Abstract
Lomaiviticins A and B are complex antitumor antibiotics that were isolated from a strain of Micromonospora. A confluence of several unusual structural features renders the lomaiviticins exceedingly challenging targets for chemical synthesis. We report an 11-step, enantioselective synthetic route to lomaiviticin aglycon. Our route proceeds by late-stage, stereoselective dimerization of two equivalent monomeric intermediates, a transformation that may share parallels with the natural products’ biosyntheses. The route we describe is scalable and convergent, and it lays the foundation for determination of the mode of action of these natural products.
Lomaiviticins A and B (1 and 2, Figure 1) are complex dimeric bacterial metabolites that were obtained as minor constituents from the fermentation broth of Micromonospora lomaivitiensis.1 The lomaiviticins, and the related monomeric isolates known as the kinamycins (4–6),2 constitute a small family of natural products which contain a diazo functional group. In 1–6, this function is embedded in a highly oxidized tetracyclic carbon skeleton, forming a diazotetrahydrobenzo[b]fluorene (diazofluorene, see structure 7). Simple diazo-containing molecules, such as diazomethane, are prone to detonation.3 Thus, the presence of this functional group within a natural product, itself formed under conditions of microbial metabolism, is remarkable. Several additional structural features of the lomaiviticins—deoxyglycoside residues, highly oxidized napthoquinones, and a sterically congested carbon–carbon bond bridging the two moieties of each metabolite—elevate their molecular complexity and render their structures singular among all known natural products.
Figure 1.
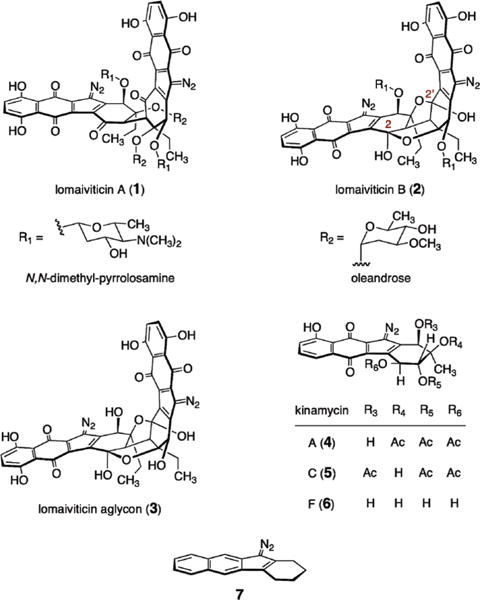
Structures of lomaiviticins A and B (1 and 2), lomaiviticin aglycon (3), kinamycins A, C, and F (4–6), and diazotetrahydrobenzo-[b]fluorene (diazofluorene, 7).
Both 1 and 2 exhibit powerful antimicrobial activities (MICs ≈ 6–25 ng/spot, plate assay), and lomaiviticin A (1) is reported to inhibit the growth of 25 cancer cell lines at low nanomolar to picomolar concentrations. The activity profile of 1 differs from those of other cancer chemotherapeutics, such as doxorubicin and mitomycin, suggesting that a distinct mechanism of action is operative.1 Model studies have provided evidence that the lomaiviticins form reactive intermediates under reducing conditions.4 However, investigations of 1 and 2 directly have been impossible to initiate because the lomaiviticins are obtained in minute quantities from their natural source,1 and a total synthesis has not yet been realized.5,6
Herein, we report an 11-step, enantioselective synthesis of lomaiviticin aglycon (3). This research addresses the decade-long problem of synthesis of the dimeric diazofluorene structure of the lomaiviticins. Many steps in our route may be executed efficiently on gram scales, as shown. The brevity and convergence of our synthesis renders it amenable to incorporation of affinity probes for target identification experiments and to the synthesis of analogues for structure–function studies.7
Our strategy to synthesize 3 was guided by the hypothesis that nature prepares the lomaiviticins by direct oxidative dimerization of monomeric diazofluorenes, as depicted in Scheme 1a. The merit of this approach in a synthetic setting is that it prognosticates the union of two identical monomers late-stage in the synthesis, thereby vastly simplifying the route to 3. However, several challenges to this bond construction are readily identified. First, under strongly acidic or basic conditions, monomers such as 8 are susceptible to a β-elimination-aromatization pathway (Scheme 1b), and this reaction manifold severely limits the chemical reagents that are compatible with such substrates. Second, as a consequence of the steric hindrance about the incipient bond, it was anticipated that the rate of dimerization may be too slow to produce workable yields of coupled products (9). This substitution pattern also made it difficult to predict the stereochemical outcome of the coupling event. Finally, although the oxidative couplings of ketones,8 their enolates,9 and their enoxysilanes10 have been used with great success in synthetic chemistry, the oxidative dimerization of diazofluorenes, such as 8, was unknown at the outset of these studies.
Scheme 1. (a) Proposed Oxidative Dimerization To Form Lomaiviticins A and B (1 and 2), (b) Elimination–Aromatization Pathway, and (c) Syntheses of the Diazofluorenes 18 and 19a.
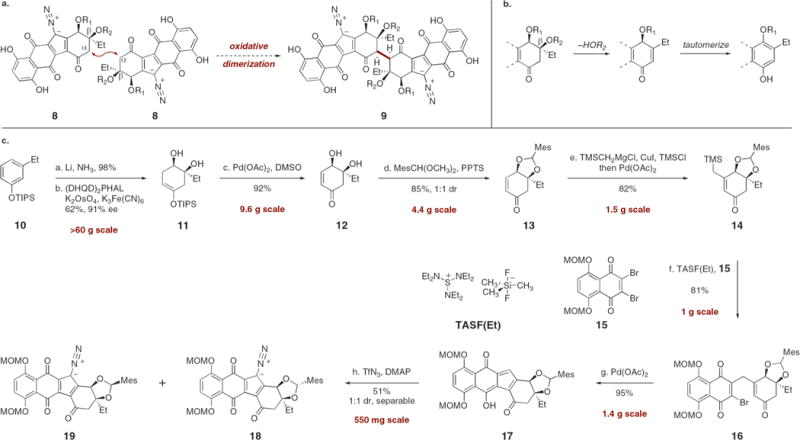
aConditions: (a) Li, NH3, t-BuOH, THF, −78 → −33 → −78 °C, 98%. (b) (DHQD)2PHAL, K2OsO4, CH3SO2NH2, t-BuOCH3–t-BuOH-H2O, −5 °C, 62%, 91% ee. (c) Pd(OAc)2, O2, DMSO, 24 °C, 92%. (d) MesCH(OCH3)2, PPTS, CH3CN, 24 → 50 °C, 85%. (e) TMSCH2MgCl, CuI, HMPA, Et3N, TMSCl, THF, −30 → −60 → −78 °C; Pd(OAc)2, CH3CN, 24 °C, 82%. (f) TASF(Et), CH2Cl2, −78 °C, 81%. (g) Pd(OAc)2, polymer-supported PPh3, Ag2CO3, toluene, 80 °C, 95%. (h) TfN3, DMAP, CH3CN, −20 °C, 51%.
Our pathway to lomaiviticin aglycon (3) began with synthesis of the monomeric diazofluorene 18 by the sequence outlined in Scheme 1c. Our route to 18 follows a pathway originally developed for the synthesis of kinamycin F (6),6c with several critical modifications to increase flexibility and material throughput. The synthesis began with silylation of the inexpensive commercial reagent 3-ethylphenol (96%, >1 mol scale).11 Birch reduction of the resulting silyl ether 10 followed by regio- and stereoselective dihydroxylation12 afforded the enoxysilane 11 in 91% ee and 61% yield over two steps (>60 g scale). In a departure from previous studies, the diol 11 was oxidized directly to the α,β-unsaturated ketone 12 by treatment with palladium acetate (10 mol %) under an atmosphere of dioxygen (92%, 9.6 g scale).13 This modification delayed installation of the protecting group, which accelerated the development of this sequence. The α,β-unsaturated ketone 12 was then protected as its mesitylaldehyde acetal (85%, 1:1 mixture of diastereomers, 4.4 g scale). The mixture of diastereomeric acetals 13 was homologated to the β-(trimethylsilylmethyl)-α,β-unsaturated ketones 14 by copper-mediated 1,4-addition of trimethylsilylmethylmagnesium chloride, trapping with chlorotrimethylsilane, and reoxidation (82%, 1.5 g scale).
The β-(trimethylsilylmethyl)-α,β-unsaturated ketones 14 and 2,3-dibromo-5,8-bis(methoxymethyloxy)naphthoquinone (15, prepared in one step from 2,3-dibromo-5,8-dihydroxynaphthoquinone)11,14 were efficiently coupled by treatment with tris-(diethylamino)sulfonium trimethyldifluorosilicate [TASF(Et)], to afford the γ-quinonylation products 16 in 81% yield (1 g scale). The products 16 were then cyclized by heating in the presence of palladium acetate and polymer-supported triphenylphosphine. Lowering the ratio of phosphine to palladium from 2.5:1 to 1.5:1 increased the yield of the cyclized products 17 to 95% (compared to 66% yield in our kinamycin synthesis;6c 1.4 g scale). Finally, diazo transfer to the cyclized products 17 (TfN3, DMAP) afforded the diazofluorenes 18 and 19 in 51% combined yield, and these were readily separated by flash column chromatography (550 mg scale).15
Transformation of 18 (or 19) to lomaiviticin aglycon (3) proved to be a formidable challenge. An exhaustive evaluation of conditions (>1500 experiments) using 18, 19, and various derivatives of each was unsuccessful. Ultimately, however, a short sequence was developed (Scheme 2a). First, the exo-mesityl diazofluorene 18 was converted to its trimethylsilyl enol ether derivative 20 by exposure to trimethylsilyl trifluoromethanesulfonate and triethylamine at 0 °C. The unpurified enoxysilane 20 was immediately re-dissolved in benzene and treated with a solution of manganese tris(hexafluoroacetylacetonate) (21)16 in benzene at 21 °C. Under these conditions, the oxidative coupling products 22 and 23 were obtained in 38% combined yield on a 100 mg scale (26% isolated yield of 22, 12% isolated yield of 23, 30% of 18 recovered).17 A third dimeric product, tentatively identified as the Cs-symmetric dimer, was detected by LC/MS analysis of the unpurified product mixture, but this compound was not formed in quantities sufficient for detailed characterization.
Scheme 2. (a) Dimerization of the exo-Mesityl Diazofluorene 18,a (b) Stereochemical Model for the Dimerization Event, and (c) Cyclization of the Ketone Isomer (25) of Lomaiviticin Aglycon (3).
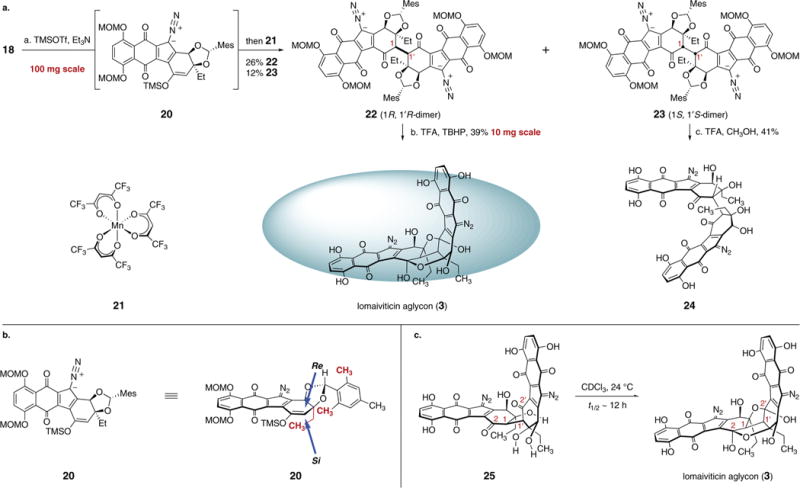
aConditions: (a) TMSOTf, Et3N, CH2Cl2, 0 °C, then 21, PhH, 21 °C, 26% 22, 12% 23, 30% 18. (b) TFA, TBHP, CH2Cl2, −35 °C, 12 h, 39%. (c) TFA, CH3OH, 50 °C, 41%.
The relative stereochemistry of the dimeric products 22 and 23 was deduced by conversion of 22 to lomaiviticin aglycon (3, vide infra). The stereoselectivity in their formation (22:23 = 2:1) is believed to originate from a nonbonded interaction between the ortho-methylarene substituents of 20 and the C-3 ethyl substituent (Scheme 2b). This interaction is proposed to orient the ethyl residue over the Si face of the enoxysilane, guiding the approach of the enoxysilanes 20 from their respective Re faces. Dimerization of the enoxysilane of 19, which contains the mesitylaldehyde acetal in the endo orientation, forms the (1S,1′S)-dimer exclusively (36% yield).11,18 This result is consistent with our stereochemical model and the inherent preference for addition exo to the cis-fused 5–6 system.
The deprotection of the (1R,1′R)-dimer 22 was successfully executed under carefully optimized conditions. Thus, slow addition of trifluoroacetic acid to a mixture of 22 and excess anhydrous tert-butylhydroperoxide19 in dichloromethane–decane solution at −35 °C, followed by extractive workup and purification, afforded lomaiviticin aglycon (3) in 39% yield (10 mg scale, 2.5 mg of 3 isolated; >15 mg of 3 has been prepared to date). Careful examination of this reaction established that the open-chain ketone isomer of lomaiviticin aglycon (25) is the product that is first formed in the deprotection of 22 (Scheme 2c). The ratio of the ketone isomer 25 to 3 was >8:1 throughout the course of the reaction (LC/MS analysis, retention times of 25 and 3 = 1.55 and 1.26 min, respectively), but these mixtures quantitatively converted to 3 upon purification by preparative thin-layer chromatography. Mixtures of 25 and 3 could be obtained by rapid isolation using flash-column chromatography,11 and HMBC experiments unequivocally established the presence of 25 [a correlation was observed between H-1/H-1′ (δ 3.34) and C-2/C-2′ (δ 190.8)]. The open-chain isomer (25) slowly converted to lomaiviticin aglycon (3) on standing in chloroform (ca. 50% conversion of 25 after 12 h at 24 °C) or, more rapidly, in methanol (full conversion within 10 min). The C-2/C-2′ carbon resonance of 3 (δ 99.4, CDCl3), which was coupled to the tertiary hydroxyl proton and to H-1/H-1′ in the HMBC spectrum, agrees well with that of lomaiviticin B (2, δ 96.5, CD3OD).1,11 These data support the assignment of the relative stereochemistry of 3 and, by inference, that of 22 and 23, as shown.
By comparison, intermediates in the (1S,1′S)-series were appreciably more robust. The (1S,1′S)-dimer 23 was deprotected by heating with trifluoroacetic acid in methanol at 50 °C, to afford the diastereomeric lomaiviticin aglycon (24, 41%). The increased stability of 24 may be attributed to the cis arrangement of the α-hydrogen and β-oxygen substituents, which is expected to decrease the rate of β-elimination. As expected, the (1S, 1′S)-diastereomer 24 showed no evidence of cyclization after standing for 2 days at 24 °C in methanol-d4.
Several additional observations that arose during the course of our studies are noteworthy. First, we found that enoxysilanes derived from diazofluorenes bearing acyclic protecting groups on the vicinal diol function were unstable above 0 °C. Attempts to induce their direct coupling (at lower temperatures) invariably resulted in elimination and aromatization, without formation of detectable levels of dimeric products. Additionally, conventional oxidants, such as copper chloride9a or ceric ammonium nitrate (CAN),10 led to rapid aromatization of the enoxysilane 20, even at −35 °C. An extensive evaluation of alternative oxidants ultimately led to the identification of manganese tris(hexafluoroacetylacetonate) (21) as uniquely effective in this bond construction. The synthesis of 21 proceeds in one step from commercial reagents,16 and the volatility of the complex allows purification of multigram quantities by sublimation (60–70 °C, 200 mTorr). The application of 21 in the oxidative coupling of enoxysilanes has not been described, to our knowledge, although Mayer has conducted detailed studies of its reactivity toward activated C–H bonds.16b The success of 21 in our studies may be attributed to two factors. First, because the manganese atom in 21 is coordinatively saturated, it cannot behave as a Lewis acid toward the β-oxygen of 20. Second, in contrast to copper chloride or CAN, the manganese complex 21 is fully soluble (and stable) in benzene. Use of this solvent in the oxidative coupling reaction may attenuate competing elimination pathways.
Supplementary Material
Acknowledgments
Financial support from Yale University, the National Science Foundation (Graduate Research Fellowship to C.M.W.), Eli Lilly, and the Searle Scholars Program is gratefully acknowledged. We thank Dr. Haiyin He (Pfizer Pharmaceuticals) and Prof. Andrew J. Phillips (Yale University) for helpful discussions. We thank Patrick Lynch for design of the cover art.
Footnotes
Supporting Information. Experimental procedures and detailed characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.He H, Ding WD, Bernan VS, Richardson AD, Ireland CM, Greenstein M, Ellestad GA, Carter GT. J Am Chem Soc. 2001;123:5362. doi: 10.1021/ja010129o. [DOI] [PubMed] [Google Scholar]
- 2.(a) Ito S, Matsuya T, Omura S, Otani M, Nakagawa A. J Antibiot. 1970;23:315. doi: 10.7164/antibiotics.23.315. [DOI] [PubMed] [Google Scholar]; (b) Hata T, Omura S, Iwai Y, Nakagawa A, Otani M. J Antibiot. 1971;24:353. doi: 10.7164/antibiotics.24.353. [DOI] [PubMed] [Google Scholar]; (c) Omura S, Nakagawa A, Yamada H, Hata T, Furusaki A, Watanabe T. Chem Pharm Bull. 1971;19:2428. doi: 10.1248/cpb.21.931. [DOI] [PubMed] [Google Scholar]; (d) Omura S, Nakagawa A, Yamada H, Hata T, Furusaki A. Chem Pharm Bull. 1973;21:931. doi: 10.1248/cpb.21.931. [DOI] [PubMed] [Google Scholar]; (e) Seaton PJ, Gould SJ. J Antibiot. 1989;42:189. doi: 10.7164/antibiotics.42.189. [DOI] [PubMed] [Google Scholar]; For a review of kinamycin biosynthesis, see:; (f) Gould SJ. Chem Rev. 1997;97:2499. doi: 10.1021/cr9600215. [DOI] [PubMed] [Google Scholar]
- 3.DeBoer TJ, Backer HJ. Organic Syntheses. Vol. 36. Wiley; New York: 1956. p. 16. [Google Scholar]
- 4.(a) Laufer RS, Dmitrienko GI. J Am Chem Soc. 2002;124:1854. doi: 10.1021/ja0167809. [DOI] [PubMed] [Google Scholar]; (b) Zeng W, Ballard TE, Tkachenko AG, Burns VA, Feldheim DL, Melander C. Bioorg Med Chem Lett. 2006;16:5148. doi: 10.1016/j.bmcl.2006.07.024. [DOI] [PubMed] [Google Scholar]; (c) Feldman KS, Eastman KJ. J Am Chem Soc. 2006;128:12562. doi: 10.1021/ja0642616. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Khdour O, Skibo EB. Org Biomol Chem. 2009;7:2140. doi: 10.1039/b903844b. [DOI] [PubMed] [Google Scholar]
- 5.For studies toward the syntheses of 1 and 2, see:; (a) Nicolaou KC, Denton RM, Lenzen A, Edmonds DJ, Li A, Milburn RR, Harrison ST. Angew Chem Int Ed. 2006;45:2076. doi: 10.1002/anie.200504466. [DOI] [PubMed] [Google Scholar]; (b) Krygowski ES, Murphy-Benenato K, Shair MD. Angew Chem Int Ed. 2008;47:1680. doi: 10.1002/anie.200704830. [DOI] [PubMed] [Google Scholar]; (c) Zhang W, Baranczak A, Sulikowski GA. Org Lett. 2008;10:1939. doi: 10.1021/ol800460a. [DOI] [PubMed] [Google Scholar]; (d) Gholap SL, Woo CM, Ravikumar PC, Herzon SB. Org Lett. 2009;11:4322. doi: 10.1021/ol901710b. [DOI] [PubMed] [Google Scholar]; (e) Nicolaou KC, Nold AL, Li H. Angew Chem Int Ed. 2009;48:5860. doi: 10.1002/anie.200902509. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Morris WJ, Shair MD. Tetrahedron Lett. 2010;51:4310. doi: 10.1016/j.tetlet.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Lee HG, Ahn JY, Lee AS, Shair MD. Chem–Eur J. 2010;16:13058. doi: 10.1002/chem.201002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For syntheses of various kinamycins, see:; (a) Lei X, Porco JA. J Am Chem Soc. 2006;128:14790. doi: 10.1021/ja066621v. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Li H, Nold AL, Pappo D, Lenzen A. J Am Chem Soc. 2007;129:10356. doi: 10.1021/ja074297d. [DOI] [PubMed] [Google Scholar]; (c) Woo CM, Lu L, Gholap SL, Smith DR, Herzon SB. J Am Chem Soc. 2010;132:2540. doi: 10.1021/ja910769j. [DOI] [PubMed] [Google Scholar]; For synthesis of (±)-O-methyl-kinamycin C, see:; (d) Kumamoto T, Kitani Y, Tsuchiya H, Yamaguchi K, Seki H, Ishikawa T. Tetrahedron. 2007;63:5189. [Google Scholar]; For studies toward the synthesis of kinamycin F (6), see:; (e) Chen N, Carriere MB, Laufer RS, Taylor NJ, Dmitrienko GI. Org Lett. 2008;10:381. doi: 10.1021/ol702650u. [DOI] [PubMed] [Google Scholar]
- 7.Wender PA, Verma VA, Paxton TJ, Pillow TH. Acc Chem Res. 2008;41:40. doi: 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins EGE, Large R. J Chem Soc, Perkin Trans. 1974;1:280. [Google Scholar]
- 9.For selected examples, see:; (a) Ito Y, Konoike T, Harada T, Saegusa T. J Am Chem Soc. 1977;99:1487. [Google Scholar]; (b) Baran PS, DeMartino MP. Angew Chem, Int Ed. 2006;45:7083. doi: 10.1002/anie.200603024. [DOI] [PubMed] [Google Scholar]; (c) DeMartino MP, Chen K, Baran PS. J Am Chem Soc. 2008;130:11546. doi: 10.1021/ja804159y. [DOI] [PubMed] [Google Scholar]
- 10.For selected examples, see:; (a) Baciocchi E, Casu A, Ruzziconi R. Tetrahedron Lett. 1989;30:3707. [Google Scholar]; (b) Avetta CT, Konkol LC, Taylor CN, Dugan KC, Stern CL, Thomson RJ. Org Lett. 2008;10:5621. doi: 10.1021/ol802516z. [DOI] [PubMed] [Google Scholar]
- 11.See Supporting Information.
- 12.Kolb HC, VanNieuwenhze MS, Sharpless KB. Chem Rev. 1994;94:2483. [Google Scholar]
- 13.(a) Ito Y, Hirao T, Saegusa T. J Org Chem. 1978;43:1011. [Google Scholar]; (b) Larock RC, Hightower TR, Kraus GA, Hahn P, Zheng D. Tetrahedron Lett. 1995;36:2423. [Google Scholar]
- 14.Huot R, Brassard P. Can J Chem. 1974;52:838. [Google Scholar]
- 15.This reaction proceeds in 75% yield, as determined by 1H NMR analysis of the unpurified reaction mixture against an internal standard. However, under all purification conditions that we have examined, the products 18 and 19 partially decompose. [Under optimized conditions, elution of analytically pure samples of 18 (or 19) resulted in 70% recovery.]
- 16.(a) Evans S, Hamnett A, Orchard AF, Lloyd DR. Faraday Discuss Chem Soc. 1972;54:227. [Google Scholar]; (b) Bryant JR, Taves JE, Mayer JM. Inorg Chem. 2002;41:2769. doi: 10.1021/ic025541z. [DOI] [PubMed] [Google Scholar]
- 17.The ratio of 22 and 23 varied somewhat between experiments but was consistently within the range 1.5–3:1. For example, in a separate 100 mg scale experiment, 22, 23, and 18 were obtained in 33%, 23%, and 15% yield, respectively.
- 18.Deprotection of the (1S,1′S)-dimer derived from 19 (TFA, CH3OH) provided 24 in 38% yield; see Supporting Information.
- 19.Myers AG, Fundy MAM, Lindstrom PA. Tetrahedron Lett. 1988;29:5609. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


