Abstract
An emphasis on quality improvement (QI) is vital to the cost-effective provision of evidence-based health care. QI projects in gastroenterology have typically focused on endoscopy to minimize or eliminate procedure-related complications or errors. However, a significant component of gastroenterology care is based on the management of chronic disease. Patients with chronic diseases are seen in many different outpatient practices in the community and academia. In an attempt to ensure that every patient receives high-quality care, major gastrointestinal societies have published guidelines on the management of common gastrointestinal complaints. However, adherence to these guidelines varies. We discuss common outpatient gastrointestinal illnesses with established guidelines for management that could benefit from active QI projects; these would ensure a consistently high standard of care for every patient.
Keywords: Quality, Improvement, Inflammatory Bowel Disease, Celiac Disease, Liver Disease
In recent years quality improvement (QI) and quality assurance (QA) have become catch phrases that are widely used throughout medicine. Although these terms are often used interchangeably, they differ in scope and relevance to day-to-day practice. QA is defined as planned, systematic activities that are implemented to ensure that a level of performance is attained. In medicine this has most often taken the form of compliance sessions and morbidity and mortality conferences, where adverse outcomes are discussed retrospectively in annual training.
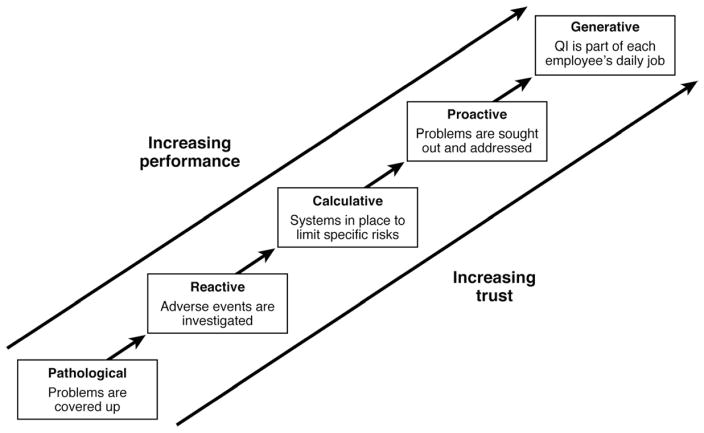
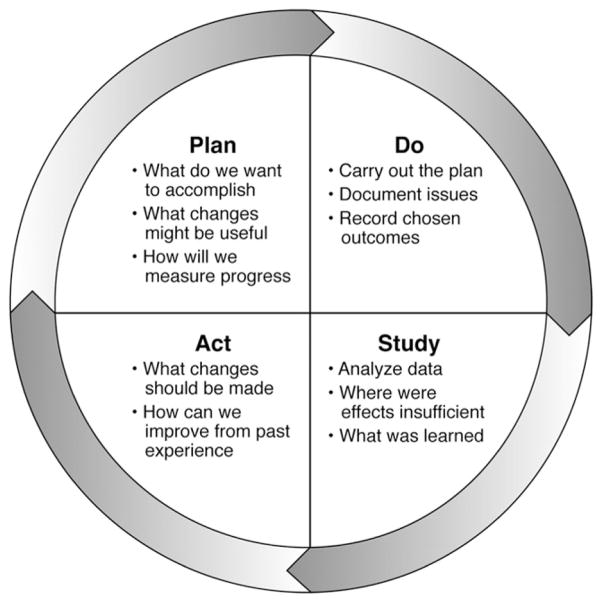
Although salutary changes often derive from such peer review, this is by nature a reactive process suited more toward avoiding aberrant events than toward raising the overall level of care (Figure 1).1 The recognition of the limitations of QA methodology in healthcare has led directly to the ascendance of QI as the accepted framework for healthcare initiatives. Exemplified by the Plan-Do-Study-Act cycle (Figure 2), QI is a measurement-driven process defined by continuous proactive efforts to improve care—not through avoidance of specific unwanted events as in QA but through manipulation of the processes of healthcare delivery.2,3 Individuals engaged in QI seek to alter the processes through which healthcare is delivered and improve outcomes by reducing unintended variation, eliminating errors, streamlining care, and enhancing communication.
Figure 1.
Evolution of a culture of QI. Adapted from Hudson P. Applying the lessons of high risk industries to healthcare. Qual Saf Health Care 2003;12(Suppl 1):i7–12.
Figure 2.
Plan-Do-Study-Act cycle. Adapted from Langley GL, Nolan KM, Nolan TW, et al. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance (2nd edition). San Francisco: Jossey-Bass Publishers, 2009.
In gastroenterology, QI initiatives have largely centered on endoscopy. Endoscopy is an excellent area for QI work because of the high volume of a limited range of invasive procedures with standardized reporting and significant associated risks and expense. Efforts in the endoscopy realm have varied4; they include patient education5–9 and recall,10 –12 optimization of preparation,13 sedation monitoring,14,15 adenoma detection,16,17 biopsy obtainment,18 –20 reporting,21 and adverse event recognition.22,23 In contrast to endoscopy, QI work in gastroenterology clinical practice has been more limited, and measures of quality of care in the inpatient and outpatient settings are less well defined.24 It is not the intent of this review to recommend guidelines discussed but to review the guidelines on specific outpatient conditions presented by major gastroenterology societies. However, QI is widely applicable to gastroenterologists as well as primary care physicians, as they try to streamline care while avoiding unwanted errors in managing a multitude of common gastrointestinal conditions.
With the growing emphasis on healthcare outcomes and accountability, it is in the interest of all groups to critically evaluate and refine delivery of care in the outpatient setting. Although the QI needs in endoscopy are relatively uniform, appropriate QI projects in outpatient clinical practice will become more dependent on specific practice characteristics. Although inpatient gastroenterology QI efforts are of great importance, because most patient care occurs in the outpatient setting, this review will focus on the most commonly encountered topics in outpatient gastroenterology, in which QI efforts can decrease practice variation and improve care. The goal of this review is to provide examples of common targets for QI work in outpatient clinical practice suitable for improvement of measurable patient outcomes as well as for accreditation and certification purposes. The QI projects we present will follow the Plan-Do-Study-Act cycle, emphasizing the iterative process and focusing on preidentified measurable outcomes.
Methods
For each outpatient gastrointestinal topic, the relevant guidelines published by the American Gastroenterological Association (AGA), American College of Gastroenterology (ACG), American Society for Gastrointestinal Endoscopy (ASGE), American Association for the Study of Liver Diseases (AASLD), and European Association for the Study of the Liver were reviewed. These guidelines were supplemented by searches within PubMed by using the terms described in the topics of interest and focusing on QI specific reviews and studies.
For specific gastrointestinal society guideline or position statement recommendations, a quality of evidence grade was reported whenever possible, which was based on an adaptation of the Standardized Guidelines of the Practice Committee of the AASLD (grade definition: grade A: homogeneous evidence from multiple well-designed randomized [therapeutic] or cohort [descriptive] controlled trials, each involving a number of participants to be of sufficient statistical power; grade B: evidence from at least 1 large well-designed clinical trial with or without randomization, from cohort or case-control analytical studies, or well-designed meta-analysis; grade C: evidence based on clinical experience, descriptive studies, or reports of expert committees; grade D: not rated).25,26 Other grading systems used in the literature were translated into these grades and denoted by an asterisk (*). When conflicting levels of evidence were reported by different gastrointestinal societies, the most recent and updated level of evidence for the recommendation was used. When a direct translation was not possible, the quality of evidence was described rather than graded.
Inflammatory Bowel Disease
Bone mineral density (BMD) tests, vaccinations, and dysplasia screens are important components of quality outpatient care for patients with inflammatory bowel disease (IBD).
Osteoporosis and Osteopenia
Patients with IBD are at increased risk for developing osteoporosis and osteopenia; about 15% of patients with IBD also have osteoporosis.26 Several risk factors for osteoporosis have been identified and include a course of steroid therapy longer than 3 months or recurring use of steroids, age >50 years, postmenopausal status, history of low-impact fracture, and hypogonadism. Using these risk factors to identify patients who should be tested for BMD led to the finding that 69% of patients with IBD were prescribed specific therapy.27 Currently, the AGA recommends dual-energy x-ray absorptiometry screening for high-risk patients (grade D).26 However, despite these recommendations and their validation in a prospective cohort, only 23% of patients with risk factors at a representative tertiary institution were tested.28
Vaccinations
Although immunosuppressive agents have significantly improved medical management of Crohn’s disease and ulcerative colitis, they increase the risk of infection, so vaccinations are important.29,30 Appropriate routine vaccinations are recommended by the Advisory Committee for Immunization Practices (Table 1).31,32 Live vaccines, such as varicella, generate concern among patients with IBD, who are likely to receive immunosuppressive therapy. Consideration of vaccination at initial visit could allow for safe vaccination before initiation of immunosuppressive therapy. Many common vaccines, such as hepatitis A virus, hepatitis B virus (HBV), pneumococcal, injectable influenza, and human papillomavirus, are recommended for individuals on or being considered for immunosuppressive regimens. Despite recommendations, vaccination of patients with IBD is underutilized in general practice.30
Table 1.
Recommended Vaccine Schedule
| Vaccine | Cohort |
|---|---|
| Influenza | All patients >50 years old, with IBD, chronic liver disease, or celiac disease, or immunosuppressed |
| Pneumococcus | All patients >65 years old, with IBD, chronic liver disease, or celiac disease, or immunosuppressed |
| Herpes zoster | All patients >60 years old, contraindicated in immunosuppressed patients |
| Varicella zoster virus | All patients with IBD if no prior infection. Contraindicated in immunosuppressed patients |
| Hepatitis A | Patients with chronic liver disease, patients with IBD |
| Hepatitis B | Patients with chronic liver disease, patients with IBD |
| Human papilloma virus | Males and females up to age 26 |
On the basis of major society guidelines, adequate bone health and infection prevention through appropriate testing and vaccination are considered important parts of outpatient IBD QI. An example of QI initiatives in these areas could include the following steps: (1) development of an evidence-based practice standard for BMD testing and vaccination in patients with IBD; (2) retrospective evaluation of appropriately tested or vaccinated patients; and (3) development or enhancement of a mechanism that increases rates of BMD testing or vaccination in appropriate individuals. Possibilities include patient-completed forms and templated notes in patients’ charts to serve as reminders to ordering physicians. Potential initiatives include automated reminder letters for vaccinations, such as influenza, that are needed on a recurring basis. For BMD testing, patients could receive a standardized test referral when they check out from the clinic. QI initiatives should also include (4) a prospective audit of IBD patients to assess rates of appropriate vaccination or referral for BMD testing and (5) evaluations for any potential shortcomings of the system. After this step, healthcare workers should return to step 3.
Screening for Dysplasia
The risk of colorectal cancer and dysplasia is increased in patients with ulcerative and Crohn’s colitis, compared with the general population. This risk of colorectal cancer is estimated to be 2% for patients who have had ulcerative colitis for 10 years or more and as high as 18% for those with the disease for 30 years.33 To reduce the risk of colorectal cancer in patients with IBD, the AGA recommends that all patients undergo surveillance colonoscopy a maximum of 8 years after onset of IBD symptoms34 (grade B*). Surveillance schedules can then be based on family history, extent and activity of disease, and the presence of primary sclerosing cholangitis or abnormal findings such as polyps and strictures.19,35,36 The sensitivity of endoscopic screening for dysplasia can be increased by including chromoendoscopy, performance by an experienced endoscopist, and adequate sampling of the colon.37 When dysplasia or cancer is found, it should be confirmed by a histology analysis by an expert gastrointestinal pathologist.36,38
An example of a QI initiative in dysplasia screening would involve the following steps: (1) establish an evidence-based practice guideline for dysplasia screening in patients with longstanding colitis; (2) evaluate the tests performed in a cohort of at-risk IBD patients to identify those who have been screened for dysplasia, have had an adequate number of biopsies analyzed, and those who are undergoing appropriate surveillance; (3) identify patient-based and system-based risk factors for insufficient dysplasia screening and develop a mechanism to improve screening by addressing these risk factors (possible approaches include sending reminder letters, which are generated at initial visit and at each procedure for surveillance colonoscopy and mailed before the suggested appointment); and (4) audit patients who are receiving dysplasia screening under the new quality intervention to evaluate any potential shortcomings and assure that the QI initiative has been modified appropriately.
Medications in Inflammatory Bowel Disease
Infliximab and other anti–tumor necrosis factor-alpha therapies increase the risk of infection and reactivation of tuberculosis (TB) and HBV.39,40 The ACG therefore recommends routine testing for TB and HBV before anti–tumor necrosis factor therapy begins.41 Although the rate of screening is increasing, there is a substantial need for QI efforts to improve these rates.42
Before patients are treated with 6-mercaptopurine or azathioprine, AGA guidelines recommend thiopurine methyltransferase testing by activity or genotype to identify patients at risk for developing severe bone marrow suppression (grade B).43 Likewise, it is equally important to monitor patients while they are receiving therapy; systems to ensure appropriate interval laboratory tests and routine follow-up examinations could be included in QI plans.
An example of a QI project to promote the safe use of appropriate medications, such as infliximab, would involve the following steps: (1) an initial retrospective review of patients who are receiving infliximab to ensure documentation of appropriate tests for TB and HBV; (2) implementation of interventions to ensure that patients were tested for these diseases before therapy began (such as a checklist for the ordering physician or the pharmacist to confirm that tests for TB and HBV have been completed before they dispense infliximab); and (3) confirmation of the efficacy of the quality initiative via prospective audit of patients who are receiving infliximab and room for further modification of the initiative to achieve 100% compliance with prescreening for HBV and TB.
Diagnosis of Irritable Bowel Syndrome
The prevalence of irritable bowel syndrome (IBS) is about 7% in North America44; it is one of the most frequent complaints among patients at outpatient gastroenterology practices. The care of patients with IBS is estimated to cost about $20 billion.44 QI in IBS care can therefore have a significant impact on patient care and healthcare resources.
Patients suspected of having IBS are routinely tested to exclude alternative organic disorders. The 2009 ACG IBS Task Force argues against the diagnosis of IBS by exclusion.44 Instead, the ACG states patients who meet the definition of IBS (abdominal pain or discomfort associated with a change in bowel habits during a period of at least 3 months) without alarm symptoms need few, if any, formal tests. Alarm symptoms such as weight loss, anemia, family history of colorectal cancer, IBD, or celiac sprue are at high risk for organic disease; the absence of these would be consistent with a diagnosis of IBS. For patients older than 50 years or with alarm symptoms, the ACG recommends colonoscopy examination (grade C*). In patients younger than 50 with no alarm symptoms, the ACG recommends against routine colonoscopy because of the low probability that these patients have IBD or colorectal neoplasia44 (grade B*). In patients with diarrhea-predominant or mixed IBS, the 2009 ACG Task Force recommends testing for celiac disease (grade B*). Glucose or lactulose breath tests can be considered, although there are no recommendations for small intestinal bacterial overgrowth tests because of insufficient evidence. Likewise, routine tests for food allergies and exclusion diets are not recommended in major society guidelines.
QI projects in IBS could focus on ensuring adequate adherence to consensus guidelines to reduce costly and invasive tests. Similar to BMD testing in IBD, a QI project for IBS could be designed as follows: (1) development of an evidence-based practice standard for appropriate initial evaluation of patients with suspected IBS; (2) retrospective evaluation of the appropriately tested patients; (3) development or enhancement of a mechanism to ensure adherence to appropriate testing; (4) prospective audit of IBS patients to assess rates of appropriate invasive diagnostic procedures; and (5) evaluations of any potential shortcomings of the system. The physician should then return to step 3.
Colorectal Cancer Screening
The overall goal of colorectal cancer screening and surveillance is to reduce mortality by removing colorectal cancer precursor lesions and detecting cancer at an early stage to enable more effective treatment. Guidelines for whom and when to screen, which are based on risk factors, have been outlined by several gastroenterology professional organizations.45
Quality measures in endoscopy are diverse and beyond the scope of this review.46 – 48 However, quality colorectal cancer screening does not end with the colonoscopy report. A continuation of the inroads made against colorectal cancer in the coming years will require aggressive interventions to endorse proper screening and surveillance throughout the population.49 Electronic reminders can help patients return on time for follow-up examinations.12 Sint Nicolaas et al50 recently identified patient awareness of colonoscopy results and surveillance recommendations as important areas for improvement. Although in this study patient communication did not correlate to improved attendance, higher rates of follow-up were noted in the departments with follow-up communication systems in place. It is common for patients to return for reexamination at shorter intervals than the major society guidelines recommend,51 which increases risks and costs. For instance, in a study of 3627 patients undergoing colorectal cancer screening, 49% were reexamined within 7 years (median, 3.1 years) of the index colonoscopy, and 38% of those with fewer than 3 small adenomas received follow-up colonoscopies within 4 years.52,53
Successful colorectal cancer screening QI projects may target adherence to consensus guidelines, with the goal of reducing the proportion of patients who return too early or too late for follow-up examinations. An appropriate practice-based QI initiative could include the following. (1) Evaluation of rates of surveillance colonoscopies would be based on retrospective review. Shortcomings in surveillance could be improved by a combination of methods. For example, automatically generated reminder letters could help to recall patients who miss recommended appointments. (2) Computer-based software that creates endoscopy records could incorporate automatic recommendations for surveillance intervals, which are based on endoscopy findings. If final surveillance intervals differ from these algorithm-based recommendations, a specific reason would be documented. (3) This QI intervention could then be prospectively evaluated to identify potential shortcomings or further ways of improvement.
Gastroesophageal Reflux Disease
The lifetime prevalence of gastroesophageal reflux disease (GERD) in the United States is 20%– 40%, making it one of the most common outpatient gastrointestinal complaints. Patients with mild-to-moderate symptoms are unlikely to have complications, and therefore the AGA considers initial empiric trial of lifestyle modification and acid suppression therapy appropriate.54,55 Most cases of GERD (70%– 80%) may be managed with these approaches; evaluation of refractory GERD is beyond the scope of this review. Two major quality issues in the management of GERD include the appropriate timing and use of endoscopic evaluation and mitigation of adverse medication effects.56
The ACG and ASGE recommend that at initial presentation, endoscopy should be reserved for patients with alarm symptoms of complicated disease, such as involuntary weight loss, dysphagia, or gastrointestinal bleeding54,57 (grade C*). Concordantly, the AGA recommends that when an empiric trial of twice-daily proton pump inhibitor (PPI) therapy has failed to provide adequate control of GERD symptoms, patients without alarm symptoms should undergo endoscopy to determine whether they have esophagitis, complications, or Barrett’s esophagus (BE) (grade B*). Any visualized area of mucosal irregularity, as well as an area of normal-appearing mucosa, should be analyzed by biopsy to identify BE, eosinophilic esophagitis, or other lesions.58 The AGA also recommends against the use of routine endoscopy to assess disease progression, because endoscopy has not been shown to decrease outcomes from esophageal cancer detection.58 Screening those at risk for BE remains controversial and has not been consistently recommended, because it has not been shown to affect the incidence or prognosis of esophageal adenocarcinoma57,59,60 (grade B*). The AGA and ACG state that screening the general population with GERD is not cost-effective,59,60 and a large number of patients with BE lack symptoms.57 The highest yield appears to be in white men older than the age of 50 with long-standing GERD symptoms (more than 10 years), although the effects of screening in this high-risk population have not been established.
Because GERD is a chronic condition, continuous medical therapy is often necessary to control symptoms and prevent complications.54 However, the cost and side effects of long-term acid suppression, particularly with PPI therapy, should be carefully considered. Recent studies have reported several clinical consequences of chronic potent acid inhibition, including decreased calcium absorption and increased rates of pneumonia and Clostridium difficile–associated colitis.58,61– 67 Few patients are reassessed on a regular basis to determine whether the PPIs are still needed, despite the fact that many patients who are receiving continuous therapy are able to have their dose modified or reduced on the basis of the presence or absence of symptoms.56 Patients with nonerosive GERD could be candidates for on-demand acid suppressive therapy, whereas those with erosive esophagitis most often require once-daily PPI therapy to reduce the risk of recurrence. The AGA recommends that long-term acid suppression therapy be titrated to the lowest effective dose needed to achieve therapeutic goals (grade A*).58
One QI initiative could focus on the appropriate referral of patients with GERD for endoscopic evaluation. (1) Consider review of the panel of patients seen in clinic for GERD to assess validity of recommendations made for or against endoscopy. (2) A mechanism to improve the appropriate rate of referral for endoscopy can be developed, which is based specifically on the presence or absence of alarm symptoms, by using a checklist or reminder system in the medical chart to assess alarm symptoms. (3) A positive result would trigger an automated endoscopy referral or require specific documentation of the medical, patient, or system reason(s) for not referring to endoscopy. (4) Once implemented, it is possible to assess the proportion of GERD patients with and without alarm symptoms who were referred for endoscopy. Imperfections of the endoscopy referral mechanism can then be evaluated for further improvement.
Another QI initiative in GERD could focus on controlling symptoms in patients who receive continuous medical therapy to identify those whose doses or frequencies of therapy might be reduced. The QI initiative would involve the following: (1) reviewing records of patients seen in the last year with GERD who receive continuous PPI therapy to identify the proportion that had a follow-up assessment of their symptoms within those 12 months; (2) developing a mechanism to increase the rate of regular symptom assessment; a system-based alert might be used to inform clinicians that a patient has been on PPI therapy for longer than 1 year, with a reminder to reassess the patient’s symptoms and need for continued PPI use at least annually and to record this in the medical record; and (3) reevaluating shortcomings of this PPI reminder system so it can be improved.
Barrett’s Esophagus
In BE metaplastic columnar epithelium, which has a predisposition to cancer, replaces the normal stratified squamous epithelium of the distal esophagus.59 The change can be of any length, is recognized as columnar mucosa at endoscopy, and is confirmed to have intestinal metaplasia by biopsy. BE is the most important identifiable risk factor for esophageal adenocarcinoma60; BE is estimated to affect 0.5%–1.5% of the general population of the United States and 5%–15% of patients with chronic GERD. The key quality issues in BE involve medical therapy, appropriate endoscopic surveillance, and treatment of dysplastic BE.
Pharmacologic acid suppression is recommended for all patients with BE, with the goal of controlling reflux symptoms.60 Although acid suppression does not appear to prevent esophageal adenocarcinoma, PPI therapy may reduce the risk for dysplasia in patients with BE; this reduction provides the rationale to also treat asymptomatic patients with a PPI.59 The objective of surveillance endoscopy for patients with BE is to identify and treat dysplasia before it progresses to esophageal adenocarcinoma, although it is not known whether surveillance actually reduces cancer incidence or mortality.59,60 In the 2011 Medical Position Statement by the AGA, the following recommendations were made (grade B*). Surveillance should be performed by using white-light endoscopy, with 4-quadrant biopsies obtained from every 2 cm of BE. Any mucosal irregularity should be analyzed separately by biopsy, and 4-quadrant biopsies should be collected from every 1 cm in areas of known or suspected dysplasia. Any dysplasia should be confirmed by an expert gastrointestinal pathologist, because the grade of dysplasia determines the appropriate surveillance interval; more advanced disease requires more frequent surveillance, because it has an increased rate of progression to cancer. Patients diagnosed with BE but no dysplasia, which is based on the initial endoscopy examination, should be reexamined by endoscopy and biopsy analysis within 1 year; if the absence of dysplasia is confirmed, further surveillance should not be performed in less than 3 years on the basis of the recommendations from the AGA and ASGE.59,68 A summary of the appropriate surveillance intervals is beyond the scope of this review.
High-grade dysplasia is the threshold for intervention in patients with BE.59 Options include intensive surveillance; endoscopic mucosal ablation with photodynamic therapy, radio-frequency ablation (RFA), cryotherapy or thermal ablation therapy, endoscopic mucosal resection (EMR), or esophagectomy. In recent years, RFA and EMR have become the most popular endoscopic approaches and can be safely offered as combination therapy for patients with confirmed high-grade dysplasia at a visible lesion (grade B*).59 In this case, EMR of the lesion is performed first, followed by RFA of the remaining Barrett’s epithelium.69 After endoscopic ablative therapy or EMR is complete, patients should be maintained on high-dose PPI therapy to allow for healing and regeneration of the neosquamous esophageal epithelium.69 Follow-up endoscopy should be performed at 8–12 weeks to assess the results of therapy and to consider repeat ablation if residual BE is found. The AGA recommends that patients who have had ablative therapy continue surveillance via biopsy analysis of the entire area where BE was detected at intervals appropriate for the highest pretreatment grade of dysplasia until there is reasonable certainty of complete ablation. Periodic surveillance thereafter is recommended by guidelines from the major societies.59
A successful QI initiative for BE could focus on promoting the appropriate endoscopic evaluation of all patients with BE by using 4-quadrant biopsies and making appropriate recommendations for continued surveillance or therapeutic intervention based on the grade of dysplasia identified. (1) Records from patients with BE would be reviewed to ascertain the proportion with each grade of dysplasia and provide recommendations for interval surveillance or treatment. (2) The rate of appropriate surveillance or treatment might be improved by computerized notification when the final pathology report is reviewed. (3) Clinicians could enter the follow-up interval or treatment referral or provide the medical, patient, or system reason(s) for opting out, and a reminder system would recall the patient. (4) Once implemented, prospective assessments can determine whether patients with BE are appropriately recommended for interval surveillance or therapeutic intervention, on the basis of their grade of dysplasia. Imperfections of this trigger and reminder system can be repeatedly evaluated for continued improvement. Similarly, in another QI project a review of all patients seen with BE could be undertaken to ensure that these patients remain on PPI therapy to reduce the risk of dysplasia and also that appropriate steps are taken to reduce the risks associated with long-term PPI therapy.
Liver Disease
Similar to patients with luminal gastrointestinal disease, definitions of quality measures are necessary for patients with advanced liver disease and cirrhosis.70 Kanwal et al71 have developed a set of evidence-based quality indicators for physicians and institutions to use as a tool in the care of patients with cirrhosis. Surveillance for hepatocellular carcinoma (HCC) and management of varices are 2 important examples of quality indicators for outpatient clinical practices that manage patients with advanced liver disease and cirrhosis.
Hepatocellular Carcinoma
The annual incidence of HCC ranges from 3% to 8% among patients with advanced liver disease,72,73 and for this reason systems are needed to ensure proper surveillance and management. Patients are often diagnosed with HCC at a late stage of tumor progression, so their tumors are large and have undergone vascular invasion and metastasis, precluding surgical resection or transplantation; subsequently, mortality is high. Surveillance with biannual ultrasound examinations appears to be cost-effective for patients with cirrhosis; they have an expected annual incidence of HCC that exceeds 1.5%/year among patients with hepatitis C and 0.2%/year among those with hepatitis B.74 Surveillance with abdominal ultrasound is recommended by both AASLD and European Association for the Study of the Liver.
Levels of alpha fetoprotein are often used with imaging analyses to identify patients with HCC, but this approach increases costs and has a high false-positive rate, so it is not recommended as a surveillance tool by AASLD.75,76 These guidelines suggest surveillance for all patients with cirrhosis and for patients with hepatitis B in these categories: Asian man >40 years, Asian woman >50 years, HBV-associated cirrhosis, African and North American blacks, and family history of HCC. Whites with low HBV activity have a low risk for developing HCC, so surveillance generally is not recommended, in contrast to white patients with high viral load and active hepatitis.75 A large randomized controlled trial that included 18,816 patients found that mortality from HCC was significantly lower after 5 years in the screened group (who received biannual ultrasound and alpha fetoprotein tests) compared with the control group (83 vs 132 per 100,000; mortality rate ratio of 0.63).77
Despite data to support the benefits of surveillance for high-risk populations, the prevalence of adequate screening is unknown but appears to be low. In a population-based retrospective cohort study of patients diagnosed with HCC by Davila et al,72,78 only 17% of patients in the Medicare database >65 years old received regular examinations for HCC, and 38% were examined inconsistently. Patients at highest risk for inadequate surveillance were those living in urban areas, with lower incomes, and receiving care at nonacademic centers.72,78 The large retrospective cohort study of Veterans Administration hepatitis C virus patients demonstrated that 88% of patients with cirrhosis did not receive guideline-based HCC surveillance. At 1 year, only 42% received guideline-based surveillance, and rates subsequently fell during the next 2–3 years of follow-up.79
QI initiatives to improve HCC outcomes need to provide a system-based approach to improve surveillance in high-risk cohorts to increase survival through earlier detection of tumors. A QI initiative for surveillance of HCC could include the following: (1) a retrospective review of HCC screening in appropriate individuals; (2) intervention, which could include sending reminders to specific patients or physicians, especially for patients at highest risk of missing examinations, or standardized, closed-loop communication with referring primary care physicians; and (3) prospective review of improvements in surveillance after set interventions; these should be assessed and alterations made as needed.
Gastroesophageal Varices
Varices develop in approximately 50% of patients with cirrhosis and are an important factor to consider in determining QI for outpatients with liver disease (second to cost and mortality from variceal bleeding). It is important to evaluate these patients by endoscopy and provide β-blocker therapies as prophylactics. The incidence of varices development is 8% per year, and development is correlated with the severity of liver disease.80,81 Patients with compensated cirrhosis without varices should be evaluated for varices development by endoscopy at 2- to 3-year intervals, patients with small varices should be evaluated every 1–2 years, and patients with decompensated cirrhosis should be evaluated every year (grade C*).82,83 The risk of variceal bleeding and liver-related mortality is significantly reduced with β-blocker therapies,84 and therapy was shown to be cost-effective in primary prophylaxis of variceal bleeding.85,86
Surveillance and treatment of varices have been found to be lower than would be suggested on the basis of guidelines.87 QI initiatives should address inadequate screening and prophylaxis. One could consider the following: (1) reviewing the numbers of routine examinations patients receive for varices and comparing these with recommendations from guidelines; (2) identifying barriers to appropriate screening; (3) creating and testing computer-based templates to document patients’ heart rates at each visit to ensure proper doses of β-blockers; and (4) creating a screening sheet for patients with advanced liver disease to improve follow-up and communication with referring physicians.
Celiac Disease
Celiac disease is common, with a prevalence of 1% or more, and has many intestinal and extraintestinal manifestations.88 In gastroenterology practice, QI efforts to address the growing celiac disease population should focus on adequate testing and monitoring. Endoscopy with small intestinal biopsy is the standard used to identify patients with celiac disease. However, the reliability of endoscopic biopsy analysis depends on the number of samples collected and the locations they were taken from; the recommended 6 biopsy samples are not regularly collected,20 and newer recommendations for collecting samples from the duodenal bulb are often not considered.89,90
Practices may consider the following: (1) establishing guidelines for biopsy analysis of individuals with known or suspected celiac disease; (2) coordinating these guidelines with the pathology group; and (3) auditing records from patients who have undergone endoscopy for signs and symptoms of celiac disease to evaluate the proportion from whom adequate biopsies were taken; and (4) further interventions could be needed if gaps in practice are noted.
Celiac disease is a lifelong disorder that affects multiple body systems. Although management guidelines vary,88 the standard of care includes patient visits to a celiac dietitian, monitoring levels of celiac-associated antibodies, and measuring bone density within the first year of diagnosis. Documentation of 1 or more of these recommendations could be used as a marker of the quality of overall care provided to patients with celiac disease. As with other disorders, it can be helpful to create practice guidelines based on local resources and patient populations, but their use should be regularly assessed and changes made as needed to improve rates of appropriate care.
Conclusions
Meaningful QI efforts should not be seen as top-down mandates from administrative leadership, accreditation bodies, or government organizations. At the same time, all of these groups have focused significant attention and energy on QI, with the intent of improving healthcare outcomes and efficiency. Although these goals are shared by all, the means to achieve them can vary substantially, and edicts from above rarely have the effects they were designed to achieve.91,92 To help ensure that gastroenterology practices are free to choose QI initiatives that best serve their specific patients and do not disrupt their clinical environment, it is vital that practices foster internally derived QI efforts.
As we have described (Table 2), there are many initiatives that can be considered “low-hanging fruit” and are well within the resources of nearly all practices. In private practice settings, administrative time can often be leveraged to facilitate these projects, whereas in academic settings, students, residents, and fellows (the latter now mandated by the Accreditation Council for Graduate Medical Education to complete a QI project during training) are generally eager to participate; these efforts increase their understanding of the discipline, directly affect patient care, and often lead to publishable data. In addition, a growing number of online resources provide information and resources to assist in developing QI initiatives (Table 3). In many cases, the cost of implementing these measures is modest and cost-effective, suggesting that substantial improvement is possible even in an era of diminishing reimbursement. In all settings, QI should be seen as a team effort of the practice as a whole. Individuals at all levels from senior clinicians to administrative staff should be encouraged to identify areas of potential risk. Leadership then has the responsibility to work with front-line clinicians to prioritize issues, assess feasibility, and allocate resources when necessary.
Table 2.
QI Process Improvement Topics
| QI topic | Outcome | Example of process improvement | |
|---|---|---|---|
| IBD | BMD evaluation | % of patients appropriately tested | Templated reminder notes in patients’ charts |
| Vaccination | % of IBD patients vaccinated | Reminder letters for recurrent vaccines | |
| Dysplasia screening | % of IBD patients appropriately screened | Automated reminder letters to patients | |
| Immunosuppressants | % of patients undergoing screening for TB and HBV before anti-TNF | Preauthorization checklist for TB/HBV screening | |
| IBS | Diagnosis of IBS | % of patients receiving appropriate work-up, including endoscopy or screening for celiac disease | Routine audits to assess % of patients receiving appropriate testing |
| Colorectal cancer | Colorectal cancer screening | % of patients screened at appropriate intervals | Automated reminder letters to patients |
| GERD | Endoscopic referral | % of patients with alarm symptoms referred for endoscopy at initial evaluation | Templated reminder alerts and automated endoscopic referral |
| Medication therapy | % of patients on PPI undergoing annual reassessment of symptoms | System-based alerts detecting chronic PPI use | |
| BE | % of patients undergoing appropriate surveillance | Mandatory interval specification at time of pathology review | |
| Liver | HCC screening | % of biannually screened at-risk patients | Electronic medical record screening sheet with reminder alerts |
| Esophageal varices | % of patients appropriately screened for esophageal varices | Automated physician alert and patient letter for surveillance endoscopy | |
| Celiac disease | BMD evaluation | % of patients appropriately tested | Templated reminder notes in patients’ charts |
TNF, tumor necrosis factor.
Table 3.
Useful Online QI Resources
| Organization | Web site | Description |
|---|---|---|
| Institute for Healthcare Improvement | http://www.ihi.org/ | Independent nonprofit organization focused on goals adapted from the Institute of Medicine’s 6 improvement aims for the healthcare system, building the will for change, cultivating promising concepts for improving patient care, and helping healthcare systems put those ideas into action. |
| Agency for Healthcare Research and Quality | http://www.ahrq.gov/ | Agency within the United States Department of Health and Human Services that supports research design to improve outcomes and quality of healthcare, reduce costs, address patient safety and medical errors, and broaden access to effective services, with the mission to improve the quality, safety, efficiency, and effectiveness of healthcare for all Americans. |
| American College of Physicians Quality Improvement Programs | http://www.acponline.org/running_practice/quality_improvement/ | Free web-based QI programs by the American College of Physicians offering expert training on implementing clinical QI tools and techniques, information on evidence-based best practices, and strategies for creating systems change and improving patient care. Also provides online QI discussion groups, library and resources, news, and Physician Quality Reporting System. |
| AGA Quarterly: Quality | http://www.gastro.org/journals-publications/quarterly-quality-newsletter | Quarterly online newsletter by the AGA focusing on quality management, patient safety, and related resources, providing information for gastroenterology practices related to delivering high-quality, safe, and efficient care. |
| Gastrointestinal Quality Improvement Consortium, Ltd | http://giquic.gi.org/ | Quality initiative by the ACG and the ASGE to provide reliable and relevant measures of endoscopic quality that give physicians meaningful information to use to improve patient care. |
Finally, it is the common experience in healthcare, as in other industries, that the benefits of attention to QI go beyond improving patient outcomes and increasing efficiency. Adoption of fundamental principles of QI in an outpatient practice or division improves overall patient care as well as practice finance and boosts workplace satisfaction, which are outcomes we can all agree on.
Abbreviations used in this paper
- AASLD
American Association for the Study of Liver Diseases
- ACG
American College of Gastroenterology
- AGA
American Gastroenterological Association
- ASGE
American Society for Gastrointestinal Endoscopy
- BE
Barrett’s esophagus
- BMD
bone mineral density
- EMR
endoscopic mucosal resection
- GERD
gastroesophageal reflux disease
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- PPI
proton pump inhibitor
- QA
quality assurance
- QI
quality improvement
- RFA
radiofrequency ablation
- TB
tuberculosis
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Hudson P. Applying the lessons of high risk industries to health care. Qual Saf Health Care. 2003;12(Suppl 1):i7–12. doi: 10.1136/qhc.12.suppl_1.i7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cleghorn GD, Headrick LA. The PDSA cycle at the core of learning in health professions education. Jt Comm J Qual Improv. 1996;22:206–212. doi: 10.1016/s1070-3241(16)30223-1. [DOI] [PubMed] [Google Scholar]
- 3.Guinane CS, Sikes JI, Wilson RK. Using the PDSA cycle to standardize a quality assurance program in a quality improvement-driven environment. Jt Comm J Qual Improv. 1994;20:696–705. doi: 10.1016/s1070-3241(16)30118-3. [DOI] [PubMed] [Google Scholar]
- 4.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–885. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 5.Ward SH, Lin K, Meyer B, et al. Increasing colorectal cancer screening among African Americans, linking risk perception to interventions targeting patients, communities and clinicians. J Natl Med Assoc. 2008;100:748–758. doi: 10.1016/s0027-9684(15)31356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christie J, Itzkowitz S, Lihau-Nkanza I, et al. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008;100:278–284. doi: 10.1016/s0027-9684(15)31240-2. [DOI] [PubMed] [Google Scholar]
- 7.Rawl SM, Champion VL, Scott LL, et al. A randomized trial of two print interventions to increase colon cancer screening among first-degree relatives. Patient Educ Couns. 2008;71:215–227. doi: 10.1016/j.pec.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman M, Borum ML. Colorectal cancer screening of African Americans by internal medicine resident physicians can be improved with focused educational efforts. J Natl Med Assoc. 2007;99:1010–1012. [PMC free article] [PubMed] [Google Scholar]
- 9.Khankari K, Eder M, Osborn CY, et al. Improving colorectal cancer screening among the medically underserved: a pilot study within a federally qualified health center. J Gen Intern Med. 2007;22:1410–1414. doi: 10.1007/s11606-007-0295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerra CE, Schwartz JS, Armstrong K, et al. Barriers of and facilitators to physician recommendation of colorectal cancer screening. J Gen Intern Med. 2007;22:1681–1688. doi: 10.1007/s11606-007-0396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw MJ, Beebe TJ, Tomshine PA, et al. A randomized, controlled trial of interactive, multimedia software for patient colonoscopy education. J Clin Gastroenterol. 2001;32:142–147. doi: 10.1097/00004836-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Leffler DA, Neeman N, Rabb JM, et al. An alerting system improves adherence to follow-up recommendations from colonoscopy examinations. Gastroenterology. 2011;140:1166–1173. doi: 10.1053/j.gastro.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Siddiqui AA, Yang K, Spechler SJ, et al. Duration of the interval between the completion of bowel preparation and the start of colonoscopy predicts bowel-preparation quality. Gastrointest Endosc. 2009;69(Suppl):700–706. doi: 10.1016/j.gie.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 14.Ko HH, Zhang H, Telford JJ, et al. Factors influencing patient satisfaction when undergoing endoscopic procedures. Gastrointest Endosc. 2009;69:883–891. doi: 10.1016/j.gie.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 15.Chung YW, Han DS, Yoo KS, et al. Patient factors predictive of pain and difficulty during sedation-free colonoscopy: a prospective study in Korea. Dig Liver Dis. 2007;39:872–876. doi: 10.1016/j.dld.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Lee A, Iskander JM, Gupta N, et al. Queue position in the endoscopic schedule impacts effectiveness of colonoscopy. Am J Gastroenterol. 2011;106:1457–1465. doi: 10.1038/ajg.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanaka MR, Deepinder F, Thota PN, et al. Adenomas are detected more often in morning than in afternoon colonoscopy. Am J Gastroenterol. 2009;104:1659–1665. doi: 10.1038/ajg.2009.249. [DOI] [PubMed] [Google Scholar]
- 18.Kottachchi D, Yung D, Marshall JK. Adherence to guidelines for surveillance colonoscopy in patients with ulcerative colitis at a Canadian quaternary care hospital. Can J Gastroenterol. 2009;23:613–617. doi: 10.1155/2009/691850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez SA, Collins JM, Knigge KL, et al. Surveillance and management of dysplasia in ulcerative colitis. Gastrointest Endosc. 2007;65:432–439. doi: 10.1016/j.gie.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 20.Lebwohl B, Kapel RC, Neugut AI, et al. Adherence to biopsy guidelines increases celiac disease diagnosis. Gastrointest Endosc. 2011;74:103–109. doi: 10.1016/j.gie.2011.03.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lieberman D, Nadel M, Smith RA, et al. Standardized colonoscopy reporting and data system: report of the Quality Assurance Task Group of the National Colorectal Cancer Roundtable. Gastrointest Endosc. 2007;65:757–766. doi: 10.1016/j.gie.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 22.Sawhney MS, Salfiti N, Nelson DB, et al. Risk factors for severe delayed postpolypectomy bleeding. Endoscopy. 2008;40:115–119. doi: 10.1055/s-2007-966959. [DOI] [PubMed] [Google Scholar]
- 23.Leffler DA, Kheraj R, Garud S, et al. The incidence and cost of unexpected hospital use after scheduled outpatient endoscopy. Arch Intern Med. 2010;170:1752–1757. doi: 10.1001/archinternmed.2010.373. [DOI] [PubMed] [Google Scholar]
- 24.Dorn SD. Gastroenterology in a new era of accountability: part 1—an overview of performance measurement. Clin Gastroenterol Hepatol. 2011;9:750–753. doi: 10.1016/j.cgh.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 25.Heathcote EJ. Management of primary biliary cirrhosis: the American Association for the Study of Liver Diseases practice guidelines. Hepatology. 2000;31:1005–1013. doi: 10.1053/he.2000.5984. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124:795–841. doi: 10.1053/gast.2003.50106. [DOI] [PubMed] [Google Scholar]
- 27.Kornbluth A, Hayes M, Feldman S, et al. Do guidelines matter? Implementation of the ACG and AGA osteoporosis screening guidelines in inflammatory bowel disease (IBD) patients who meet the guidelines’ criteria. Am J Gastroenterol. 2006;101:1546–1550. doi: 10.1111/j.1572-0241.2006.00571.x. [DOI] [PubMed] [Google Scholar]
- 28.Etzel JP, Larson MF, Anawalt BD, et al. Assessment and management of low bone density in inflammatory bowel disease and performance of professional society guidelines. Inflamm Bowel Dis. 2011;17:2122–2129. doi: 10.1002/ibd.21601. [DOI] [PubMed] [Google Scholar]
- 29.Ferkolj I. How to improve the safety of biologic therapy in Crohn’s disease. J Physiol Pharmacol. 2009;60(Suppl 7):67–70. [PubMed] [Google Scholar]
- 30.Melmed GY, Ippoliti AF, Papadakis KA, et al. Patients with inflammatory bowel disease are at risk for vaccine-preventable illnesses. Am J Gastroenterol. 2006;101:1834–1840. doi: 10.1111/j.1572-0241.2006.00646.x. [DOI] [PubMed] [Google Scholar]
- 31.Wasan SK, Baker SE, Skolnik PR, et al. A practical guide to vaccinating the inflammatory bowel disease patient. Am J Gastroenterol. 2010;105:1231–1238. doi: 10.1038/ajg.2009.733. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. [Accessed April 1, 2012];General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) 2011 Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm?s_cid=rr6002a1_e.
- 33.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farraye FA, Odze RD, Eaden J, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:738–745. doi: 10.1053/j.gastro.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 35.Collins PD, Mpofu C, Watson AJ, et al. Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease. Cochrane Database Syst Rev. 2006;2:CD000279. doi: 10.1002/14651858.CD000279.pub3. [DOI] [PubMed] [Google Scholar]
- 36.Itzkowitz SH, Present DH. Consensus conference: colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314–321. doi: 10.1097/01.mib.0000160811.76729.d5. [DOI] [PubMed] [Google Scholar]
- 37.Ullman TA. Colonoscopic surveillance in inflammatory bowel disease. Curr Opin Gastroenterol. 2005;21:585–588. doi: 10.1097/01.mog.0000174219.07957.36. [DOI] [PubMed] [Google Scholar]
- 38.Farraye FA, Odze RD, Eaden J, et al. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746–774. doi: 10.1053/j.gastro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 39.Shale MJ, Seow CH, Coffin CS, et al. Review article: chronic viral infection in the anti-tumour necrosis factor therapy era in inflammatory bowel disease. Aliment Pharmacol Ther. 2010;31:20–34. doi: 10.1111/j.1365-2036.2009.04112.x. [DOI] [PubMed] [Google Scholar]
- 40.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 41.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–524. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 42.Vaughn BP, Doherty GA, Gautam S, et al. Screening for tuberculosis and hepatitis B prior to the initiation of anti-tumor necrosis therapy. Inflamm Bowel Dis. 2012;18:1057–1063. doi: 10.1002/ibd.21824. [DOI] [PubMed] [Google Scholar]
- 43.Lichtenstein GR, Abreu MT, Cohen R, et al. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:935–939. doi: 10.1053/j.gastro.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 44.American College of Gastroenterology Task Force on Irritable Bowel Syndrome. Brandt LJ, Chey WD, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(Suppl 1):S1–S35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 45.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 46.Stebbing JF. Quality assurance of endoscopy units. Best Pract Res Clin Gastroenterol. 2011;25:361–370. doi: 10.1016/j.bpg.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Faigel DO, Pike IM, Baron TH, et al. Quality indicators for gastrointestinal endoscopic procedures: an introduction. Am J Gastroenterol. 2006;101:866–872. doi: 10.1111/j.1572-0241.2006.00677.x. [DOI] [PubMed] [Google Scholar]
- 48.Petersen BT. Quality assurance for endoscopists. Best Pract Res Clin Gastroenterol. 2011;25:349–360. doi: 10.1016/j.bpg.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Vital signs: colorectal cancer screening among adults aged 50–75 years—United States 2008. MMWR Morb Mortal Wkly Rep. 2010;59:808–812. [PubMed] [Google Scholar]
- 50.Sint Nicolaas J, de Jonge V, Cahen DL, et al. Awareness of surveillance recommendations among patients with colorectal adenomas. Clin Gastroenterol Hepatol. 2012;10:405–411. doi: 10.1016/j.cgh.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 51.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Schoen RE, Pinsky PF, Weissfeld JL, et al. Utilization of surveillance colonoscopy in community practice. Gastroenterology. 2010;138:73–81. doi: 10.1053/j.gastro.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laiyemo AO, Pinsky PF, Marcus PM, et al. Utilization and yield of surveillance colonoscopy in the continued follow-up study of the polyp prevention trial. Clin Gastroenterol Hepatol. 2009;7:562–567. doi: 10.1016/j.cgh.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeVault KR, Castell DO American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastro-esophageal reflux disease. Am J Gastroenterol. 2005;100:190–200. doi: 10.1111/j.1572-0241.2005.41217.x. [DOI] [PubMed] [Google Scholar]
- 55. [Accessed April 1, 2012];ICSI health care guideline: initial management of dyspepsia and GERD. 2006 Available at: http://www.icsi.org/dyspepsia_gerd/dyspepsia__9.html.
- 56.GERD Work Group. [Accessed April 1, 2012];Gastroesophageal reflux disease (GERD) physician performance measurement set. 2007 Available at: http://www.ama-assn.org/ama1/pub/upload/mm/pcpi/gerdws122107.pdf.
- 57.Standards of Practice Committee. Lichtenstein DR, Cash BD, et al. Role of endoscopy in the management of GERD. Gastrointest Endosc. 2007;66:219–224. doi: 10.1016/j.gie.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 58.Kahrilas PJ, Shaheen NJ, Vaezi MF, et al. American Gastroenterological Association medical position statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383–1391. doi: 10.1053/j.gastro.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 59.American Gastroenterological Association. Spechler SJ, Sharma P, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084–1091. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 60.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 61.Herzig SJ, Howell MD, Ngo LH, et al. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA. 2009;301:2120–2128. doi: 10.1001/jama.2009.722. [DOI] [PubMed] [Google Scholar]
- 62.Hauben M, Horn S, Reich L, et al. Association between gastric acid suppressants and Clostridium difficile colitis and community-acquired pneumonia: analysis using pharmacovigilance tools. Int J Infect Dis. 2007;11:417–422. doi: 10.1016/j.ijid.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Dial S, Delaney JA, Barkun AN, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989–2995. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 64.Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784–790. doi: 10.1001/archinternmed.2010.89. [DOI] [PubMed] [Google Scholar]
- 65.Alkhatib AA, Elkhatib FA, Khatib OF. Gastric acid-reducing medications and clopidogrel: what are the latest FDA recommendations? Am J Gastroenterol. 2010;105:1211. doi: 10.1038/ajg.2009.722. [DOI] [PubMed] [Google Scholar]
- 66.Johnson CE, Freel CD, Kornbluth S. Features of programmed cell death in intact Xenopus oocytes and early embryos revealed by near-infrared fluorescence and real-time monitoring. Cell Death Differ. 2010;17:170–179. doi: 10.1038/cdd.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spechler SJ, Fitzgerald RC, Prasad GA, et al. History, molecular mechanisms, and endoscopic treatment of Barrett’s esophagus. Gastroenterology. 2010;138:854–869. doi: 10.1053/j.gastro.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570–580. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 69.Lekakos L, Karidis NP, Dimitroulis D, et al. Barrett’s esophagus with high-grade dysplasia: focus on current treatment options. World J Gastroenterol. 2011;17:4174–4183. doi: 10.3748/wjg.v17.i37.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lai M, Afdhal NH. Health care quality measurement in the care of patients with cirrhosis. Clin Gastroenterol Hepatol. 2010;8:649–650. doi: 10.1016/j.cgh.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 71.Kanwal F, Kramer J, Asch SM, et al. An explicit quality indicator set for measurement of quality of care in patients with cirrhosis. Clin Gastroenterol Hepatol. 2010;8:709–717. doi: 10.1016/j.cgh.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 72.Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154:85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 73.Franceschi S, Raza SA. Epidemiology and prevention of hepatocellular carcinoma. Cancer Lett. 2009;286:5–8. doi: 10.1016/j.canlet.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 74.Wong GL, Wong VW, Tan GM, et al. Surveillance programme for hepatocellular carcinoma improves the survival of patients with chronic viral hepatitis. Liver Int. 2008;28:79–87. doi: 10.1111/j.1478-3231.2007.01576.x. [DOI] [PubMed] [Google Scholar]
- 75.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- 78.Davila JA, Morgan RO, Richardson PA, et al. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–141. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154:85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 80.Groszmann RJ, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254–2261. doi: 10.1056/NEJMoa044456. [DOI] [PubMed] [Google Scholar]
- 81.Merli M, Nicolini G, Angeloni S, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol. 2003;38:266–272. doi: 10.1016/s0168-8278(02)00420-8. [DOI] [PubMed] [Google Scholar]
- 82.Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol. 2007;102:2086–2102. doi: 10.1111/j.1572-0241.2007.01481.x. [DOI] [PubMed] [Google Scholar]
- 83.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823–832. doi: 10.1056/NEJMra0901512. [DOI] [PubMed] [Google Scholar]
- 84.D’Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis. 1999;19:475–505. doi: 10.1055/s-2007-1007133. [DOI] [PubMed] [Google Scholar]
- 85.Saab S, DeRosa V, Nieto J, et al. Costs and clinical outcomes of primary prophylaxis of variceal bleeding in patients with hepatic cirrhosis: a decision analytic model. Am J Gastroenterol. 2003;98:763–770. doi: 10.1111/j.1572-0241.2003.07392.x. [DOI] [PubMed] [Google Scholar]
- 86.Teran JC, Imperiale TF, Mullen KD, et al. Primary prophylaxis of variceal bleeding in cirrhosis: a cost-effectiveness analysis. Gastroenterology. 1997;112:473–482. doi: 10.1053/gast.1997.v112.pm9024301. [DOI] [PubMed] [Google Scholar]
- 87.Moodley J, Lopez R, Carey W. Compliance with practice guidelines and risk of a first esophageal variceal hemorrhage in patients with cirrhosis. Clin Gastroenterol Hepatol. 2010;8:703–708. doi: 10.1016/j.cgh.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 88.Leffler D. Celiac disease diagnosis and management: a 46-year-old woman with anemia. JAMA. 2011;306:1582–1592. doi: 10.1001/jama.306.14.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Evans KE, Aziz I, Cross SS, et al. A prospective study of duodenal bulb biopsy in newly diagnosed and established adult celiac disease. Am J Gastroenterol. 2011;106:1837–1842. doi: 10.1038/ajg.2011.171. [DOI] [PubMed] [Google Scholar]
- 90.Elli L, Bardella MT. Duodenal bulb biopsies and celiac disease. Gastrointest Endosc. 2011;73:1069. doi: 10.1016/j.gie.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 91.Gee A. Concerns raised over new US resident physician work hours. Lancet. 2011;378:758. doi: 10.1016/s0140-6736(11)61361-5. [DOI] [PubMed] [Google Scholar]
- 92.Horwitz LI. Why have working hour restrictions apparently not improved patient safety? BMJ. 2011;342:d1200. doi: 10.1136/bmj.d1200. [DOI] [PMC free article] [PubMed] [Google Scholar]