Both amino acids and macromolecular organic solids can be synthesized from simple molecules in small solar system bodies.
Keywords: amino acid, meteorite, asteroid, aqueous alteration, insoluble organic matter, carbonaceous chondrite
Abstract
The exogenous delivery of organic molecules could have played an important role in the emergence of life on the early Earth. Carbonaceous chondrites are known to contain indigenous amino acids as well as various organic compounds and complex macromolecular materials, such as the so-called insoluble organic matter (IOM), but the origins of the organic matter are still subject to debate. We report that the water-soluble amino acid precursors are synthesized from formaldehyde, glycolaldehyde, and ammonia with the presence of liquid water, simultaneously with macromolecular organic solids similar to the chondritic IOM. Amino acid products from hydrothermal experiments after acid hydrolysis include α-, β-, and γ-amino acids up to five carbons, for which relative abundances are similar to those extracted from carbonaceous chondrites. One-pot aqueous processing from simple ubiquitous molecules can thus produce a wide variety of meteoritic organic matter from amino acid precursors to macromolecular IOM in chondrite parent bodies.
INTRODUCTION
Amino acids are important ingredients of life that would have been delivered to Earth by extraterrestrial sources, such as comets and meteorites (1). Amino acids are found in aqueously altered carbonaceous chondrites in good part in the form of derivatives and/or precursors that release amino acids after acid hydrolysis (2). Most of the organic carbon (>70 weight %) in carbonaceous chondrites exists in the form of solvent insoluble organic matter (IOM) with complex macromolecular structures (3). However, the origins of organic matter in meteorites are still subject to debate. Complex macromolecular organic matter can be produced by either photolysis of interstellar ices (4) or aqueous chemistry in planetesimals (5). A classic view of the origin of amino acids in meteorites is that they were formed during parent body aqueous alteration by the Strecker reaction from aldehydes, ammonia, and HCN, but the Strecker reaction can only produce α-amino acids, which represent only a fraction of the amino acids in carbonaceous chondrites (3). Alternative amino acid synthesis scenarios are ultraviolet (UV) photolysis (6, 7), cosmic ray bombardment of interstellar ices (8), and Fischer-Tropsch type (FTT) syntheses in the solar nebula and/or parent bodies (9). Spark discharge experiments on CH4, N2, and H2O with traces of NH3 have also produced relative abundances of amino acids similar to those in the Murchison meteorite, although energy sources for meteorite parent bodies may include high-energy irradiation, shock waves, and other sources (10). Interstellar photochemistry may well explain the formation of refractory organic matter in comets, including potential cometary materials, such as anhydrous chondritic interplanetary dust particles and ultracarbonaceous micrometeorites (11, 12). On the other hand, the principal source of Earth’s volatiles, inferred from hydrogen and nitrogen isotopic compositions, was probably aqueously altered carbonaceous chondrites (13), where aqueous alteration played a critical role in the inventory and variety of organic matter (14). To understand the final state of organics in planetesimals before their delivery to the early Earth, we focused on the synthesis of amino acids during aqueous alteration, which ubiquitously occurred on the parent bodies of carbonaceous chondrites that contain abundant organics (15). Here, we demonstrate one-pot synthesis of a complex suite of amino acids simultaneously with IOM via hydrothermal experiments simulating the aqueous processing as it occurred in planetesimals.
Cody et al. (5) proposed that condensation of formaldehyde in the presence of liquid water could yield insoluble organic solids similar to those found in chondritic meteorites through the formose reaction and subsequent condensation and carbonization. Further studies have shown that ammonia can be incorporated into this reaction, which could then produce organic solids containing imidazole, pyridine, and pyrrole structures (16). Some previous studies showed that the hydrothermal reaction of formaldehyde and ammonia could produce amino acids (17, 18), but these reaction products were only α-amino acids.
We conducted hydrothermal experiments isothermally at 90°C up to 250°C for 72 hours with a starting solution containing formaldehyde, glycolaldehyde (the simplest condensate of formaldehyde), ammonia, and water with a molar ratio of C/N/H2O (7.2:0.72:100) and some Ca(OH)2 (0.36), following our previous method of organic solid synthesis (16). This starting composition was selected on the basis of the assumption that organic matter in carbonaceous chondrites and comets was formed from a common precursor material that originated in the outer solar system and/or the interstellar medium (5, 19, 20); thus, the original volatile composition of carbonaceous chondrite parent bodies was close to that of comets, which typically contain H2CO/NH3/H2O (0.4 to 4:0.5 to 1.5:100) (including short-period and Oort cloud comets) (21). Glycolaldehyde has also been detected in the comet C/2014 Q2 (Lovejoy) with a concentration of 0.016% relative to H2O (22).
RESULTS
We studied the amino acid products of our experiments by high-performance liquid chromatography (HPLC) and the 150°C experiment additionally by ultraperformance liquid chromatography with fluorescence detection/quadrupole time-of-flight hybrid mass spectrometry (UPLC-FD/QToF-MS). We also performed control experiments under the same experimental conditions but without adding ammonia. Only trace amounts of amino acids (up to 5 μM) were detected after acid hydrolysis in the control experiments, and the amino acid concentrations are shown as blank-corrected values listed in Table 1. The major amino acids obtained after acid hydrolysis were glycine, alanine, β-alanine, and α- and γ-aminobutyric acid (ABA) (~30 to 1080 μM each in the 150°C samples) (Figs. 1 and 2). Small amounts of β-ABA, α- and β-aminoisobutyric acid (AIB), and glutamic acid were also found (~4 to 30 μM each in the 150°C samples). We assessed the degree of contamination by considering the relative abundances of the d- and l-enantiomers of each amino acid [including protein amino acids (aspartic acid, glutamic acid, serine, alanine, and valine) and a nonprotein amino acid (β-ABA)] in the experimental product for the 150°C run by UPLC-FD/QToF-MS (Table 1 and Fig. 1), because amino acids produced abiotically yield an equal abundance between enantiomers, whereas only l-amino acids are used to form proteins in the biosphere. Our result suggests that valine is present only as l-enantiomer (l-homochirality) and thus is likely to be derived from biological contaminants but that the other amino acids are experimental products, as indicated by their racemic enantiomeric ratios (d/l ≈ 1).
Table 1. Amino acid abundance.
Summary of the average amino acid abundance (micromolar) of the blank-corrected 6 M HCl acid-hydrolyzed reaction product solution from the hydrothermal experiment containing ammonia, formaldehyde, and glycolaldehyde heated at each temperature for 72 hours and analyzed by HPLC and UPLC-FD/QToF-MS. Quantification of the amino acids included background-level corrections using a control sample treated under the same experimental conditions without the presence of ammonia. The associated errors are SDs. The amino acid solutions were derivatized by ο-phthaldialdehyde (OPA)/N-acetyl-l-cysteine (NAC) derivatization (15 min for UPLC/QToF-MS and in-line for HPLC) and identified by comparison to the retention time of the amino acid standard run on the same day. The abundance (micromolar) of each amino acid was acquired by the peak area integration of the corresponding representative mass/charge ratio (m/z). 2nd, second run of the hydrothermal experiments; 3rd, third run of the hydrothermal experiments; free, amino acid analyses conducted without acid hydrolysis. Enantiomers could not be separated under the present chromatographic conditions for β-AIB and α-ABA. Asp, aspartic acid; Glu, glutamic acid; Ser, serine; Thr, threonine; Gly, glycine; Ala, alanine; Iva, isovaline; Val, valine; n.d., not detected.
| Peak no. | Amino acid | Concentration in micromolar | |||||||
| HPLC | UPLC/QToF-MS | ||||||||
| 90°C | 150°C | 150°C 2nd | 150°C free | 150°C 2nd (free) | 200°C | 250°C | 150°C 3rd | ||
| 1 | d-Asp | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | <3.4 |
| 2 | l-Asp | 2.6 ± 0.3 | |||||||
| 3 | l-Glu | 58 | 24 | 33 | n.d. | n.d. | 8–14 | 12.3 | 9 ± 3.4 |
| 4 | d-Glu | 11.8 ± 0.6 | |||||||
| 5 | d-Ser | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2.5 ± 0.3 |
| 6 | l-Ser | <0.1 | |||||||
| 7 | d-Thr | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | <0.1 |
| 8 | l-Thr | <0.1 | |||||||
| 9 | Gly | 249 | 331 | 278 | 31 | 22 | 272–308 | 53.0 | 344.1 ± 11 |
| 10 | β-Ala | 199 | 171 | 187 | 40 | 30 | 38–42 | n.d. | 218.9 ± 6.3 |
| 11 | d-Ala | 495 | 479 | 534 | 247 | 182 | 687–759 | n.d. | 635.8 ± 13 |
| 12 | l-Ala | 444.8 ± 11.6 | |||||||
| 13 | γ-ABA | 16 | 31 | 48 | n.d. | 2 | 132–143 | 133.3 | 53.4 ± 9.9 |
| 14 | d,l-β-AIB | 19 | 6 | 9 | n.d. | n.d. | n.d. | n.d. | <0.1 |
| 15 | d-β-ABA | 13.1 ± 0.3 | |||||||
| 16 | l-β-ABA | 13.4 ± 0 | |||||||
| 17 | α-AIB | 3.9 ± 0.1 | |||||||
| 18 | d,l-α-ABA | 78 | 100 | 53 | n.d. | n.d. | 140–191 | n.d. | 38.2 ± 3.2 |
| 19 | d-Iva | <0.1 | |||||||
| 20 | l-Iva | <0.1 | |||||||
| 21 | l-Val | n.d. | n.d. | n.d. | n.d. | n.d. | 35–64 | n.d. | <10.9 |
| 22 | d-Val | <1.0 | |||||||
| Total | 1115 | 1141 | 1142 | 319 | 235 | 1313–1521 | 199 | 1792 | |
Fig. 1. UPLC/QToF-MS chromatograms.
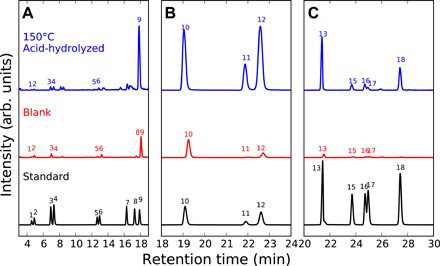
Representative UPLC/QToF-MS chromatograms for the 6 M HCl acid-hydrolyzed (total) reaction product solution from the hydrothermal experiment with ammonia, formaldehyde, and glycolaldehyde heated at 150°C for 72 hours, the control blank sample, and the amino acid standard solution. Chromatograms show derivatized positive ionization m/z values of (A) 395.0913, 409.1069, 367.0964, 381.112, and 337.0858, (B) 351.1015 (C3 amino acids), and (C) 365.1171 (C4 amino acids), respectively. Each subplot is scaled against the highest peak within the section, and thus, the peak sizes are proportional between the samples. The peak numbers correspond to the amino acids listed in Table 1. arb. units, arbitrary units.
Fig. 2. Amino acid yields (concentrations in micromolar) in the reaction products after acid hydrolysis.
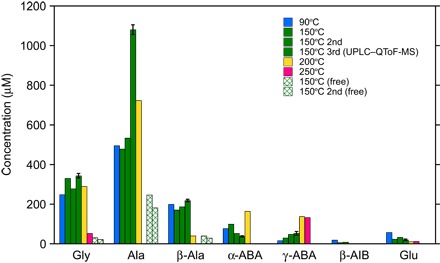
The yields without acid hydrolysis are also shown for the 150°C product. To demonstrate reproducibility and errors, the results of duplicate hydrothermal experiments for 150°C are shown, and duplicate analytical runs of the same sample are also shown for 200°C.
The amino acid abundances are much higher in the acid-hydrolyzed fraction than in the nonhydrolyzed fraction (“free” amino acids; Table 1 and Fig. 2), which suggests that most of the amino acid experimental products are present as the bound form. In the case of glycine, especially, the yield was increased by over 10 times after hydrolysis. This suggests that amino acid precursor molecules containing amides (–CO–NH–; for example, one of the simplest glycine precursors could be hydantoin) were produced during hydrothermal treatment and that amino acids were released via acid hydrolysis through breaking the amide bonds. In the acid-hydrolyzed fraction, alanine was the dominant product at 90°, 150°, and 200°C, followed by glycine and β-alanine (Fig. 2). At 250°C, γ-ABA was the most abundant amino acid product; however, the total amino acid yields (<190 μM) were much lower than that of the other experiments (>1000 μM). This observation is consistent with our previous results (16), indicating that most water-soluble molecules condensed into large macromolecular insoluble materials at high temperatures and that alkyl chains in the products increased as temperature increased. Abundant γ-ABA produced in the 250°C experiment can thus be explained by its higher thermodynamic stability, as compared to alanine, β-alanine, and α-ABA (23). The amino acid synthesis pathway involved in this hydrothermal experiment does not include other α-preferred syntheses, such as the Strecker reactions, because there is no systematic preference for α-amino acids (Fig. 2). FTT reactions were suggested to explain the presence of small, straight-chain, N-terminal (n-ω-amino) amino acids in the thermally altered meteorites (24, 25). The FTT reactions are catalytic processes that produce hydrocarbons from CO and H2 in the presence of catalysts and thus are not involved in our hydrothermal experiments.
DISCUSSION
The aqueously altered chondritic parent bodies were initially composed of icy dust containing silicates, and the ices mainly contained H2O, CO, and CO2 with some formaldehyde and ammonia (21). Decay of radioactive nuclides (most likely 26Al) and/or the occurrence of transient events, such as impact shocks (26), could have produced heat that melted the ices contained in the planetesimals to produce liquid water (27). The conditions of the aqueous alteration have been estimated to be 0° to 80°C (pH 6 to 12) for CM2 chondrites, 20° to 150°C (pH 7 to 10) for CI1 chondrites, ~120°C for CM1 chondrites, 50° to 150°C for CR chondrites, 0° to 340°C for CO and CV chondrites, and up to 260°C for ordinary chondrites (15), although these temperatures are rather uncertain. These warm and slightly alkaline conditions are preferable for the formose reaction to occur (28), and further condensation and carbonization produced complex macromolecular organic solids (5, 16). As shown in the present study, intermediate soluble components produced during these reactions can be the precursors of amino acids extracted by acid hydrolysis.
The yield of amino acids after acid hydrolysis of our experimental products, ~20 μg of glycine relative to 20 mg of organic solids (16), is consistent with that in the Murchison meteorite, where yields of glycine and IOM are 7 μg/g and 20 mg/g, respectively (29). The free amino acid abundances in the 150°C samples are 21 to 28%, and these values are somewhat less than the 38 to 64% observed in CI-CM-CR chondrites (30). The total amino acid abundances (after acid hydrolysis) vary among different meteoritic classes and petrologic types, roughly CR3 > CM2 > CI1 > CM1 ~ CR1, whereas CR2 shows significant variation. For example, the total amino acid abundances were 4.3 to 6.8 parts per million (ppm) for CI1, 0.66 to 0.71 ppm for CM1, and 5.8 to 21 ppm for CM2, and, for CRs, 0.9 to 1.6 ppm for GRO 95577 (CR1), 4.8 ppm for Renazzo (CR2), 180 to 320 ppm for EET 92042 (CR2), 249 ppm for GRA 95229 (CR2), 81 ppm for QUE 99177 (CR3), and 3.8 ppm for Shişr 033 (CR) (30–33). Thus, extended aqueous alteration (petrologic type 1) might have partially destroyed amino acids. The high amino acid concentration in the CR2 and CR3 chondrites that are considered by some workers to be the most primitive carbonaceous chondrites (19, 33) indicates that aqueous alteration is not the only amino acid synthetic pathway. UV/cosmic ray irradiation of interstellar ice could also produce the amino acids commonly found in meteorites (6–8).
Meanwhile, chondrites that have experienced even mild thermal metamorphism have lower amino acid concentrations; for example, amino acid concentrations after acid hydrolysis are 0.26 to 2.4 ppm in CO3 chondrites (24, 25), 0.7 to 6.1 ppm in CV3 chondrites (24), 1.1 to 3.3 ppm in LL3 ordinary chondrites (25), and 2.7 to 3.2 nmol/g (roughly 0.3 ppm) in CI-like chondrites that have experienced short-term heating (34, 35). The parent bodies of these meteorites have probably undergone heating at temperatures up to 700°C (36). The lower yields of amino acids in these chondrites are consistent with the experimental results in the 250°C experiment that produced significantly lower concentrations of amino acids than the experiments, which were heated to a lesser extent. It is commonly held that, although aqueous alteration can produce amino acids, and thermal alteration can also produce some amino acids, the combination of both processes can have a destructive effect. However, our experiments indicate that this reaction involving both aqueous and heating (up to 250°C) processes can also produce some amino acids. Higher temperatures are more favorable for the synthesis of some amino acids, such as γ-ABA.
Figure 3 compares the relative abundances of each amino acid normalized with respect to glycine on a molar basis in the reaction products and those extracted from the most pertinent groups of chondritic meteorites by acid hydrolysis (24, 25, 29–33, 37–41). There are no significant differences in the amino acid contents of the 90° to 200°C experimental products, suggesting that the reaction temperature in this range does not significantly affect the amino acid distribution. The amino acid distributions in the reaction products at 90° to 200°C are similar to those of the aqueously altered CI-CM-CR chondrites except for a significantly lower α-AIB abundance (Fig. 3A). In addition, in CI chondrites, the abundance of β-alanine is higher, and that of α-ABA is lower, than those of the reaction products and CM and CR chondrites (Fig. 3A). Heating duration, starting materials, pH, and catalytic effects of inorganic phases may be responsible for the small variations in the amino acids in CI-CM-CR meteorites, as well as some contributions from preexisting amino acid species produced by interstellar chemistry.
Fig. 3. Amino acid abundances relative to glycine (acid-hydrolyzed) on a molar basis.
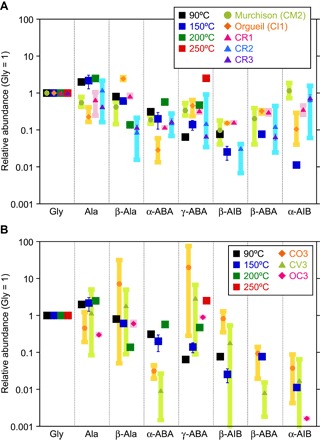
Experimental products are compared with those extracted from (A) CM, CI, and CR carbonaceous chondrites and (B) CO3 and CV3 carbonaceous chondrites and type 3 ordinary chondrites (OC). β-ABA and α-AIB were only analyzed for the 150°C sample with the UPLC/QToF-MS method. For the 150°C product, the average of three experiments with 1σ error is shown. The Murchison meteorite data are the average of seven analyses (29–32, 37–39) with maximum and minimum values. The Orgueil meteorite data are the average of four analyses (30–32, 38) with maximum and minimum values. CR1 data are the average of two analyses of GRO 95577 (30, 33) with maximum and minimum values. CR2 data are the average of eight analyses for four meteorites [Renazzo (31); EET 92042 (30, 33); GRA 95229 (33, 52); LAP 02342 (41)] with maximum and minimum values. CR3 data are from the QUE 99177 meteorite (30). CO3 data are the average of five meteorites [MIL 05013, DOM 08006, and ALHA77307 (CO3.0) (24); Colony (CO3.0) and Ornans (CO3.4) (25)] with maximum and minimum values. CV3 data are the average of six analyses for five meteorites [Allende (31); Allende, ALH 84028, LAP 02206, LAR 06317, and GRA 06101 (24)] with maximum and minimum values. Type 3 ordinary chondrite (OC3) data are the average of two meteorites [Bishunpur (LL3.15) and Chainpur (LL3.4) (25)] with maximum and minimum values.
On the other hand, the relative abundances of γ-ABA to glycine from the thermally metamorphosed carbonaceous chondrites [for example, CO3 and CV3 (24, 25, 31)] are somewhat higher than that from the experimental products, specifically at lower temperatures (Fig. 3B). This observation is consistent with the increasing γ-ABA abundances with increasing experimental temperatures. However, the abundance of α-ABA from these chondrites is lower than that from the experimental products. This is likely because non–α-amino acids are thermodynamically more stable than the α-amino acids, that is, γ-ABA > β-alanine > α-ABA (23). Meanwhile, ordinary chondrites [Bishunpur (LL3.15) and Chainpur (LL3.4) (25)] have relative abundances comparable to the experimental products (Fig. 3B). The relative abundance of α-AIB in the 150°C experimental product falls between CO, CV, and ordinary chondrites, which can again be explained by the thermodynamic stability of α-AIB, and thus, it can only be found in the meteorites that are heated to a lesser extent.
Note that the wide variations of amino acid abundances in meteorites are likely influenced by the complex chemical reactions that occurred on the parent asteroids, as well as earlier in the solar nebula, and still earlier in the precursor interstellar molecular cloud. For the thermally metamorphosed chondrites, there could be some contributions from n-ω-amino acids produced by FTT reactions in their parent bodies (24).
l-enantiomeric excesses in meteoritic amino acids (42) are not explained in our experiments. However, adsorption on asymmetric mineral surfaces and their catalytic effects could have produced enantiomeric excesses during aqueous alteration within the parent asteroids, with possible contributions from UV circularly polarized light interactions with precursor molecules in the interstellar medium (43, 44). Amino acids produced via the one-pot aqueous chemistry should be further processed by other mechanisms to produce the chiral asymmetry observed for meteoritic amino acids.
High δD values [up to 3600 per mil (‰)] reported in meteoritic amino acids (45) can be explained by considering that the starting molecules (H2CO and NH3) with high D/H ratios originated from low-temperature interstellar chemistry. Interstellar formaldehyde was reported to have high D/H ratios; for example, the [HDCO]/[H2CO] abundance ratio in molecular clouds is 0.01 to 0.03 (46), and the [D2CO]/[H2CO] abundance ratio in star-forming regions in interstellar medium is 0.02 to 0.4 (47). These values are sufficiently high, as compared to δD = 3600‰ (D/H ~ 0.0007), even if some hydrogen has exchanged with D-poor water in parent bodies. The δ13C values of individual amino acids from the Murchison meteorite range from +4.9 to +52.8‰, and this variability can be interpreted to indicate that the synthetic histories of amino acids may have been diverse, even within groups of compounds with very similar functional group composition (48). The α-amino acids show a clear decreasing trend in their 13C abundance with increasing carbon chain length, suggesting kinetically controlled syntheses of higher homologs from lower ones, although β-amino acids show the opposite trend (48). We expect that formose reactions based on the addition of carbon in our experiments follow the former trend, and thus, we postulate that the experimental products should exhibit 13C/12C fractionation in individual amino acids. A future study is needed to validate this hypothesis.
The hydrothermal reactions of formaldehyde, glycolaldehyde, and ammonia led to the production of α-, β-, and γ-amino acids, in addition to previously reported IOM-like organic solids (16). It is also reported that alkylpyridines were produced by the reaction of aldehydes and ammonia through aldol condensation and Chichibabin-type synthesis on simulated meteorite parent bodies under alkaline conditions (49). The one-pot aqueous chemistry in planetesimals, starting from ubiquitous simple molecules, is thus a plausible scenario to explain the wide variety of organic matter found in the aqueously altered chondrites.
MATERIALS AND METHODS
Hydrothermal experiments
The hydrothermal experiments were conducted following the method for the organic solid synthesis reported previously (16, 50); the protocol was also based on previous work by Cody et al. (5) and Ricardo et al. (51). The starting solution contained paraformaldehyde at 2 mmol (60 mg), glycolaldehyde at 1 mmol (60 mg), Ca(OH)2 [15 mg; given the low solubility in water, Ca(OH)2 exists largely as a solid], 4.9 M ammonium hydroxide (81 μl providing an N/C atomic ratio of 0.1), and pure water (1 ml). Paraformaldehyde was obtained from Alfa Aesar, and glycolaldehyde (dimer) was obtained from Sigma-Aldrich. Calcium hydroxide [Ca(OH)2] and ammonium hydroxide were obtained from Wako Pure Chemical. Paraformaldehyde hydrolyzed very rapidly at high pH to yield pure formaldehyde. Ca(OH)2 was added to make the starting solution with a pH in the range of 11.5 to 11.7, and Ca(OH)2 also acted as a catalyst of the formose reaction (5, 28, 51). Each solution charged into glass tubes that were flame-sealed and then heated isothermally at 90°, 150°, 200°, and 250°C for 72 hours in an oven (ETTAS HTO-450S). After heating, the residues were separated by centrifugation. Water used in this synthetic experiment and the following analytical procedures was purified with a Millipore Milli-Q system.
Amino acid analysis with HPLC
The liquid phases of the reaction products were then acid-hydrolyzed with 6 M HCl [12 M HCl (0.5 ml) was added to a 0.5-ml sample] at 110°C for 24 hours to convert them into their corresponding amino acids. Following the acid hydrolysis, the sample was dried using centrifugal drying at 60°C for approximately 9 hours. The hydrolyzed fraction was dissolved in 0.1 M HCl (2 ml) and was applied to a column packed with cation-exchange resin (Bio-Rad AG 50W-X8 Resin, 200 to 400 meshes, hydrogen form). The resin (~2 to 3 ml) was prewashed with 1 M HCl and water (20 ml each) and 1 M NaOH and water (20 ml each), followed by conditioning with 1 M HCl and water (20 ml each). A 1-ml aliquot of 0.1 M HCl sample solution was induced to the column after filtration with DISMIC-3CP (0.45 μm) and rinsed with 0.1 M HCl (1 ml) and water (20 ml). The solution containing amino acids was then eluted with 10% NH3 aqueous solution (15 ml) and water (20 ml). The NH3 eluate was dried using centrifugal drying at 60°C for approximately 5 hours. The dried sample was dissolved in 0.5 ml of water. A summary of the analytical procedures is shown in fig. S1. All glassware was baked in air at 500°C for at least 3 hours before use in all experiments.
Ten-microliter or 20-μl aliquot of the sample solution was then analyzed using an HPLC system, which was set up for amino acid analyses. The HPLC system was equipped with a system controller (Shimadzu SCL-10A), HPLC pumps (Shimadzu LC-20AD and LC-10ATvp), a polystyrene-type ion exchange column [Shimadzu Shim-pack ISC-07/S1504Na; 4.0 mm (inner diameter) × 15 cm; particle diameter, 7 μm] in a column heater (Sugai U-620 type 50) maintained at 55°C, and a fluorescence detector (Shimadzu RF-20Axs) with a 358-nm excitation wavelength and a 450-nm emission wavelength. A post-column derivatization was used with the solution of OPA (0.104 g/liter), NAC (0.65 g/liter), Na2CO3 (40.7 g/liter), H3BO3 (13.5 g/liter), K2SO4 (18.8 g/liter), and polyoxyethylene lauryl ether (0.2 g/liter). Gradient elution was performed using a Shimadzu amino acid mobile phase kit (Na type), with the gradients as follows: (A) 0.2 M sodium citrate, ethanol, and perchloric acid (pH 3.20); (B) 0.6 M sodium citrate, boric acid, and sodium hydroxide (pH 10.0); and (C) 0.2 M sodium hydroxide (for conditioning purposes). The flow rate of the carrier was 0.300 ml/min with (A) and (B) gradients shown in fig. S2. All samples were filtrated with DISMIC-3CP 0.45-μm membrane filters before analysis.
Commercial amino acid standard solutions (Wako Amino Acids Mixture Standard Solution, Type B and Type AN-2; table S1) were used for peak identifications. To prepare standard solutions for LC-FD/ToF-MS analysis, individual amino acid standards were dissolved in water and combined to create an amino acid mixture standard solution (containing 1 μM each of d,l-aspartic acid, d,l-glutamic acid, d,l-serine, d,l-threonine, glycine, β-alanine, d,l-alanine, d,l-α-ABA, d,l-β-ABA, γ-ABA, α-AIB, d,l-β-AIB, d,l-isovaline, d,l-valine, ε-amino-n-caproic acid, d,l-isoleucine, and d,l-leucine).
Amino acid analysis with UPLC/MS
Ten microliters of the 10-fold diluted hydrothermal experimental product of the 150°C experiment and the associated control sample were treated with the same preparation protocol (acid hydrolysis and desalting), as outlined in the previous section. The dried, desalted samples were redissolved in 100 μl of water. Twenty microliters of the samples was derivatized with OPA/NAC fluorescent derivatization immediately before analysis, as previously described (39).
The amino acid abundances and distributions were measured by UPLC-FD/QToF-MS at NASA Johnson Space Center (JSC) using a Waters ACQUITY UPLC and a Waters ACQUITY fluorescence detector connected to a Waters Xevo G2-XS QTof. Twenty-five microliters of the derivatized samples was separated using a Waters BEH C18 column (2.1 × 50 mm; 1.7-μm particle size), followed by a second Waters BEH phenyl column (2.1 × 150 mm; 1.7-μm particle size). Chromatographic conditions were as follows: column temperature, 30°C; flow rate, 150 μl/min; solvent A [50 mM ammonium formate and 8% methanol (pH 8.0)]; solvent B (methanol); gradient time (%B): 0 min (0), 35 min (55), and 45 min (100). The electrospray and mass spectrometer conditions have been described in detail by Glavin et al. (39). Amino acids were identified by comparison to known standards using the detected masses at the expected chromatographic retention times.
Supplementary Material
Acknowledgments
Funding: This work was supported by the Astrobiology Center of National Institutes of Natural Sciences (grant number AB271015). Y.K. was supported by Japan Society for the Promotion of Science KAKENHI (grant number JP15K17794) and The Mitsubishi Foundation. Q.H.S.C. acknowledges support from the NASA Postdoctoral Program at the JSC, administered by Universities Space Research Association through a contract with NASA. M.E.Z. was supported by the NASA Cosmochemistry Program. Author contributions: Y.K. coordinated the experiments and wrote the manuscript with assistance from Q.H.S.C. and S.T. Y.K. conducted hydrothermal experiments and HPLC analyses. Q.H.S.C. conducted UPLC/QToF-MS analyses. All authors contributed to the data interpretation and commented on the paper. Competing interests: The authors declare that they have no competing interests. Data availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from Y.K.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/3/e1602093/DC1
fig. S1. Experimental and analytical scheme.
fig. S2. Mobile phase gradient for the amino acid analyses.
fig. S3. HPLC chromatograms of amino acids in the standard solution and the reaction products from the hydrothermal experiments.
fig. S4. The 0- to 40-min region of the UPLC-FD chromatograms for the 6 M HCl acid-hydrolyzed reaction product solution from the hydrothermal experiment containing ammonia, formaldehyde, and glycolaldehyde heated at 150°C for 72 hours, the control blank sample, and the amino acid standard solution, measured at NASA JSC.
fig. S5. Mass spectra of the OPA/NAC-derivatized amino acids with an m/z of 337.0858 (corresponds to glycine), 351.1015 (alanine), 365.1171 (C4 amino acids; for example, AIB), 367.0964 (serine), 379.1328 (C5 amino acids; for example, valine), 381.112 (threonine), 393.1484 (C6 amino acids; for example, leucine), 395.0913 (aspartic acid), and 409.1069 (glutamic acid).
table S1. Wako Amino Acids Mixture Standard Solution (0.1 M HCl).
REFERENCES AND NOTES
- 1.Chyba C., Sagan C., Endogenous production, exogenous delivery and impact-shock synthesis of organic-molecules: An inventory for the origins of life. Nature 355, 125–132 (1992). [DOI] [PubMed] [Google Scholar]
- 2.Cronin J. R., Acid-labile amino acid precursors in the Murchison meteorite 1: Chromatographic fractionation. Orig. Life 7, 337–342 (1976). [DOI] [PubMed] [Google Scholar]
- 3.Pizzarello S., The chemistry of life’s origin: A carbonaceous meteorite perspective. Acc. Chem. Res. 39, 231–237 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Greenberg J. M., Li A., Mendoza-Gómez C. X., Schutte W. A., Gerakines P. A., de Groot M., Approaching the interstellar grain organic refractory component. Astrophys. J. 455, L177–L180 (1995). [Google Scholar]
- 5.Cody G. D., Heying E., Alexander C. M. O., Nittler L. R., Kilcoyne A. L. D., Sandford S. A., Stroud R. M., Establishing a molecular relationship between chondritic and cometary organic solids. Proc. Natl. Acad. Sci. U.S.A. 108, 19171–19176 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein M. P., Dworkin J. P., Sandford S. A., Cooper G. W., Allamandola L. J., Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 416, 401–403 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Muñoz Caro G., Meierhenrich U. J., Schutte W. A., Barbier B., Arcones Segovia A., Rosenbauer H., Thiemann W. H.-P., Brack A., Greenberg J. M., Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature 416, 403–406 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Kasamatsu T., Kaneko T., Saito T., Kobayashi K., Formation of organic compounds in simulated interstellar media with high energy particles. Bull. Chem. Soc. Jpn. 70, 1021–1026 (1997). [Google Scholar]
- 9.Pizzarello S., Catalytic syntheses of amino acids and their significance for nebular and planetary chemistry. Meteorit. Planet. Sci. 47, 1291–1296 (2012). [Google Scholar]
- 10.Wolman Y., Haverland W. J., Miller S. L., Nonprotein amino acids from spark discharges and their comparison with the Murchison meteorite amino acids. Proc. Natl. Acad. Sci. U.S.A. 69, 809–811 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duprat J., Dobrică E., Engrand C., Aléon J., Marrocchi Y., Mostefaoui S., Meibom A., Leroux H., Rouzaud J.-N., Gounelle M., Robert F., Extreme deuterium excesses in ultracarbonaceous micrometeorites from central Antarctic snow. Science 328, 742–745 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Floss C., Stadermann F. J., Bradley J., Dai Z. R., Bajt S., Graham G., Carbon and nitrogen isotopic anomalies in an anhydrous interplanetary dust particle. Science 303, 1355–1358 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Alexander C. M. O., Bowden R., Fogel M. L., Howard K. T., Herd C. D. K., Nittler L. R., The provenances of asteroids, and their contributions to the volatile inventories of the terrestrial planets. Science 337, 721–723 (2012). [DOI] [PubMed] [Google Scholar]
- 14.S. Pizzarello, G. W. Cooper, G. J. Flynn, The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles, in Meteorites and the Early Solar System II, D. S. Lauretta, H. Y. McSween Jr., Eds. (University of Arizona Press, 2006), pp. 625–651. [Google Scholar]
- 15.A. J. Brearley, The action of water, in Meteorites and the Early Solar System II, D. S. Lauretta, H. Y. McSween Jr., Eds. (University of Arizona Press, 2006), pp. 587–624. [Google Scholar]
- 16.Kebukawa Y., Kilcoyne A. L. D., Cody G. D., Exploring the potential formation of organic solids in chondrites and comets through polymerization of interstellar formaldehyde. Astrophys. J. 771, 19 (2013). [Google Scholar]
- 17.Fox S. W., Windsor C. R., Synthesis of amino acids by the heating of formaldehyde and ammonia. Science 170, 984–986 (1970). [DOI] [PubMed] [Google Scholar]
- 18.Marshall W. L., Hydrothermal synthesis of amino acids. Geochim. Cosmochim. Acta 58, 2099–2106 (1994). [Google Scholar]
- 19.Alexander C. M. O., Fogel M., Yabuta H., Cody G. D., The origin and evolution of chondrites recorded in the elemental and isotopic compositions of their macromolecular organic matter. Geochim. Cosmochim. Acta 71, 4380–4403 (2007). [Google Scholar]
- 20.Herd C. D. K., Blinova A., Simkus D. N., Huang Y., Tarozo R., Alexander C. M. O., Gyngard F., Nittler L. R., Cody G. D., Fogel M. L., Kebukawa Y., Kilcoyne A. L. D., Hilts R. W., Slater G. F., Glavin D. P., Dworkin J. P., Callahan M. P., Elsila J. E., De Gregorio B. T., Stroud R. M., Origin and evolution of prebiotic organic matter as inferred from the Tagish Lake meteorite. Science 332, 1304–1307 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Charnley S. B., Rodgers S. D., Interstellar reservoirs of cometary matter. Space Sci. Rev. 138, 59–73 (2008). [Google Scholar]
- 22.Biver N., Bockelée-Morvan D., Moreno R., Crovisier J., Colom P., Lis D. C., Sandqvist A., Boissier J., Despois D., Milam S. N., Ethyl alcohol and sugar in comet C/2014 Q2 (Lovejoy). Sci. Adv. 1, e1500863 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitadai N., Predicting thermodynamic behaviors of non-protein amino acids as a function of temperature and pH. Origins Life Evol. B. 46, 3–18 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Burton A. S., Elsila J. E., Callahan M. P., Martin M. G., Glavin D. P., Johnson N. M., Dworkin J. P., A propensity for n-ω-amino acids in thermally altered Antarctic meteorites. Meteorit. Planet. Sci. 47, 374–386 (2012). [Google Scholar]
- 25.Chan H.-S., Martins Z., Sephton M. A., Amino acid analyses of type 3 chondrites Colony, Ornans, Chainpur, and Bishunpur. Meteorit. Planet. Sci. 47, 1502–1516 (2012). [Google Scholar]
- 26.Rubin A. E., Petrologic evidence for collisional heating of chondritic asteroids. Icarus 113, 156–167 (1995). [Google Scholar]
- 27.MacPherson G. J., Davis A. M., Zinner E. K., The distribution of Al-26 in the early solar-system—A reappraisal. Meteoritics 30, 365–386 (1995). [Google Scholar]
- 28.Kopetzki D., Antonietti M., Hydrothermal formose reaction. New J. Chem. 35, 1787–1794 (2011). [Google Scholar]
- 29.Cronin J., Pizzarello S., Amino acids in meteorites. Adv. Space Res. 3, 5–18 (1983). [DOI] [PubMed] [Google Scholar]
- 30.Glavin D. P., Callahan M. P., Dworkin J. P., Elsila J. E., The effects of parent body processes on amino acids in carbonaceous chondrites. Meteorit. Planet. Sci. 45, 1948–1972 (2010). [Google Scholar]
- 31.Botta O., Glavin D. P., Kminek G., Bada J. L., Relative amino acid concentrations as a signature for parent body processes of carbonaceous chondrites. Orig. Life Evol. Biosph. 32, 143–163 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Martins Z., Hofmann B. A., Gnos E., Greenwood R. C., Verchovsky A., Franchi I. A., Jull A. J. T., Botta O., Glavin D. P., Dworkin J. P., Ehrenfreund P., Amino acid composition, petrology, geochemistry, 14C terrestrial age and oxygen isotopes of the Shişr 033 CR chondrite. Meteorit. Planet. Sci. 42, 1581–1595 (2007). [Google Scholar]
- 33.Martins Z., Alexander C. M. O’D., Orzechowska G. E., Fogel M. L., Ehrenfreund P., Indigenous amino acids in primitive CR meteorites. Meteorit. Planet. Sci. 42, 2125–2136 (2007). [Google Scholar]
- 34.Burton A. S., Grunsfeld S., Elsila J. E., Glavin D. P., Dworkin J. P., The effects of parent-body hydrothermal heating on amino acid abundances in CI-like chondrites. Polar Sci. 8, 255–263 (2014). [Google Scholar]
- 35.Chan Q. H. S., Chikaraishi Y., Takano Y., Ogawa N. O., Ohkouchi N., Amino acid compositions in heated carbonaceous chondrites and their compound-specific nitrogen isotopic ratios. Earth Planets Space 68, 1–13 (2016). [Google Scholar]
- 36.G. R. Huss, A. E. Rubin, J. N. Grossman, Thermal metamorphism in chondrites, in Meteorites and the Early Solar System II, D. S. Lauretta, H. Y. McSween Jr., Eds. (University of Arizona Press, 2006), pp. 567–586. [Google Scholar]
- 37.Burton A. S., Glavin D. P., Elsila J. E., Dworkin J. P., Jenniskens P., Yin Q.-Z., The amino acid composition of the Sutter’s Mill CM2 carbonaceous chondrite. Meteorit. Planet. Sci. 49, 2074–2086 (2014). [Google Scholar]
- 38.Ehrenfreund P., Glavin D. P., Botta O., Cooper G., Bada J. L., Extraterrestrial amino acids in Orgueil and Ivuna: Tracing the parent body of Cl type carbonaceous chondrites. Proc. Natl. Acad. Sci. U.S.A. 98, 2138–2141 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glavin D. P., Dworkin J. P., Aubrey A., Botta O., Doty J. H. III, Martins Z., Bada J. L., Amino acid analyses of Antarctic CM2 meteorites using liquid chromatography-time of flight-mass spectrometry. Meteorit. Planet. Sci. 41, 889–902 (2006). [Google Scholar]
- 40.Glavin D. P., Dworkin J. P., Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc. Natl. Acad. Sci. U.S.A. 106, 5487–5492 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pizzarello S., Holmes W., Nitrogen-containing compounds in two CR2 meteorites: 15N composition, molecular distribution and precursor molecules. Geochim. Cosmochim. Ac. 73, 2150–2162 (2009). [Google Scholar]
- 42.Cronin J. R., Pizzarello S., Enantiomeric excesses in meteoritic amino acids. Science 275, 951–955 (1997). [DOI] [PubMed] [Google Scholar]
- 43.Pizzarello S., Groy T. L., Molecular asymmetry in extraterrestrial organic chemistry: An analytical perspective. Geochim. Cosmochim. Acta 75, 645–656 (2011). [Google Scholar]
- 44.Pizzarello S., Yarnes C. T., Enantiomeric excesses of chiral amines in ammonia-rich carbonaceous meteorites. Earth Planet. Sci. Lett. 443, 176–184 (2016). [Google Scholar]
- 45.Pizzarello S., Huang Y. S., The deuterium enrichment of individual amino acids in carbonaceous meteorites: A case for the presolar distribution of biomolecule precursors. Geochim. Cosmochim. Acta 69, 599–605 (2005). [Google Scholar]
- 46.Loren R. B., Wootten A., High-excitation lines of deuterated formaldehyde (HDCO) in the Orion Molecular Cloud. Astrophys. J. 299, 947–955 (1985). [Google Scholar]
- 47.Loinard L., Castets A., Ceccarelli C., Lefloch B., Benayoun J.-J., Caux E., Vastel C., Dartois E., Tielens A. G. G. M., Doubly deuterated formaldehyde in star-forming regions: An observational approach. Planet. Space Sci. 50, 1205–1213 (2002). [Google Scholar]
- 48.Pizzarello S., Huang Y., Fuller M., The carbon isotopic distribution of Murchison amino acids. Geochim. Cosmochim. Acta 68, 4963–4969 (2004). [Google Scholar]
- 49.Yamashita Y., Naraoka H., Two homologous series of alkylpyridines in the Murchison meteorite. Geochem. J. 48, 519–525 (2014). [Google Scholar]
- 50.Kebukawa Y., Cody G. D., A kinetic study of the formation of organic solids from formaldehyde: Implications for the origin of extraterrestrial organic solids in primitive Solar System objects. Icarus 248, 412–423 (2015). [Google Scholar]
- 51.Ricardo A., Carrigan M. A., Olcott A. N., Benner S. A., Borate minerals stabilize ribose. Science 303, 196 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Pizzarello S., Huang Y., Alexandre M. R., Molecular asymmetry in extraterrestrial chemistry: Insights from a pristine meteorite. Proc. Natl. Acad. Sci. U.S.A. 105, 3700–3704 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/3/e1602093/DC1
fig. S1. Experimental and analytical scheme.
fig. S2. Mobile phase gradient for the amino acid analyses.
fig. S3. HPLC chromatograms of amino acids in the standard solution and the reaction products from the hydrothermal experiments.
fig. S4. The 0- to 40-min region of the UPLC-FD chromatograms for the 6 M HCl acid-hydrolyzed reaction product solution from the hydrothermal experiment containing ammonia, formaldehyde, and glycolaldehyde heated at 150°C for 72 hours, the control blank sample, and the amino acid standard solution, measured at NASA JSC.
fig. S5. Mass spectra of the OPA/NAC-derivatized amino acids with an m/z of 337.0858 (corresponds to glycine), 351.1015 (alanine), 365.1171 (C4 amino acids; for example, AIB), 367.0964 (serine), 379.1328 (C5 amino acids; for example, valine), 381.112 (threonine), 393.1484 (C6 amino acids; for example, leucine), 395.0913 (aspartic acid), and 409.1069 (glutamic acid).
table S1. Wako Amino Acids Mixture Standard Solution (0.1 M HCl).


