
Self administration of nicotine or alcohol was done in 16 chambers housed in sound-attenuating boxes operated by a Med Associates (Georgia, VT) interface system. The interior dimensions of the plexiglas chambers were 30 × 21 × 21 cm. Each chamber was equipped with two infusion pumps, one that delivered alcohol into a drinking well, and one that delivered nicotine solution through an IV catheter line. The chambers had two retractable levers. During alcohol SA, appropriate lever responding activated the infusion pump (Razel Sci., Stamford, CT) for 5 sec., delivering 0.19 ml of alcohol solution into a drinking receptacle, which was accompanied by a flashing white cue light (0.5 s on, 0.5 s off) above the lever during the 30 sec timeout period. During nicotine SA, appropriate lever responding activated the infusion pump for 0.5 s delivering the nicotine solution (in a volume of 10 μl/100 g body weight) via the IV catheter which was accompanied by continuous illumination of the white cue light above the lever for the 30 sec timeout period. During alcohol SA, only the alcohol-associated lever was present, while during nicotine SA, only the nicotine-associated lever was present. The chambers were also equipped with a red house light that was illuminated during the SA session, located near the top of the chamber opposite the levers.
Catheter and sham catheter surgery
Rats were anaesthetized using isoflurane/oxygen. Incision sites were treated with a local anesthetic (0.1 ml bupivacaine, 0.125%, s.c.). Penicillin (30 000 U, i.m.) was administered as an antibiotic prior to surgery and buprenorphine (0.01 mg/kg, s.c.) as an analgesic after surgery. Catheters were implanted into the right jugular vein as previously described (Corrigall and Coen 1989) and exited between the scapulae and was attached to the modified 22-gauge cannula connected to the fluid swivel system. After the 1 week recovery period, catheters were flushed daily with 0.1 ml of a sterile heparin-saline solution (50 U/ml). Catheter patency was tested weekly by i.v. injections of sodium methohexital (0.05 mg/kg). The data from animals that did not show rapid anesthesia following i.v. methohexital injection were excluded from analysis. Animals receiving sham catheter surgery (those that received only alcohol SA) were anesthetized and treated with analgesics and antibiotics in the same way, received a 1 cm long incision between the scapulae that was then sutured.
Procedures
Alcohol SA training
All rats were first trained to self-administer alcohol. They received daily limited access sessions (30 min) with the choice between water and alcohol in Richter tubes, with 5 d each at 3, 6 and 12 %. Animals were then trained on operant alcohol SA (12% w/v alcohol) in daily 1 h sessions. At session initiation, the houselight was illuminated, one of the retractable levers was extended, and appropriate responding resulted in alcohol delivery. Animals were trained in this way at FR1 for 4 d. The mean ± SEM numbers of alcohol reinforcements, lever presses and level of alcohol intake (g/kg/1h) over the last 2 d of this training were respectively, 21±1, 28±2 and 1±0.1. At the end of each session, the drinking wells were checked for the presence of unconsumed alcohol. If present, it was aspirated with a 1 ml syringe and its volume measured and recorded. This value was used in the calculation of the number of reinforcements consumed.
Animals were then assigned to 1 of 3 groups, matched according to the mean numbers of alcohol reinforcements received over the last 2 days of alcohol SA. Animals in groups that would self-administer nicotine, or alcohol + nicotine received catheter surgery, while those in the group that would self-administer only alcohol received sham catheter surgery, and then 1 week of recovery.
Training for alcohol and nicotine (daily dual access) SA or alcohol or nicotine SA alone
Animals then received SA sessions with alcohol (0.19 ml of 8% w/v/delivery), nicotine (15 μg/kg/infusion), or alcohol and nicotine according the following groups. Alcohol alone (Alc alone): Animals received daily 1 h alcohol SA sessions, as described above: 5 d at FR1, 3 d at FR2 and 4 d at FR3. Nicotine alone (Nic alone): Rats received daily 1 h nicotine SA sessions with the same FR progression across days. In order to control for circadian effects on drug SA, half of the animals in Alcohol and Nicotine alone groups were run in the morning, and the other half in the afternoon. Nicotine and Alcohol (Nic and Alc): Animals received daily dual access alcohol and nicotine SA sessions on the same day with the same FR progression as above, with the sessions were separated by about 5 h. The animals in the Nic and Alc group were divided into 2 further matched groups, one group (Nic-Alc) received the nicotine SA session first and then the alcohol SA session, while the other (Alc-Nic) received alcohol SA first, and then nicotine SA. During these alternating access sessions, the concentration of alcohol was reduced to 8% (w/v) from 12% in order to avoid ceiling effects, as this lower dose produces sub maximal responding. The nicotine infusion dose used throughout the experiment (15 μg/kg/infusion) was selected for the same reason.
SA of nicotine and/or alcohol on a sequential, alternating multiple schedule
After nicotine SA and/or alcohol SA became stable in the Alc alone, Nic alone and Nic and Alc groups in the daily dual access sessions, animals received sessions with the sequential alternating multiple schedule. For these sessions, animals retained the same drug SA condition as during the daily dual access. The design of the multiple schedule was adapted from studies examining alcohol, nicotine or cocaine vs. sucrose SA (Czachowski et al. 1999; Stairs et al. 2010).
Nic alone and Alc alone groups
Animals were placed in the boxes, the houselight was turned on and one of the levers (associated with either nicotine or alcohol) was extended for a 5 min period; appropriate responding (FR3) resulted in drug delivery with cue and timeout conditions as during training. The lever was then retracted and the houselight was turned off during the next 5 min period. These periods alternated for the duration of the 2 h session.
Nic and Alc
At the start of each session, the houselight was turned on and a lever extended, for a 5 min period during which animals could press for alcohol or nicotine at FR3. During the next 5 min period, this lever was retracted, and the lever for the other drug extended, and animals were allowed to press for the other drug for 5 min. These periods alternated in this way for the duration of the 2 h session. The animals in the Nic and Alc group were assigned to 2 subgroups, “Nic first” that received opportunity to self-administer nicotine during the first 5 min interval and “Alcohol first” that receive alcohol first. These groups comprised equal numbers of animals from the above Alc-Nic and Nic-Alc groups during the daily dual SA phase. After this initial test with the multiple schedule, animals received 2 days of daily dual access sessions, as described above, prior to the initiation of the naltrexone testing on the multiple schedule.
Test of the effects of naltrexone on responding for alcohol and/or nicotine on a multiple schedule
Animals were administered vehicle (saline) or naltrexone at doses of 0.3 or 1 mg/kg 15 min prior to the alternating access multiple schedule sessions. These doses used are based on previous reports (Corrigall and Coen 1991; Dhaher et al. 2012; Le et al. 1999). Vehicle and the two doses of naltrexone were administered in counterbalanced order with each test separated by at least 2 d, on which animals received daily dual access sessions.
Drugs
Alcohol (Commercial Alcohols Incorporated, Tiverton, ON) was diluted with tap water and is the concentration is expressed as weight/volume (w/v). Nicotine bitartrate solutions (Sigma) for IV SA were prepared daily using sterile saline, and their pH was adjusted to 6.8-7.2. Naltrexone HCl was diluted in saline vehicle and injected IP in a volume of 1 ml/kg. Drug doses are expressed as base.
Data analysis and presentation
For the data from the daily dual access training phase, nicotine and alcohol reinforcements and lever presses were collapsed across the days at each FR and analyzed with one-way ANOVA separately for each drug, using the between factor of Group (for the alcohol data: Alc alone, Nic-Alc and Alc-Nic; for the nicotine data: Nic alone, Nic-Alc and Alc-Nic). Nicotine and alcohol reinforcements and lever presses during the multiple schedule sessions prior to the tests with naltrexone were analyzed with one way ANOVAs with the between factors of Group (for nicotine: Nic alone, Alc first, Nic first, for alcohol: Alc alone, Alc first, Nic first). Statistical tests on the alternating access sessions when naltrexone was administered were done separately within each group using the within factor of naltrexone dose. Significant effects from ANOVA (p values < 0.05) were followed by post-hoc tests using the Newman-Keuls procedure.
Results
Daily dual access to nicotine and alcohol SA
Figure 1 shows the data from operant sessions when animals received daily dual access to alcohol and nicotine in separate sessions on the same day (Alc-Nic and Nic Alc), or single sessions with only alcohol (Alc only) or nicotine (Nic only), at the different FR requirements. Alcohol is shown in panel A, and Nicotine in panel B. The data from the animals receiving both alcohol and nicotine is grouped according to the order that the daily alcohol and nicotine SA sessions occurred (nicotine SA first (Nic-Alc) or alcohol SA first (Alc-Nic)). The top panel shows total numbers of nicotine or alcohol reinforcements received during the 1 h sessions, and the bottom panel shows the total numbers of lever presses made. Data are collapsed across the days at each FR (5 days at FR1, 3 days at FR2 and 4 days at FR3).
Figure 1. Self-administration (SA) in animals receiving daily SA sessions with alcohol alone, nicotine alone, alcohol followed by nicotine or nicotine followed by alcohol.
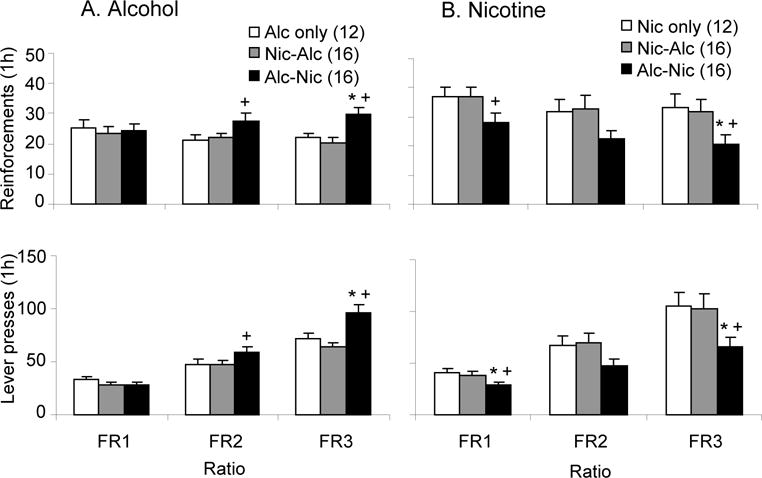
A. Mean (± sem) number alcohol reinforcements earned and lever presses made by animals in the different SA conditions in the daily SA sessions. Animals were assigned to one of three groups and received an alcohol SA session (Alc only), a nicotine SA session followed by an alcohol SA session (Nic-Alc), or an alcohol SA session followed by a nicotine SA session (Alc-Nic). Alcohol reinforcements were 0.19 ml of 8 % w/v alcohol, and for nicotine 15 μg/kg/infusion. B. Mean (± sem) number of nicotine reinforcements earned and lever presses. Animals received a nicotine SA session (Nic only), a nicotine SA session followed by an alcohol SA session (Nic-Alc), or an alcohol SA session followed by a nicotine SA session (Alc-Nic). * Significantly different from Alc only or Nic only group; + significantly different from Nic-Alc group (p’s<0.05). For this and the remaining figures, values in parentheses are the group N’s.
Alcohol
Animals that received daily alcohol SA prior to nicotine SA received significantly greater numbers of alcohol reinforcements and lever pressed significantly more for alcohol at FR2 and FR3, compared to those receiving access to only alcohol or that received nicotine SA prior to alcohol SA. This was reflected in significant effects of Group on alcohol reinforcements at FR2 (F(2,43)=3.75, p<0.05) and FR3 (F(2,43)=12.24, p<0.05) and on lever presses for alcohol at FR2 (F(2,43)=3.51, p<0.05) and FR3 (F(2,43)=13.29, p<0.05).
Nicotine
Animals that received alcohol SA prior to nicotine SA received significantly fewer numbers of nicotine reinforcements and lever pressed significantly less for nicotine compared to those that self-administered only nicotine or those that received alcohol SA after nicotine at FR1 and FR3. In the statistical analysis, this was reflected in significant effects of Group at FR1 (F(2,42)=3.97, p<0.05) and FR3 (F(2,42)=5.39, p<0.05) on nicotine reinforcements and at FR1 (F(2,4)=4.75, p<0.05) and FR3 (F(2,43)=5.30, p<0.05) for lever presses for nicotine.
SA of nicotine and/or alcohol on a sequential alternating multiple schedule
Effect of alternating access to nicotine on alcohol SA
Figure 2, left panel, shows the effects of nicotine SA in alternating 5 min sessions on numbers of alcohol reinforcements received and lever pressing. The initial analysis of these data did not reveal any significant effects of order (alcohol or nicotine in the first access period), so the data from both order groups were merged. Compared to animals self-administering alcohol alone, those receiving alternating access to nicotine and alcohol received significantly higher numbers of alcohol reinforcements (F(1,39)=12.55, p<0.05) (A) and lever pressed more (F(1,39)=16.39, p<0.05) (B).
Figure 2. SA in animals on a multiple schedule receiving alternating 5 min access periods to alcohol and nicotine or to nicotine or alcohol alone.
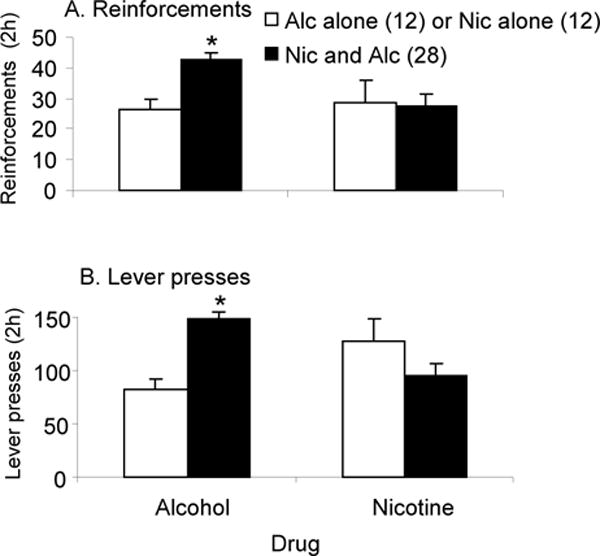
A. Mean (± sem) number of alcohol (left) and nicotine reinforcements (right) earned by animals receiving alternating access to alcohol alone (Alc alone), nicotine alone (Nic alone) or alcohol and nicotine together (Nic and Alc). B. Mean (± sem) number of lever presses for alcohol (left) and nicotine (right) made by animals receiving alternating access to alcohol alone (Alc alone), nicotine alone (Nic alone) (white bars) or nicotine and alcohol (Nic and Alc) (black bars). * Significantly different from (p<0.05).
Effect of alternating access to alcohol on nicotine SA
The right panel of Figure 2 shows the effects of alcohol SA in alternating 5 min sessions on nicotine intake and lever pressing. Since there were no significant effects of whether nicotine or alcohol was available first in these alternating access sessions, the data from both order groups were merged. The numbers of nicotine reinforcements received (A) and numbers of lever presses (B) made by animals receiving alternating access to alcohol and nicotine did not differ significantly from animals receiving access to nicotine alone (p>0.05).
Effect of naltrexone on nicotine SA and/or alcohol SA under sequential, alternating access conditions
Figure 3, left panel, shows the effects of alternating access to nicotine on naltrexone-induced reductions in numbers of alcohol reinforcements received (top) and lever pressing (bottom). One-way ANOVA on the data from animals receiving alternating access to both alcohol and nicotine (Nic + Alc) revealed a significant effect of naltrexone dose on alcohol reinforcements (F(2,79)=14.44, p<0.05) and lever presses (F(2,79)=7.26, p<0.05). Both doses of naltrexone significantly reduced numbers of alcohol reinforcements and lever presses for alcohol compared to the vehicle condition in these animals. Naltrexone modestly reduced numbers of alcohol reinforcements and lever presses in animals self-administering alcohol alone (Alc alone), but these effects did not reach statistical significance (respectively, p= 0.084 and 0.085). The results of an ANOVA done including the factor of Group, revealed there was a significant effect of Group on alcohol SA; overall, animals with alternating access to both drugs lever pressed more (F(1,70)=14.11, p<0.05) and received more alcohol reinforcements (F(1,70)=8.59, p<0.05).
Figure 3. Effects of naltrexone on SA in animals on a multiple schedule receiving alternating 5 min access periods to alcohol and nicotine or to nicotine or alcohol alone.
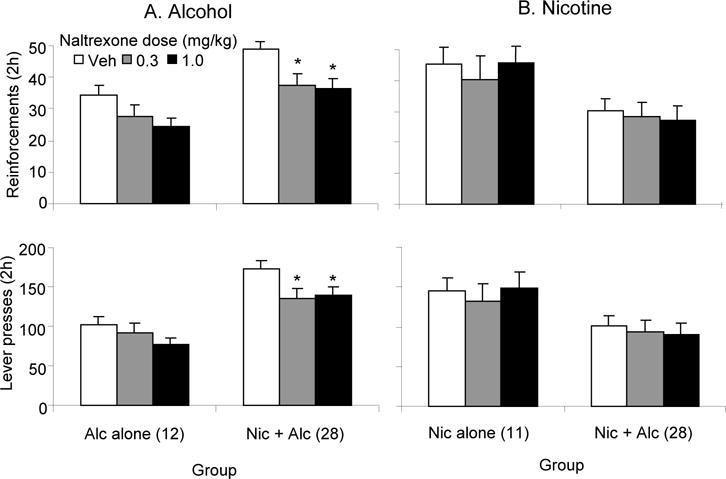
Mean (± sem) number alcohol (A) or nicotine (B) reinforcements earned and lever presses made by animals receiving alternating access to alcohol alone (Alc alone), nicotine alone (Nic alone) or nicotine and alcohol (Nic + Alc) following injections of vehicle or different doses of naltrexone (0.3, 1.0 mg/kg). Animals received vehicle and each dose of naltrexone in counterbalanced order. * Significantly different from vehicle group (p<0.05).
Figure 3, right panel, shows the effects of alternating access to alcohol SA on the effects of naltrexone on nicotine reinforcements received (top) and lever pressing (bottom). Naltrexone did not significantly affect nicotine reinforcements or lever presses in animals receiving alternating access to nicotine and alcohol (Nic + Alc) or in those receiving nicotine alone (Nic alone) (p’s >0.05). ANOVA with the factor of group revealed that, overall, animals with alternating access to both alcohol and nicotine lever pressed less (F(1,67)=5.94, p<0.05), and received fewer reinforcements of nicotine (F(1,67)=5.92, p<0.05) than did animals self-administering nicotine alone.
Discussion
The key findings of our study were that alcohol SA was increased in animals that also received exposure to nicotine SA. This effect of nicotine SA occurred under 2 different conditions: daily, sequential limited access sessions for alcohol and nicotine, and during SA sessions when alcohol and nicotine were self-administered in alternating 5 min access periods. We also demonstrated the nicotine SA enhanced naltrexone-induced reductions in alcohol intake under conditions of alternating access. This is the first demonstration that the voluntary SA of nicotine increases alcohol SA, and that such co-administration of nicotine enhances the effects of a drug on alcohol SA.
The effects of passive administration of nicotine on alcohol intake have been extensively studied in rodents. Acute injections of nicotine do not affect, or decrease alcohol intake upon initial exposure, but when given repeatedly, can increase alcohol intake over days (Bito-Onon et al. 2011; Le et al. 2000). From these data, we hypothesized that nicotine SA sessions prior to alcohol SA sessions would increase alcohol intake during the daily dual sessions. We found instead that animals receiving SA sessions with alcohol prior to SA sessions with nicotine self-administered more alcohol than those self-administering alcohol alone. Animals receiving access to nicotine followed by alcohol self-administered the same amount of alcohol as those receiving alcohol alone. The increases in alcohol consumption were evident 1 week after initiation of the daily dual access sessions. The time course of the emergence of this effect is in part consistent with work showing that the effects of passive nicotine injections on alcohol SA take days to develop (Bito-Onon et al. 2011; Le et al. 2000)., although this may be confounded in our study by the escalating demand requirements during the FR training.
Recent work has shown that the effects of acute, noncontingent injections of nicotine on alcohol SA are highly dependent on when the alcohol SA test is conducted. These studies reported that nicotine-induced increases in alcohol SA occurred 3 or 4 h after nicotine injection (Doyon et al. 2013; Hauser et al. 2012). Doyon et al. found that the increased drinking seen 3 h after nicotine injection was accompanied by significantly reduced alcohol-induced activation of the mesolimbic dopamine (DA) system. This is a projection system originating in the ventral tegmental area (VTA) and projecting to the nucleus accumbens that is critical to the reinforcing effects of drugs of abuse, including alcohol and nicotine(Wise 2002). Nicotine injections resulted in decreased alcohol-induced DA release in the nucleus accumbens and decreased alcohol-induced increases in DA neuronal firing in the VTA (Doyon et al. 2013). These effects were shown to be long-lasting, persisting for up to 40 h. The mechanism underlying this was shown to involve nicotine-induced corticosterone release, that resulted in enhanced GABAergic inhibition of DA cell firing (Doyon et al. 2013). Increased self-administration of alcohol observed 3 h after nicotine injection was inferred to occur because animals would self-administer more alcohol to correct the deficit in DA cell firing.
This mechanism is not supported by our data, as we found increased alcohol intake in animals receiving alcohol access prior to nicotine access during the daily dual sessions. In addition, we saw no effect of daily nicotine SA on alcohol intake when the sessions occurred 5 h prior to the daily alcohol SA. Although the reasons for this are not known, it is possible that differences between the two paradigms may contribute to the discrepant results. Doyon et al. administered a high, bolus dose of nicotine non-contingently, whereas the animals in our study received a lower dose of nicotine that was administered voluntarily.
We also report that access to alcohol and nicotine SA in alternating 5 min periods resulted in significantly increased alcohol SA. This occurred irrespective of whether alcohol or nicotine was presented first during the alternating access sessions. In our previous study, we found that simultaneous access to oral alcohol and i.v. nicotine reduced nicotine intake, while alcohol intake stayed the same (Le et al. 2010). We hypothesized that the lack of a positive effect of nicotine on alcohol intake occurred due to the fact that most of the alcohol is consumed early in the session, while nicotine intake occurs steadily across the session. Therefore, alcohol intake ceases before significant, sustained circulating levels of nicotine are achieved. The alternating procedure we employed in the current study, that restricted alcohol and nicotine intake to 5 min periods, overcame this problem, and with it we demonstrated increases in alcohol SA as a result of nicotine SA.
A mechanism consistent with the enhancement of alcohol intake produced by SA of nicotine during the alternating access sessions involves nicotine’s acute activating effects on the mesolimbic DA system. Acute injections of nicotine induce DA release in the nucleus accumbens (Damsma et al. 1989; Tizabi et al. 2007). This nicotine-induced activation of DA transmission may drive alcohol consumption through amplification of the rewarding impact of alcohol.
Another mechanism that could explain the effects of nicotine SA on alcohol SA is a modification of brain opioids by chronic nicotine exposure. Alcohol intake is reduced by antagonists of the mu opioid receptor (Hyytia and Kiianmaa 2001; Kim et al. 2000; Krishnan-Sarin et al. 1998; Mhatre and Holloway 2003) and the delta opioid receptor (Hyytia and Kiianmaa 2001; Le et al. 1993; Nielsen et al. 2008). Chronic nicotine changes delta opioid receptor (McCarthy et al. 2010) and mu opioid receptor sensitivity (Galeote et al. 2006; Marco et al. 2007; Walker et al. 1998; Walters et al. 2005; Wewers et al. 1999). It is possible that the chronic nicotine exposure experienced by the animals in our study potentiated its effects on alcohol intake through a change in these brain opioid systems.
There is evidence that alcohol exposure can affect nicotinic acetylcholinergic receptors (nAChRs), and this may be a mechanism that contributed to our findings. A hallmark effect of nicotine administration is a rapid desensitization of nAChRs (Brody et al. 2006; Picciotto et al. 2008). Alcohol can affect both nAChR number and the effects of nicotine on nAChR number, although the direction of these effects of alcohol are not completely clear (Dohrman and Reiter 2003; Marszalec et al. 1999). These results suggest that alcohol’s effects on nicotine-induced nAChR desensitization is a potential mechanism through which the two drugs may interact.
In laboratory animals, little work has been done on the effects of alcohol on nicotine intake. We found modest decreases in nicotine SA in response to daily passive injections of alcohol (0.25 and 0.5 g/kg) given 15 min prior to nicotine SA (Wang 2003). We subsequently showed that simultaneous access to alcohol SA at the same time as nicotine SA also resulted in reduced nicotine SA (Le et al. 2010). Consistent with this, in the present study, alcohol SA in limited access sessions prior to nicotine SA sessions reduced nicotine SA.
We did not observe significant effects of alcohol on nicotine intake in the alternating access, multiple schedule sessions, although there was a slight trend towards decreased responding for nicotine. There was, however, an overall reduction in nicotine intake in animals self-administering both drugs in the vehicle condition in the naltrexone test with alternating access. The reasons for this difference are not known. One possibility is that, at the time of the naltrexone tests, animals would have had more multiple schedule sessions. Another is that the animal attrition that occurred between the two tests contributed to the difference.
We found that exposure to nicotine during the alternating access sessions increased the efficacy of naltrexone in reducing alcohol SA. This likely occurred through modifications of the brain opioid systems. As mentioned, naltrexone is more effective in reducing drinking in nicotine-dependent heavy drinkers or alcoholics, compared to those that are not dependent on nicotine (Fucito et al. 2012; King et al. 2009), and these results are paralleled our the present findings in rats. A PET study reported that in alcoholic subjects, nicotine dependence was positively related to the ability of naltrexone to block delta opioid receptors (Weerts et al. 2008). This is supported by the observation of changes in delta binding produced by chronic nicotine administration in mice (McCarthy et al. 2010). These data suggest that exposure to nicotine induces changes in the brain delta receptor system making it more susceptible to blockade by naltrexone, which would explain the smoking-induced enhancement in the effects of naltrexone on drinking, and our present finding that nicotine SA enhanced the suppressive effects of naltrexone on alcohol SA.
We observed modest reductions in the alcohol intake of animals self-administering alcohol alone, but these effects did not reach statistical significance. This contrasts to previous reports of naltrexone-induced reduction of alcohol SA in non-dependent rats at a similar dose range (Le et al. 1999; Steensland et al. 2007; Williams and Broadbridge 2009). Since alcohol dependence has been shown to increase the efficacy of naltrexone in reducing drinking (Czachowski and Delory 2009), we may have found a significant effect of naltrexone if the animals in our study had greater alcohol intake.
Naltrexone did not affect nicotine intake under any experimental condition, which is consistent with the preclinical literature suggesting that naltrexone alters nicotine intake only at high, non specific doses (e.g. 3 mg/kg) (Corrigall et al. 1988). In contrast, the clinical literature suggests that heavy alcohol use is associated with increased effectiveness of naltrexone on smoking (King et al. 2009). From these clinical observations, we could speculate the alcohol intake of the animals in our study was not high enough to produce increased sensitivity to naltrexone on nicotine intake.
Methodological considerations
Although, we did not measure plasma nicotine or alcohol of the animals in our study, previous work form our lab and others suggest the animals self-administered pharmacologically relevant levels of alcohol and nicotine, in line with the amounts commonly taken by humans. Lister Hooded rats self-administering the same dose of nicotine as in our study were reported to have mean plasma nicotine levels of 66 ng/ml. In human smokers, plasma nicotine levels peak at 15-40 ng/ml with continuous smoking (Feyerabend et al. 1985; Russell et al. 1986). Therefore, the circulating nicotine levels of the animals in the present study are in line with those seen in human smokers. The animals in our study self-administering alcohol alone received and consumed about 20-25 alcohol reinforcements during the daily dual and alternating access sessions. In a previous study (Marinelli et al. 2007), we found that this level of intake resulted in blood alcohol levels of about 70 mg%. This corresponds to the blood alcohol level in humans seen after about 4 standard drinks (McKee et al. 2008; Udo et al. 2013).
A concern in the study is that during the alternating access phase, the animals in the control groups (alcohol or nicotine alone) received 5 min drug access periods, followed by 5 min periods during which there was no reinforcer and no lever. A criticism is that the responding of these animals could be potentially affected by frustration. Although this cannot be completely ruled out, it is unlikely that it affected responding, as we noted that the amounts of alcohol and nicotine consumed by animals in the alcohol and nicotine alone groups during the 2 h alternating access sessions (total of 1 h drug access) did not differ significantly from what they consumed in the 1 h-long daily dual access sessions, when they had continuous access to the levers.
A potential confound is that nicotine-induced increases in alcohol intake we observed did not occur due to effects on the reinforcing properties of alcohol, but rather due to effects on the pharmacokinetics of alcohol. This is unlikely as acute or chronic systemic injections of nicotine had no effect on the elimination of alcohol, administered i.v. (Hisaoka and Levy 1985). Although marked effects of nicotine on alcohol absorption have been described, these studies administered both drugs in a single bolus intragastrically, a manner that would maximize any effects of nicotine on absorption of alcohol from the gut (Chen et al. 2001; Parnell et al. 2006). This is unlikely to have occurred in our study, as nicotine was self-administered directly into the circulation and in small doses. Little is known about the effects of alcohol on nicotine pharmacokinetics, but it is not likely a factor in our study, as nicotine was self-administered i.v.
A potential explanation for increased alcohol SA in animals receiving nicotine after alcohol SA during the daily dual sessions is that the alcohol consumed served as an anticipatory cue for the later availability of nicotine. Although cued anticipatory increases have been shown for drugs and natural reinforcers, we think it unlikely that alcohol came to be a cue to signal nicotine availability. This is because the nicotine SA sessions occurred about 5 h after the alcohol SA session. Most studies demonstrating cue-induced anticipation of another drug have presented the cue and reinforcer in closer temporal contiguity (Palmatier et al. 2005).
Circadian effects on alcohol intake may also explain in part the increased alcohol intake in the animals self-administering nicotine after alcohol during the daily dual access SA sessions. For example, in the “Drinking in the Dark” paradigm, high levels of alcohol intake in mice are noted 2 h after onset of darkness (Giardino and Ryabinin 2013; Thiele and Navarro 2013). This would correspond roughly to the time of the alcohol SA sessions of the animals receiving alcohol SA sessions before the nicotine SA sessions during daily dual access in the present study.
Conclusions
In the present study we have established and validated a sequential access model of alcohol and nicotine SA in rats. We report that animals can be successfully trained to self-administer relevant levels of alcohol and nicotine using this model. We also demonstrated that nicotine SA can increase alcohol SA under these conditions. We further demonstrate the utility of the model in showing that the effects of naltrexone on alcohol intake are enhanced in animals that have sequential access to alcohol and nicotine SA. This demonstrates that this sequential access model is a useful procedure to study the interaction between alcohol and nicotine SA.
Acknowledgments
This work was supported by a grant from the NIAAA (AA13108) to A.D. Lê.
References
- Acheson A, Mahler SV, Chi H, de Wit H. Differential effects of nicotine on alcohol consumption in men and women. Psychopharmacology (Berl) 2006;186:54–63. doi: 10.1007/s00213-006-0338-y. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend. 2006;81:197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Kostowski W, Koros E. Ethanol-reinforced behaviour in the rat: effects of naltrexone. Eur J Pharmacol. 1999;374:321–327. doi: 10.1016/s0014-2999(99)00245-9. [DOI] [PubMed] [Google Scholar]
- Bito-Onon JJ, Simms JA, Chatterjee S, Holgate J, Bartlett SE. Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addict Biol. 2011;16:440–9. doi: 10.1111/j.1369-1600.2010.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry. 2006;63:907–15. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ, Parnell SE, West JR. Nicotine decreases blood alcohol concentration in neonatal rats. Alcohol Clin Exp Res. 2001;25:1072–7. [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–8. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Opiate antagonists reduce cocaine but not nicotine self-administration. Psychopharmacology (Berl) 1991;104:167–70. doi: 10.1007/BF02244173. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Herling S, Coen KM. Evidence for opioid mechanisms in the behavioral effects of nicotine. Psychopharmacology (Berl) 1988;96:29–35. doi: 10.1007/BF02431529. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Delory MJ. Acamprosate and naltrexone treatment effects on ethanol and sucrose seeking and intake in ethanol-dependent and nondependent rats. Psychopharmacology (Berl) 2009;204:335–48. doi: 10.1007/s00213-009-1465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Samson HH, Denning CE. Independent ethanol- and sucrose-maintained responding on a multiple schedule of reinforcement. Alcohol Clin Exp Res. 1999;23:398–403. [PubMed] [Google Scholar]
- Damsma G, Day J, Fibiger HC. Lack of tolerance to nicotine-induced dopamine release in the nucleus accumbens. Eur J Pharmacol. 1989;168:363–268. doi: 10.1016/0014-2999(89)90798-x. [DOI] [PubMed] [Google Scholar]
- Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8:1465–70. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- Dhaher R, Toalston JE, Hauser SR, Bell RL, McKinzie DL, McBride WJ, Rodd ZA. Effects of naltrexone and LY255582 on ethanol maintenance, seeking, and relapse responding by alcohol-preferring (P) rats. Alcohol. 2012;46:17–27. doi: 10.1016/j.alcohol.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrman DP, Reiter CK. Ethanol modulates nicotine-induced upregulation of nAChRs. Brain Res. 2003;975:90–8. doi: 10.1016/s0006-8993(03)02593-9. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Dong Y, Ostroumov A, Thomas AM, Zhang TA, Dani JA. Nicotine decreases ethanol-induced dopamine signaling and increases self-administration via stress hormones. Neuron. 2013;79:530–40. doi: 10.1016/j.neuron.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health. 2006;29:162–71. [PMC free article] [PubMed] [Google Scholar]
- Feyerabend C, Ings RM, Russel MA. Nicotine pharmacokinetics and its application to intake from smoking. Br J Clin Pharmacol. 1985;19:239–47. doi: 10.1111/j.1365-2125.1985.tb02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, Park A, Gulliver SB, Mattson ME, Gueorguieva RV, O’Malley SS. Cigarette smoking predicts differential benefit from naltrexone for alcohol dependence. Biol Psychiatry. 2012;72:832–8. doi: 10.1016/j.biopsych.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Marinelli PW, Le AD. Biological processes underlying co-use of alcohol and nicotine: neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Res Health. 2006;29:186–92. [PMC free article] [PubMed] [Google Scholar]
- Galeote L, Kieffer BL, Maldonado R, Berrendero F. Mu-opioid receptors are involved in the tolerance to nicotine antinociception. J Neurochem. 2006;97:416–23. doi: 10.1111/j.1471-4159.2006.03751.x. [DOI] [PubMed] [Google Scholar]
- Giardino WJ, Ryabinin AE. CRF1 receptor signaling regulates food and fluid intake in the drinking-in-the-dark model of binge alcohol consumption. Alcohol Clin Exp Res. 2013;37:1161–70. doi: 10.1111/acer.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glautier S, Clements K, White JA, Taylor C, Stolerman IP. Alcohol and the reward value of cigarette smoking. Behav Pharmacol. 1996;7:144–154. [PubMed] [Google Scholar]
- Hauser SR, Getachew B, Oster SM, Dhaher R, Ding ZM, Bell RL, McBride WJ, Rodd ZA. Nicotine modulates alcohol-seeking and relapse by alcohol-preferring (P) rats in a time-dependent manner. Alcohol Clin Exp Res. 2012;36:43–54. doi: 10.1111/j.1530-0277.2011.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–76. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hisaoka M, Levy G. Kinetics of drug action in disease states XI: effect of nicotine on the pharmacodynamics and pharmacokinetics of phenobarbital and ethanol in rats. J Pharm Sci. 1985;74:412–5. doi: 10.1002/jps.2600740409. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin Exp Res. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Kandel D, Chen K, Warner LA, Kessler RC, Grant B. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the U.S. population. Drug Alcohol Depend. 1997;44:11–29. doi: 10.1016/s0376-8716(96)01315-4. [DOI] [PubMed] [Google Scholar]
- Kim SG, Stromberg MF, Kim MJ, Volpicelli JR, Park JM. The effect of antagonists selective for mu- and delta-opioid receptor subtypes on alcohol consumption in C57BL/6 mice. Alcohol. 2000;22:85–90. doi: 10.1016/s0741-8329(00)00109-9. [DOI] [PubMed] [Google Scholar]
- King A, Cao D, Vanier C, Wilcox T. Naltrexone decreases heavy drinking rates in smoking cessation treatment: an exploratory study. Alcohol Clin Exp Res. 2009;33:1044–50. doi: 10.1111/j.1530-0277.2009.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, McNamara P, Angstadt M, Phan KL. Neural substrates of alcohol-induced smoking urge in heavy drinking nondaily smokers. Neuropsychopharmacology. 2010;35:692–701. doi: 10.1038/npp.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Meyer PJ. Naltrexone alteration of acute smoking response in nicotine-dependent subjects. Pharmacol Biochem Behav. 2000;66:563–72. doi: 10.1016/s0091-3057(00)00258-6. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Wand GS, Li XW, Portoghese PS, Froehlich JC. Effect of mu opioid receptor blockade on alcohol intake in rats bred for high alcohol drinking. Pharmacology Biochemistry and Behavior. 1998;59:627. doi: 10.1016/s0091-3057(97)00474-7. [DOI] [PubMed] [Google Scholar]
- Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Le AD, Lo S, Harding S, Juzytsch W, Marinelli PW, Funk D. Coadministration of intravenous nicotine and oral alcohol in rats. Psychopharmacology (Berl) 2010;208:475–86. doi: 10.1007/s00213-009-1746-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–44. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Quan B, Chow S. The effects of selective blockade of delta and mu opiate receptors on alcohol consumption by C57BL/6 mice in a restricted access paradigm. Brain Res. 1993;630:330–333. doi: 10.1016/0006-8993(93)90672-a. [DOI] [PubMed] [Google Scholar]
- Le AD, Wang A, Harding S, Juzytsch W, Shaham Y. Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology. 2003;168:216. doi: 10.1007/s00213-002-1330-9. [DOI] [PubMed] [Google Scholar]
- Liu X, Palmatier MI, Caggiula AR, Sved AF, Donny EC, Gharib M, Booth S. Naltrexone attenuation of conditioned but not primary reinforcement of nicotine in rats. Psychopharmacology (Berl) 2009;202:589–98. doi: 10.1007/s00213-008-1335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EM, Granstrem O, Moreno E, Llorente R, Adriani W, Laviola G, Viveros MP. Subchronic nicotine exposure in adolescence induces long-term effects on hippocampal and striatal cannabinoid-CB1 and mu-opioid receptors in rats. Eur J Pharmacol. 2007;557:37–43. doi: 10.1016/j.ejphar.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–55. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Marszalec W, Aistrup GL, Narahashi T. Ethanol-nicotine interactions at alpha-bungarotoxin-insensitive nicotinic acetylcholine receptors in rat cortical neurons. Alcohol Clin Exp Res. 1999;23:439–45. [PubMed] [Google Scholar]
- McCarthy MJ, Zhang H, Neff NH, Hadjiconstantinou M. Desensitization of delta-opioid receptors in nucleus accumbens during nicotine withdrawal. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-2028-z. [DOI] [PubMed] [Google Scholar]
- McKee SA, O’Malley SS, Shi J, Mase T, Krishnan-Sarin S. Effect of transdermal nicotine replacement on alcohol responses and alcohol self-administration. Psychopharmacology (Berl) 2008;196:189–200. doi: 10.1007/s00213-007-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhatre M, Holloway F. Micro1-opioid antagonist naloxonazine alters ethanol discrimination and consumption. Alcohol. 2003;29:109–16. doi: 10.1016/s0741-8329(03)00021-1. [DOI] [PubMed] [Google Scholar]
- Nielsen CK, Simms JA, Pierson HB, Li R, Saini SK, Ananthan S, Bartlett SE. A novel delta opioid receptor antagonist, SoRI-9409, produces a selective and long-lasting decrease in ethanol consumption in heavy-drinking rats. Biol Psychiatry. 2008;64:974–81. doi: 10.1016/j.biopsych.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nil R, Buzzi R, Battig K. Effects of single doses of alcohol and caffeine on cigarette smoke puffing behavior. Pharmacol Biochem Behav. 1984;20:583–90. doi: 10.1016/0091-3057(84)90308-3. [DOI] [PubMed] [Google Scholar]
- Olausson P, Ericson M, Lof E, Engel JA, Soderpalm B. Nicotine-induced behavioral disinhibition and ethanol preference correlate after repeated nicotine treatment. Eur J Pharmacol. 2001;417:117–123. doi: 10.1016/s0014-2999(01)00903-7. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Wilkinson JL, Metschke DM, Bevins RA. Stimulus properties of nicotine, amphetamine, and chlordiazepoxide as positive features in a pavlovian appetitive discrimination task in rats. Neuropsychopharmacology. 2005;30:731–41. doi: 10.1038/sj.npp.1300629. [DOI] [PubMed] [Google Scholar]
- Parnell SE, West JR, Chen WJ. Nicotine decreases blood alcohol concentrations in adult rats: a phenomenon potentially related to gastric function. Alcohol Clin Exp Res. 2006;30:1408–13. doi: 10.1111/j.1530-0277.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–42. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, Lawhon D. Potentiation of nicotine reward by alcohol. Alcohol Clin Exp Res. 2002;26:1930–1. doi: 10.1097/01.ALC.0000040982.92057.52. [DOI] [PubMed] [Google Scholar]
- Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, Lawhon D. Psychopharmacological interactions between nicotine and ethanol. Nicotine Tob Res. 2004;6:133–44. doi: 10.1080/14622200310001656957. [DOI] [PubMed] [Google Scholar]
- Russell MA, Jarvis MJ, West RJ. Use of urinary nicotine concentrations to estimate exposure and mortality from passive smoking in non-smokers. Br J Addict. 1986;81:275–81. doi: 10.1111/j.1360-0443.1986.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Sayette M. The effects of alcohol on cigarette craving. Alcohol Clin Exp Res. 2002;26:1925–7. doi: 10.1097/01.ALC.0000040961.08770.AD. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Samson HH. Repeated nicotine injections decrease operant ethanol self-administration. Alcohol. 2002;28:1–7. doi: 10.1016/s0741-8329(02)00238-0. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Neugebauer NM, Bardo MT. Nicotine and cocaine self-administration using a multiple schedule of intravenous drug and sucrose reinforcement in rats. Behav Pharmacol. 2010;21:182–93. doi: 10.1097/FBP.0b013e32833a5c9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–23. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Navarro M. “Drinking in the dark” (DID) procedures: A model of binge-like ethanol drinking in non-dependent mice. Alcohol. 2013 doi: 10.1016/j.alcohol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Bai L, Copeland RL, Jr, Taylor RE. Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol. 2007;42:413–6. doi: 10.1093/alcalc/agm057. [DOI] [PubMed] [Google Scholar]
- Udo T, Harrison EL, Shi J, Tetrault J, McKee SA. A preliminary study on the effect of combined nicotine replacement therapy on alcohol responses and alcohol self-administration. Am J Addict. 2013;22:590–7. doi: 10.1111/j.1521-0391.2013.12014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Zernig G, Young AM. In vivo apparent affinity and efficacy estimates for mu opiates in a rat tail-withdrawal assay. Psychopharmacology (Berl) 1998;136:15–23. doi: 10.1007/s002130050534. [DOI] [PubMed] [Google Scholar]
- Walters CL, Cleck JN, Kuo YC, Blendy JA. Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron. 2005;46:933–43. doi: 10.1016/j.neuron.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Wang AJ. Master’s Thesis. University of Toronto; 2003. Nicotine and ethanol interactions. [Google Scholar]
- Weerts EM, Kim YK, Wand GS, Dannals RF, Lee JS, Frost JJ, McCaul ME. Differences in delta- and mu-opioid receptor blockade measured by positron emission tomography in naltrexone-treated recently abstinent alcohol-dependent subjects. Neuropsychopharmacology. 2008;33:653–65. doi: 10.1038/sj.npp.1301440. [DOI] [PubMed] [Google Scholar]
- Wewers ME, Dhatt RK, Snively TA, Tejwani GA. The effect of chronic administration of nicotine on antinociception, opioid receptor binding and met-enkelphalin levels in rats. Brain Res. 1999;822:107–13. doi: 10.1016/s0006-8993(99)01095-1. [DOI] [PubMed] [Google Scholar]
- Williams KL, Broadbridge CL. Potency of naltrexone to reduce ethanol self-administration in rats is greater for subcutaneous versus intraperitoneal injection. Alcohol. 2009;43:119–26. doi: 10.1016/j.alcohol.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]


