Abstract
A recent unprecedented outbreak of Zika virus (ZIKV) in the Americas has been associated with microcephaly and other congenital malformations in infants as well as Guillain-Barre syndrome in adults. The development of a safe and effective ZIKV vaccine is therefore an urgent global health priority. Promising data from preclinical vaccine studies in mice and monkeys suggest that an effective vaccine will likely be possible, but important scientific challenges remain. Here we review the current state of ZIKV vaccine development. We discuss different vaccination strategies and, in the context of what has been learnt from other flavivirus vaccines, we highlight challenges facing clinical evaluation of ZIKV vaccine candidates.
Introduction
ZIKV is a flavivirus that was first isolated in 1947 from a rhesus monkey in the Zika forest of Uganda. It is an enveloped, positive-sense RNA virus that shares similarities with other flaviviruses, including dengue virus (DENV), West Nile virus (WNV), yellow fever virus (YFV), Japanese encephalitis virus (JEV), and tick-borne encephalitis virus (TBEV). The unprecedented ZIKV outbreak in the Americas (Fauci and Morens, 2016; Petersen et al., 2016) prompted the World Health Organization to declare this epidemic a public health emergency of international concern from February 1 to November 18, 2016. ZIKV continues to spread in the Americas and in Asia, and the potential for explosive spread and the devastating clinical consequences in maternofetal infections underscore the urgency of the development of a ZIKV vaccine. ZIKV causes fetal microcephaly, intrauterine growth retardation, and other congenital malformations (Brasil et al., 2016a; Honein et al., 2017; Johansson et al., 2016; Melo et al., 2016; Mlakar et al., 2016; Rasmussen et al., 2016) and has also been associated with the neurologic disorder Guillain-Barre syndrome in adults (Brasil et al., 2016b; Cao-Lormeau et al., 2016; Dos Santos et al., 2016; Parra et al., 2016).
Animal models have been developed that recapitulate certain key aspects of ZIKV pathogenesis. Fetal pathology has been observed in ZIKV infected pregnant female mice (Cugola et al., 2016; Li et al., 2016; Miner et al., 2016; Yockey et al., 2016) and monkeys (Adams Waldorf et al., 2016). ZIKV appears to target placental and fetal tissues in both humans and monkeys (Driggers et al., 2016; Dudley et al., 2016) as well as cortical neural progenitor cells in the central nervous system (Cugola et al., 2016; Garcez et al., 2016; Li et al., 2016; Miner et al., 2016; Osuna et al., 2016; Qian et al., 2016), which presumably contribute to fetal pathology and neuropathology.
In this review, we highlight recent advances in ZIKV immunology and vaccine development. Preclinical vaccine studies in mice and monkeys have suggested that multiple ZIKV vaccine platforms may be highly efficacious. Early phase clinical trials have begun, and clinical efficacy trials are planned. We also review current challenges and key issues facing clinical ZIKV development.
Immunology of ZIKV Infection and Insights from Preclinical Vaccine Studies
Our understanding of the immune response to ZIKV is rapidly advancing but still far from complete. ZIKV is believed to induce both innate and adaptive immune responses that limit viral replication. The importance of innate immunity to ZIKV infection is evidenced by the enhanced susceptibility of mice lacking type I interferon (IFN) activity (Lazear et al., 2016; Yockey et al., 2016). ZIKV infection also induces IFN-induced transmembrane proteins 1 and 2 (IFITM1 and IFITM3) (Savidis et al., 2016), which have been shown to restrict viral replication. However, many questions remain as to the innate immune mechanisms that detect and respond to ZIKV.
There is an emerging consensus that ZIKV induces robust antibody responses that often cross-react with other flaviviruses, particularly DENV (Stettler et al., 2016), although it appears that there is only a single ZIKV serotype (Dowd et al., 2016a). ZIKV-specific neutralizing antibodies have been described, and monoclonal antibodies against various targets have been reported by several laboratories (Dejnirattisai et al., 2016; Sapparapu et al., 2016; Stettler et al., 2016; Swanstrom et al., 2016; Wang et al., 2016; Zhao et al., 2016). Recently, a potent neutralizing antibody, ZIKV-117, directed against a quaternary epitope on the ZIKV envelope (Env) protein dimer-dimer interface was shown to block ZIKV infection in mice and to limit early viral replication and fetal pathology when administered following ZIKV infection of pregnant mice (Sapparapu et al., 2016). Cross-reactive neutralizing antibodies against ZIKV and DENV have also been shown to bind conserved epitopes on the two viruses and have been shown to enhance viral replication in vitro (Charles and Christofferson, 2016; Dejnirattisai et al., 2016) and in mice (Stettler et al., 2016), raising the possibility that such cross-reactive antibodies might have the potential for antibody-dependent enhancement of disease.
Much remains to be learned about the cellular immune responses to ZIKV. CD4+ and CD8+ T cell responses to capsid (Cap) and Env have been observed in ZIKV-infected monkeys (Dudley et al., 2016; Osuna et al., 2016), and T cell responses to nonstructural protein 1 (NS1) and Env have been reported in ZIKV-infected humans (Stettler et al., 2016). In mice, CD8+ T cells appear to play a role in controlling ZIKV replication (Elong Ngono et al., 2017), but the importance of T cell responses in vaccine protection remains unclear.
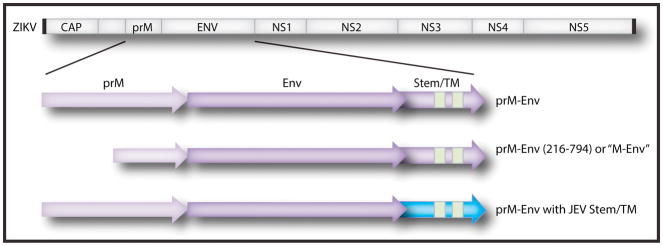
Our group demonstrated the proof-of-concept that vaccines can protect against ZIKV challenge in both mice (Larocca et al., 2016) and rhesus monkeys (Abbink et al., 2016). We developed DNA vaccines expressing the ZIKV pre-membrane (prM) and Env proteins with the signal peptide deleted for enhanced expression (prM-Env amino acids 216–794; also known as “M-Env”) (Figure 1), adenovirus (Ad) vectors expressing this same immunogen, and purified inactivated virus (PIV) vaccines. All three vaccine platforms induced ZIKV-specific neutralizing antibodies and protected both mice and rhesus monkeys against challenge with ZIKV strains from Brazil and Puerto Rico (Abbink et al., 2016; Larocca et al., 2016). In rhesus monkeys, the ZIKV PIV vaccine and the Ad vector-based vaccine induced neutralizing antibodies after a single immunization and proved more immunogenic than the DNA vaccine, although two immunizations with DNA vaccines induced sufficient neutralizing antibody titers to protect. In a subsequent study, two immunizations of DNA vaccines expressing prM-Env (Figure 1) were similarly shown to protect rhesus monkeys against ZIKV challenge (Dowd et al., 2016b). Thus, multiple vaccine platforms can provide robust protection against ZIKV challenge in both rodents and primates. However, since these immunocompetent animal models generally do not develop clinical disease, the capacity of vaccines to prevent clinical complications of ZIKV infection remains to be determined. Nevertheless, these findings raise considerable optimism that the development of a ZIKV vaccine for humans will likely be possible.
Figure 1. ZIKV genomic structure and candidate vaccine immunogens.
The pre-membrane (prM) and envelope (Env) proteins are shown in light and dark purple, respectively. Candidate immunogens that are currently being evaluated include full-length prM-Env, prM-Env (amino acids 216–794) with the “pr” cleavage peptide deleted (also termed “M-Env”) (Abbink et al., 2016; Larocca et al., 2016), and prM-Env with the JEV Env stem and transmembrane (TM) regions highlighted in blue (Dowd et al., 2016b).
Adoptive transfer studies have also shown that purified IgG from vaccinated animals confer passive protection in both mice and monkeys, providing further evidence that vaccine-induced antibodies represent a key mechanism of protection (Abbink et al., 2016; Larocca et al., 2016). Protection in passive transfer studies required serum microneutralization (MN50) titers of approximately 100 in both rodents and primates, and this correlate of protection provides a quantitative benchmark for future preclinical and clinical studies. Analogous correlates of protection based on neutralizing antibody titers have been reported in humans for other flavivirus vaccines, including JEV, TBEV, and YFV (Hombach et al., 2005; Kreil et al., 1997; Mason et al., 1973), suggesting the plausibility and generalizability of this correlate. Depletion of CD4+ and/or CD8+ T lymphocytes in vaccinated mice did not compromise protective efficacy against ZIKV challenge (Larocca et al., 2016), suggesting that vaccine-elicited T cells were not required for protection in this model, although antiviral T cells may be relevant for virologic control in other settings (Elong Ngono et al., 2017).
These data suggest that neutralizing antibodies may be a generalizable immune correlate of protection for ZIKV vaccines. The specific MN50 titer required for protection in mice and monkeys may be dependent on the experimental specifics of the models, such as the challenge dose, but nevertheless this provides a potentially useful immunologic benchmark for early phase clinical trials. Future preclinical studies will need to evaluate the potential impact of baseline immunity to DENV and other flaviviruses on the immunogenicity and protective efficacy of ZIKV vaccines, as well as the efficacy of candidate ZIKV vaccines to prevent congenital Zika syndrome in pregnant female animals.
Strategies for Clinical ZIKV Vaccine Development
Preclinical ZIKV vaccine studies have provided a strong proof-of-concept that the development of an effective ZIKV vaccine should be possible. Clinical development of ZIKV vaccines is now underway and is uniquely collaborative, focused, and efficient, with initiation of multiple phase 1 clinical trials within the first year of recognition of the ZIKV epidemic. PIV vaccines have previously been developed for other flaviviruses, including JEV and TBEV (Halstead and Thomas, 2011; Jarmer et al., 2014). DNA and PIV vaccines are particularly attractive for ZIKV, given the theoretical safety advantages of non-replicating vaccines in women who might be or might become pregnant. Other ZIKV vaccines that are in preclinical stages of development include RNA vaccines, recombinant vector-based vaccines, and purified protein subunit vaccines.
Vaccinations in a phase 1 clinical trial of the plasmid DNA vaccine (VRC-ZKADNA085-00-VP; VRC5288) produced by the Vaccine Research Center, National Institute of Allergy and Infectious Diseases (VRC/NIAID) (NCT02840487) started on August 3, 2016 in 80 flavivirus naïve men and women between the ages of 18 and 35 at low risk of acquiring ZIKV infection in the United States. This plasmid DNA vaccine is based on the H/PF/213 ZIKV strain and expresses full-length prM-Env containing the JEV stem and transmembrane region (Figure 1). Inclusion of the JEV stem and transmembrane region led to increased secretion of subviral particles, but preclinical studies in rhesus monkeys showed that this modification (VRC5288) reduced immunogenicity and protective efficacy compared to the unmodified prM-Env (VRC5283) (Dowd et al., 2016b; Pierson and Graham, 2016). This ongoing phase 1 clinical trial will enroll four groups of 20 volunteers each who will receive 4 mg of the VRC5288 DNA vaccine by the intramuscular route at weeks 0 and 8; weeks 0 and 12; weeks 0, 4, and 8; or weeks 0, 4, and 20. The primary objective is safety, and the secondary objective is immunogenicity, including ZIKV-specific neutralizing antibody titers described in assays developed at Walter Reed Army Institute of Research (WRAIR) (Larocca et al., 2016) and at NIAID (Dowd et al., 2016b) and that were protective in preclinical studies. The VRC also plans to initiate a phase 1 clinical trial of the VRC5283 DNA vaccine.
Inovio Pharmaceuticals started vaccinations in phase 1 clinical trials of another candidate plasmid DNA vaccine expressing prM-Env (GLS-5700) in 40 flavivirus naïve volunteers in the United States and Canada on July 26, 2016 and in 160 flavivirus experienced volunteers in Puerto Rico on August 29, 2016 (NCT02809443, NCT02887482). This DNA vaccine demonstrated efficacy in immunocompromised mice with evidence that plasma IgG was the correlate of protection.
Three phase 1 studies have also recently been launched by the U.S. Army, NIAID, and Beth Israel Deaconess Medical Center (BIDMC) to evaluate the safety and immunogenicity of the ZIKV PIV (ZPIV) vaccine produced by WRAIR. This vaccine is based on the PRVABC59 ZIKV strain from Puerto Rico. It was grown in Vero cells and was formalin inactivated, purified, and adjuvanted with alum. WRAIR initiated vaccinations in the first-in-human study of ZPIV on November 7, 2016 using 5 micrograms of vaccine that will be given by intramuscular injection on days 0 and 28 in 25 flavivirus-naïve volunteers and subsequently in two additional groups of 25 volunteers that will be pre-immunized with licensed vaccines for JEV or YFV to assess safety and immunogenicity in flavivirus-naïve and flavivirus-experienced individuals (NCT02963909). St. Louis University started vaccinations in a parallel dose de-escalation study on November 14, 2016 (NCT02952833), and BIDMC initiated a regimen acceleration study on November 21, 2016 (NCT02937233). The BIDMC study will assess dosing schedules of days 0 and 28, days 0 and 14, days 0 and 7, and a single ZPIV immunization on day 0. Future plans include initiation of a phase 1 ZPIV trial in Puerto Rico early in 2017 in volunteers who have natural baseline exposure to other flaviviruses, including DENV. The VRC is also planning to boost volunteers who received their DNA vaccine with ZPIV, based on previous experience with DNA prime, inactivated virus boost regimens for influenza virus (Ledgerwood et al., 2011).
NIAID is planning to advance their ZIKV DNA vaccine into efficacy trials in the Americas in 2017 if the ongoing phase 1 studies demonstrate safety and immunogenicity with neutralizing antibody titers that have been shown to protect monkeys. However, the potency and durability of immune responses elicited by DNA vaccines in humans may prove limited. This effort is further challenged by the rapidly changing incidence and localization of the ZIKV outbreak in the Americas. Intensive epidemiologic surveys in both human and mosquito populations are underway to develop the most efficient network of clinical research sites in ZIKV affected zones to execute ZIKV vaccine efficacy studies. Consideration is also being given to nonclassical study designs that would allow for rapid shifting of resources and focus from clinical sites with lower ZIKV incidence to those with higher ZIKV incidence.
WRAIR, NIAID, and BARDA are also working with Sanofi Pasteur, Emergent BioSolutions, and Takeda to advance the ZPIV vaccine into phase 2 and 3 studies if the phase 1 studies meet safety and immunogenicity benchmarks. Unlike gene-based vaccines such as plasmid DNA and RNA vaccines, the ZPIV vaccine requires longer for manufacturing to produce a sufficient amount for efficacy studies. It is likely that once ZPIV manufacturing has been completed, the ZPIV vaccine will also be tested in the clinical trial network that is being developed in the Americas (and perhaps Asia) for gene-based ZIKV vaccines.
Safety and immunogenicity data from the ongoing phase 1 clinical trials are expected soon. Given the timelines and inherent uncertainties of the clinical development process, it is important to develop a variety of vaccine platforms for ZIKV. In addition to ZPIV and DNA vaccines, RNA based vaccines, recombinant vector-based vaccines, and purified protein subunit vaccines are also currently in development, with planned pre-clinical evaluation and potential advancement into phase 1 clinical trials.
Lessons from Other Flavivirus Vaccines
There is an extensive history of successful flavivirus vaccine development, and correlates and surrogates of protection have been defined for some of these vaccines. Human vaccines have been licensed for YFV, JEV, TBEV, and DENV (Ishikawa et al., 2014). The diversity of successful flavivirus vaccines provides important insights into ZIKV vaccine development. Three JEV vaccines have demonstrated clinical efficacy but deliver different antigenic components, including a whole live attenuated virus, a recombinant chimeric live attenuated viruses, and a purified inactivated virus (Bista et al., 2001; Guirakhoo et al., 1999; Tauber et al., 2007). Each is suspected to generate varying levels of cell-mediated immunity, based on the fact the majority of T cell epitopes are in the non-structural proteins. Each also varies in the doses and schedules used and differs in the durability of protection. What is common among all the vaccines is that all three vaccines generate a neutralizing antibody response against the E protein that is believed to be important for protective efficacy (Who, 2016).
Plotkin defined a correlate of protection or predictor of protection as an immune response which statistically correlates with and is responsible for protection (Plotkin, 2010). The response may be either a mechanistic or a non-mechanistic correlate of protection. A non-mechanistic correlate does not define how a specific immune response is protective but does predict protection through its relationship with an immune response that is mechanistically responsible for protection. A mechanistic correlate of protection has a causal relationship with protection. Correlates of protection have been identified in a number of ways, including passive administration of antibody, analysis of immune responses following natural infection, analysis of immune responses in experimental vaccine recipients who were protected vs. not protected, evaluation of human infection and disease models, and evaluation of interventions in animal models. A surrogate of protection is an immune response that substitutes for the true immunologic correlate of protection (Plotkin, 2008). Thus, an immune function responsible for protection is a correlate, while a response that is measurable but not functional in a protective response is a surrogate.
JEV, TBEV, and YFV vaccines all have quantitative correlates or surrogates of protection. Neutralizing antibodies are the correlates for the YFV and JEV vaccines, while total binding antibody responses and neutralizing antibodies are correlates for the TBEV vaccine (Hombach et al., 2005; Kreil et al., 1997; Mason et al., 1973). For YFV, 0.7 neutralization units (equivalent to a 1:5 neutralizing antibody titer) is considered protective. Neutralizing units are generated by completing assays with a constant dilution of serum (antibody) while varying the amount of virus it is required to neutralize (Spector and Tauraso, 1968). Neutralizing antibody assays use the opposite approach, constant viral concentration with varying dilutions of serum. An actual sero-protective level for YFV has not yet been calculated. For JEV, a neutralizing antibody titer of 1:10 is considered protective, although 1:5 was initially considered as protective for the live attenuated virus vaccine. TBEV has ELISA and neutralizing antibody correlates of 125 ELISA Units and 1:10, respectively (Venturi et al., 2006). A single DENV vaccine has now been licensed in more than 10 countries (not including the U.S.), but a correlate has not yet been determined for this vaccine. Cross reactivity and other DENV-specific assay limitations have proven challenging, including an apparent disconnect between robust neutralizing antibody titers following vaccination and clinical efficacy (Sabchareon et al., 2012).
Challenges Facing Clinical Development of ZIKV Vaccines
Despite the unprecedented pace of preclinical and early clinical studies, important challenges remain for the clinical development of ZIKV vaccines. Development pathways and challenges for ZIKV vaccines have recently been reviewed (Marston et al., 2016; Thomas et al., 2016). The experience with other flavivirus vaccines combined with currently available preclinical data for ZIKV vaccines suggest that neutralizing antibodies will likely be important in protection against ZIKV (Abbink et al., 2016; Dowd et al., 2016b; Larocca et al., 2016). However, it remains to be determined whether a ZIKV vaccine will need to achieve true sterilizing immunity to protect a pregnant woman and her fetus from congenital ZIKV syndrome, which could be a higher bar than protecting a non-pregnant adult against ZIKV viremia. A more detailed understanding of potential viral reservoirs, such as in semen, will also be needed (Atkinson et al., 2017; Matheron et al., 2016).
Vaccine safety is critical for any prophylactic vaccine. ZIKV vaccine candidates will need to demonstrate an acceptable local and systemic safety profile. Injection site redness, swelling, and pain will need to be minimal, and the occurrence of fever, muscle aches, headache, and other systemic symptoms will need to be similar to or less than currently licensed vaccines. In addition, a ZIKV vaccine will also likely need to demonstrate safety across a broad age range, it should not cause Guillain-Barre Syndrome or other neurologic adverse events, and it should not be transmissible from vaccine recipients to non-recipients. Moreover, there should be no difference in the safety profile between baseline flavivirus-naïve and flavivirus-experienced recipients, and vaccination should not be contraindicated in pregnant women.
Assessment of vaccine immunogenicity will likely involve evaluation of both humoral and cellular responses, but it is anticipated that most development decisions will be based on neutralizing antibody titers. Licensed vaccines for other flaviviruses have validated neutralizing antibody correlates of protection, suggesting that the same concept may apply for ZIKV, and preclinical studies support this correlate as discussed above (Abbink et al., 2016; Dowd et al., 2016b; Larocca et al., 2016).
Demonstrating vaccine efficacy may be challenging for a ZIKV vaccine. The ostensibly simple task of diagnosing ZIKV infection in the context of a clinical trial with participants at risk of ZIKV exposure is non-trivial. Only 20% of patients with ZIKV infection develop symptoms, and the typical clinical symptoms of rash and low-grade fever are non-specific (Duffy et al., 2009). Serologic testing for ZIKV infection is also confounded by cross-reactive antibodies to other flaviviruses, such as DENV (Rabe et al., 2016). While detection of ZIKV RNA is highly specific, the rapid clearance of viremia complicates nucleic acid detection as the sine quo non for infection (Rabe et al., 2016). Technical solutions are being pursued for improved case definitions of ZIKV infection in the context of vaccine development, but these solutions will need to be developed quickly.
Most vaccine developers are focusing on an initial target product profile for a ZIKV vaccine that includes women of childbearing age given significant fetal morbidity in the first trimester of pregnancy (Brasil et al., 2016a) and also includes men given the possibility of sexual transmission of the virus (D’Ortenzio et al., 2016). As a result of safety concerns for women who might become pregnant during or shortly after vaccination, the use of certain vaccine approaches, such as live attenuated vaccines, will likely not be initially favored and may be used in later target product profiles for populations such as children prior to sexual debut.
How will clinical trials of ZIKV vaccines unfold? Recent trials of DENV vaccines in the Americas built substantial clinical trial capability in regions where ZIKV is currently being transmitted (Hadinegoro et al., 2015; Villar et al., 2015). If ZIKV transmission intensity remains high in these areas, then these existing clinical trial sites could be re-purposed for ZIKV vaccine trials. However, if ZIKV transmission subsides by the time vaccine efficacy trials are initiated, then alternative study designs, such as the ring vaccination approach used in the Ebola ça Suffit! vaccine study in Guinea might need to be employed (Henao-Restrepo et al., 2015). It is also unclear if ZIKV will become endemic and demonstrate seasonal transmission patterns, or if transmission will be epidemic and will become episodic with interim periods of relative quiescence. Moreover, the incidence of microcephaly and other major fetal abnormalities are relatively rare (Brasil et al., 2016a; Johansson et al., 2016), and thus efficacy studies would need to be exceedingly large to be powered to detect vaccine prevention of these important clinical endpoints. Designing efficacy trials only to measure prevention of ZIKV infection may be more tractable, but the mild clinical course of disease in the vast majority of cases and the challenges associated with serologic diagnosis pose unique difficulties. Moreover, there is also a concern regarding studies that measure vaccine efficacy with endpoints that do not substantially contribute to the public health burden. Human challenge studies have also been proposed to evaluate ZIKV vaccine efficacy, but ethical questions exist regarding this approach.
ZIKV vaccine developers may seek regulatory approval to pursue less conventional clinical endpoint efficacy trials utilizing adaptive trial designs. They may also seek licensure establishing the case for safety and efficacy outside the context of a randomized controlled clinical endpoint efficacy trial using accelerated approval mechanisms or the animal rule. In either instance, there would likely be a requirement for post-licensure demonstration of clinical benefit.
Can Antibodies Enhance Clinical Disease?
The possibility of antibody-dependent enhancement (ADE) of clinical disease is also a theoretical concern for ZIKV vaccines. The risk of severe DENV infection is significantly higher in people experiencing a secondary infection with a heterologous DENV serotype (Burke et al., 1988; Russell et al., 1967). Immune enhancement has been proposed as the pathologic mechanism for these rare (2–4% of secondary infections) but severe clinical phenotypes. It has been suggested that antibodies from the first infection may fail to neutralize the second infecting virus and actually facilitate the second virus’s infection of target cells such as monocytes and macrophages (Halstead, 2003). It is possible that when epitopes are insufficiently occupied, antibodies may increase viral uptake through interactions with immunoglobulin Fc receptors, resulting in increased levels of viremia (Rothman, 2011). The increased viral burden promotes a proinflammatory environment, coagulation dysfunction, and damage to vasculature endothelial cell linings. Epidemiologic observations, in vitro data, and limited small animal studies offer support for this theory, but data are conflicting and the relevance of these observations for humans remains unknown (Halstead et al., 1973; Kliks et al., 1988; Kliks et al., 1989; Laoprasopwattana et al., 2005; Libraty et al., 2009; Pinto et al., 2015; Vaughn et al., 2000).
Whether or not ADE will prove clinically relevant for ZIKV infection in humans remains to be determined. It has been speculated that severe clinical manifestations of ZIKV, when they occur, may be due to immune enhancement. It has also been postulated that primary infection with DENV or another flavivirus may induce antibodies that cross-react with a subsequent “secondary” infection with ZIKV and result in increased viral burden and a cascade of deleterious immunologic and clinical events (Dejnirattisai et al., 2016). It has also been suggested that ZIKV antibodies may impact subsequent DENV infection by an analogous mechanism (Kawiecki and Christofferson, 2016). Definitive data addressing these concepts in humans are currently not available.
Recent studies have shown that DENV-specific sera and monoclonal antibodies can increase ZIKV replication both in vitro (Barba-Spaeth et al., 2016; Charles and Christofferson, 2016; Dejnirattisai et al., 2016) and in mice (Stettler et al., 2016). However, passive transfer of sub-protective doses of neutralizing antibodies prior to ZIKV challenge did not result in enhancement of ZIKV replication or disease in mice or monkeys (Abbink et al., 2016; Larocca et al., 2016). Moreover, we have recently observed that monkeys that were primed with DENV or YFV and subsequently challenged with ZIKV did not exhibit increased ZIKV replication or adverse clinical outcomes as compared to flavivirus-naïve controls (unpublished data), and similar data has been reported online by the University of Wisconsin (https://zika.labkey.com/project/OConnor/ZIKV-013/begin.view). Large prospective clinical trials will likely be required to evaluate the possibility of immune enhancement to ZIKV in humans.
Conclusions
The pace of preclinical and early clinical development of ZIKV vaccines has been unprecedented. In less than a year, proof-of-concept preclinical studies have demonstrated that multiple vaccine platforms, including DNA vaccines, purified inactivated virus vaccines, and recombinant vector-based vaccines, provide robust protection in rodent and primate challenge models. Moreover, adoptive transfer studies have shown that protection can be mediated by vaccine-elicited antibodies. Multiple phase 1 clinical trials have been initiated, and clinical efficacy trials are planned. However, unique challenges will need to be addressed in the clinical development of ZIKV vaccines. Key issues facing ZIKV vaccine development include the unclear durability of protective immune responses in humans, the potential impact of baseline cross-reactive antibodies to other flaviviruses, the need to protect against congenital Zika syndrome, and the difficulty designing and conducting efficacy trials in the context of a rapidly changing epidemic. As clinical development of a variety of ZIKV vaccine candidates moves forward, basic and preclinical research will undoubtedly continue to characterize ZIKV immunology and pathogenesis.
Acknowledgments
We acknowledge support from the U.S. Military Research and Materiel Command and the U.S. Military HIV Research Program; the National Institutes of Health (AI095985, AI096040, AI100663, AI124377); and the Ragon Institute of MGH, MIT, and Harvard. The views expressed in this manuscript are those of the authors and do not represent the official views of the Department of the Army or the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, Kirilova M, Li Z, Ng’ang’a D, Nanayakkara O, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353:1129–1132. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, Studholme C, Boldenow E, Vornhagen J, Baldessari A, Dighe MK, Thiel J, Merillat S, et al. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med. 2016;22:1256–1259. doi: 10.1038/nm.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson B, Thorburn F, Petridou C, Bailey D, Hewson R, Simpson AJ, Brooks TJ, Aarons EJ. Presence and Persistence of Zika Virus RNA in Semen, United Kingdom, 2016. Emerging infectious diseases. 2017;23 doi: 10.3201/eid2304.161692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Loriere E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, et al. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature. 2016;536:48–53. doi: 10.1038/nature18938. [DOI] [PubMed] [Google Scholar]
- Bista MB, Banerjee MK, Shin SH, Tandan JB, Kim MH, Sohn YM, Ohrr HC, Tang JL, Halstead SB. Efficacy of single-dose SA 14-14-2 vaccine against Japanese encephalitis: a case control study. Lancet. 2001;358:791–795. doi: 10.1016/s0140-6736(01)05967-0. [DOI] [PubMed] [Google Scholar]
- Brasil P, Pereira JP, Jr, Raja Gabaglia C, Damasceno L, Wakimoto M, Ribeiro Nogueira RM, Carvalho de Sequeira P, Machado Siqueira A, Abreu de Carvalho LM, Cotrim da Cunha D, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro - Preliminary Report. N Engl J Med. 2016a doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P, Sequeira PC, Freitas AD, Zogbi HE, Calvet GA, de Souza RV, Siqueira AM, de Mendonca MC, Nogueira RM, de Filippis AM, et al. Guillain-Barre syndrome associated with Zika virus infection. Lancet. 2016b;387:1482. doi: 10.1016/S0140-6736(16)30058-7. [DOI] [PubMed] [Google Scholar]
- Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AS, Christofferson RC. Utility of a Dengue-Derived Monoclonal Antibody to Enhance Zika Infection In Vitro. PLoS currents. 2016;8 doi: 10.1371/currents.outbreaks.4ab8bc87c945eb41cd8a49e127082620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, Benazzato C, Almeida N, Pignatari GC, Romero S, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, Maquart M, Descamps D, Damond F, Leparc-Goffart I. Evidence of Sexual Transmission of Zika Virus. N Engl J Med. 2016;374:2195–2198. doi: 10.1056/NEJMc1604449. [DOI] [PubMed] [Google Scholar]
- Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, Sakuntabhai A, Cao-Lormeau VM, Malasit P, Rey FA, et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol. 2016 doi: 10.1038/ni.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos T, Rodriguez A, Almiron M, Sanhueza A, Ramon P, de Oliveira WK, Coelho GE, Badaro R, Cortez J, Ospina M, et al. Zika Virus and the Guillain-Barre Syndrome - Case Series from Seven Countries. N Engl J Med. 2016;375:1598–1601. doi: 10.1056/NEJMc1609015. [DOI] [PubMed] [Google Scholar]
- Dowd KA, DeMaso CR, Pelc RS, Speer SD, Smith AR, Goo L, Platt DJ, Mascola JR, Graham BS, Mulligan MJ, et al. Broadly Neutralizing Activity of Zika Virus-Immune Sera Identifies a Single Viral Serotype. Cell reports. 2016a;16:1485–1491. doi: 10.1016/j.celrep.2016.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, DeMaso CR, Castilho LR, Abbink P, Boyd M, Nityanandam R, et al. Rapid development of a DNA vaccine for Zika virus. Science. 2016b;354:237–240. doi: 10.1126/science.aai9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jaaskelainen AJ, Smura T, Rosenberg A, Hill DA, DeBiasi RL, Vezina G, et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N Engl J Med. 2016 doi: 10.1056/NEJMoa1601824. [DOI] [PubMed] [Google Scholar]
- Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, Mohns MS, Breitbach ME, Rasheed MN, Newman CM, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nature communications. 2016;7:12204. doi: 10.1038/ncomms12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- Elong Ngono A, Vizcarra EA, Tang WW, Sheets N, Joo Y, Kim K, Gorman MJ, Diamond MS, Shresta S. Mapping and Role of the CD8+ T Cell Response During Primary Zika Virus Infection in Mice. Cell Host Microbe. 2017;21:35–46. doi: 10.1016/j.chom.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci AS, Morens DM. Zika Virus in the Americas--Yet Another Arbovirus Threat. N Engl J Med. 2016;374:601–604. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352:816–818. doi: 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- Guirakhoo F, Zhang ZX, Chambers TJ, Delagrave S, Arroyo J, Barrett AD, Monath TP. Immunogenicity, genetic stability, and protective efficacy of a recombinant, chimeric yellow fever-Japanese encephalitis virus (ChimeriVax-JE) as a live, attenuated vaccine candidate against Japanese encephalitis. Virology. 1999;257:363–372. doi: 10.1006/viro.1999.9695. [DOI] [PubMed] [Google Scholar]
- Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N Engl J Med. 2015;373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Advances in virus research. 2003;60:421–467. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- Halstead SB, Chow JS, Marchette NJ. Immunological enhancement of dengue virus replication. Nature: New biology. 1973;243:24–26. [PubMed] [Google Scholar]
- Halstead SB, Thomas SJ. New Japanese encephalitis vaccines: alternatives to production in mouse brain. Expert Rev Vaccines. 2011;10:355–364. doi: 10.1586/erv.11.7. [DOI] [PubMed] [Google Scholar]
- Henao-Restrepo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, Camacho A, Carroll MW, Doumbia M, Draguez B, Duraffour S, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet. 2015;386:857–866. doi: 10.1016/S0140-6736(15)61117-5. [DOI] [PubMed] [Google Scholar]
- Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine. 2005;23:5205–5211. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, Ahmad N, Macdonald J, Evert N, Bingham A, et al. Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. JAMA. 2017;317:59–68. doi: 10.1001/jama.2016.19006. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Yamanaka A, Konishi E. A review of successful flavivirus vaccines and the problems with those flaviviruses for which vaccines are not yet available. Vaccine. 2014;32:1326–1337. doi: 10.1016/j.vaccine.2014.01.040. [DOI] [PubMed] [Google Scholar]
- Jarmer J, Zlatkovic J, Tsouchnikas G, Vratskikh O, Strauss J, Aberle JH, Chmelik V, Kundi M, Stiasny K, Heinz FX. Variation of the specificity of the human antibody responses after tick-borne encephalitis virus infection and vaccination. J Virol. 2014;88:13845–13857. doi: 10.1128/JVI.02086-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MA, Mier YT-RL, Reefhuis J, Gilboa SM, Hills SL. Zika and the Risk of Microcephaly. N Engl J Med. 2016 doi: 10.1056/NEJMp1605367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawiecki AB, Christofferson RC. Zika Virus-Induced Antibody Response Enhances Dengue Virus Serotype 2 Replication In Vitro. J Infect Dis. 2016 doi: 10.1093/infdis/jiw377. [DOI] [PubMed] [Google Scholar]
- Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg. 1989;40:444–451. doi: 10.4269/ajtmh.1989.40.444. [DOI] [PubMed] [Google Scholar]
- Kreil TR, Burger I, Bachmann M, Fraiss S, Eibl MM. Antibodies protect mice against challenge with tick-borne encephalitis virus (TBEV)-infected macrophages. Clin Exp Immunol. 1997;110:358–361. doi: 10.1046/j.1365-2249.1997.4311446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoprasopwattana K, Libraty DH, Endy TP, Nisalak A, Chunsuttiwat S, Vaughn DW, Reed G, Ennis FA, Rothman AL, Green S. Dengue Virus (DV) enhancing antibody activity in preillness plasma does not predict subsequent disease severity or viremia in secondary DV infection. J Infect Dis. 2005;192:510–519. doi: 10.1086/431520. [DOI] [PubMed] [Google Scholar]
- Larocca RA, Abbink P, Peron JP, Zanotto PM, Iampietro MJ, Badamchi-Zadeh A, Boyd M, Ng’ang’a D, Kirilova M, Nityanandam R, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016;536:474–478. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood JE, Wei CJ, Hu Z, Gordon IJ, Enama ME, Hendel CS, McTamney PM, Pearce MB, Yassine HM, Boyington JC, et al. DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials. Lancet Infect Dis. 2011;11:916–924. doi: 10.1016/S1473-3099(11)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, Zhang N, Shi L, Qin CF, Xu Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell. 2016 doi: 10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Libraty DH, Acosta LP, Tallo V, Segubre-Mercado E, Bautista A, Potts JA, Jarman RG, Yoon IK, Gibbons RV, Brion JD, et al. A prospective nested case-control study of Dengue in infants: rethinking and refining the antibody-dependent enhancement dengue hemorrhagic fever model. PLoS Med. 2009;6:e1000171. doi: 10.1371/journal.pmed.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston HD, Lurie N, Borio LL, Fauci AS. Considerations for Developing a Zika Virus Vaccine. N Engl J Med. 2016;375:1209–1212. doi: 10.1056/NEJMp1607762. [DOI] [PubMed] [Google Scholar]
- Mason RA, Tauraso NM, Spertzel RO, Ginn RK. Yellow fever vaccine: direct challenge of monkeys given graded doses of 17D vaccine. Applied microbiology. 1973;25:539–544. doi: 10.1128/am.25.4.539-544.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheron S, d’Ortenzio E, Leparc-Goffart I, Hubert B, de Lamballerie X, Yazdanpanah Y. Long-Lasting Persistence of Zika Virus in Semen. Clin Infect Dis. 2016;63:1264. doi: 10.1093/cid/ciw509. [DOI] [PubMed] [Google Scholar]
- Melo AS, Aguiar RS, Amorim MM, Arruda MB, Melo FO, Ribeiro ST, Batista AG, Ferreira T, Dos Santos MP, Sampaio VV, et al. Congenital Zika Virus Infection: Beyond Neonatal Microcephaly. JAMA neurology. 2016;73:1407–1416. doi: 10.1001/jamaneurol.2016.3720. [DOI] [PubMed] [Google Scholar]
- Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, Omange R, Best K, Luo M, Hraber PT, et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med. 2016 doi: 10.1038/nm.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra B, Lizarazo J, Jimenez-Arango JA, Zea-Vera AF, Gonzalez-Manrique G, Vargas J, Angarita JA, Zuniga G, Lopez-Gonzalez R, Beltran CL, et al. Guillain-Barre Syndrome Associated with Zika Virus Infection in Colombia. N Engl J Med. 2016;375:1513–1523. doi: 10.1056/NEJMoa1605564. [DOI] [PubMed] [Google Scholar]
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med. 2016;374:1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- Pierson TC, Graham BS. Zika Virus: Immunity and Vaccine Development. Cell. 2016;167:625–631. doi: 10.1016/j.cell.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AK, Brien JD, Lam CY, Johnson S, Chiang C, Hiscott J, Sarathy VV, Barrett AD, Shresta S, Diamond MS. Defining New Therapeutics Using a More Immunocompetent Mouse Model of Antibody-Enhanced Dengue Virus Infection. MBio. 2015;6:e01316–01315. doi: 10.1128/mBio.01316-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe IB, Staples JE, Villanueva J, Hummel KB, Johnson JA, Rose L, Mts Hills S, Wasley A, Fischer M, et al. Interim Guidance for Interpretation of Zika Virus Antibody Test Results. MMWR Morbidity and mortality weekly report. 2016;65:543–546. doi: 10.15585/mmwr.mm6521e1. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N Engl J Med. 2016;374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11:532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- Russell PK, Udomsakdi S, Halstead SB. Antibody response in dengue and dengue hemorrhagic fever. Japanese journal of medical science & biology. 1967;20(Suppl):103–108. [PubMed] [Google Scholar]
- Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- Sapparapu G, Fernandez E, Kose N, Cao B, Fox JM, Bombardi RG, Zhao H, Nelson CA, Bryan AL, Barnes T, et al. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature. 2016 doi: 10.1038/nature20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidis G, Perreira JM, Portmann JM, Meraner P, Guo Z, Green S, Brass AL. The IFITMs Inhibit Zika Virus Replication. Cell reports. 2016;15:2323–2330. doi: 10.1016/j.celrep.2016.05.074. [DOI] [PubMed] [Google Scholar]
- Spector S, Tauraso NM. Yellow fever virus. I. Development and evaluation of a plaque neutralization test. Applied microbiology. 1968;16:1770–1775. doi: 10.1128/am.16.11.1770-1775.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, et al. Specificity, cross-reactivity and function of antibodies elicited by Zika virus infection. Science. 2016 doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- Swanstrom JA, Plante JA, Plante KS, Young EF, McGowan E, Gallichotte EN, Widman DG, Heise MT, de Silva AM, Baric RS. Dengue Virus Envelope Dimer Epitope Monoclonal Antibodies Isolated from Dengue Patients Are Protective against Zika Virus. MBio. 2016;7 doi: 10.1128/mBio.01123-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber E, Kollaritsch H, Korinek M, Rendi-Wagner P, Jilma B, Firbas C, Schranz S, Jong E, Klingler A, Dewasthaly S, et al. Safety and immunogenicity of a Vero-cell-derived, inactivated Japanese encephalitis vaccine: a non-inferiority, phase III, randomised controlled trial. Lancet. 2007;370:1847–1853. doi: 10.1016/S0140-6736(07)61780-2. [DOI] [PubMed] [Google Scholar]
- Thomas SJ, L’Azou M, Barrett AD, Jackson NA. Fast-Track Zika Vaccine Development - Is It Possible? N Engl J Med. 2016;375:1212–1216. doi: 10.1056/NEJMp1609300. [DOI] [PubMed] [Google Scholar]
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- Venturi G, Mel R, Marchi A, Mancuso S, Russino F, Pra GD, Papa N, Bertiato G, Fiorentini C, Ciufolini MG. Humoral immunity and correlation between ELISA, hemagglutination inhibition, and neutralization tests after vaccination against tick-borne encephalitis virus in children. Journal of virological methods. 2006;134:136–139. doi: 10.1016/j.jviromet.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Villar L, Dayan GH, Arredondo-Garcia JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramirez JO, Carrasquilla G, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2015;372:113–123. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- Wang Q, Yang H, Liu X, Dai L, Ma T, Qi J, Wong G, Peng R, Liu S, Li J, et al. Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus. Sci Transl Med. 2016;8:369ra179. doi: 10.1126/scitranslmed.aai8336. [DOI] [PubMed] [Google Scholar]
- Who. Japanese Encephalitis Vaccines: WHO position paper, February 2015--Recommendations. Vaccine. 2016;34:302–303. doi: 10.1016/j.vaccine.2015.07.057. [DOI] [PubMed] [Google Scholar]
- Yockey LJ, Varela L, Rakib T, Khoury-Hanold W, Fink SL, Stutz B, Szigeti-Buck K, Van den Pol A, Lindenbach BD, Horvath TL, et al. Vaginal Exposure to Zika Virus during Pregnancy Leads to Fetal Brain Infection. Cell. 2016;166:1247–1256. e1244. doi: 10.1016/j.cell.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Fernandez E, Dowd KA, Speer SD, Platt DJ, Gorman MJ, Govero J, Nelson CA, Pierson TC, Diamond MS, et al. Structural Basis of Zika Virus-Specific Antibody Protection. Cell. 2016;166:1016–1027. doi: 10.1016/j.cell.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]