Abstract
Purpose
The purpose of this study was to determine if increased intraluminal pressure is the damaging factor which reduces flow mediated dilation (FMD) in young, healthy subjects following resistance exercise to maximal exertion.
Hypothesis
Attenuating the rise in brachial artery pressure during weight lifting by placing a blood pressure cuff on the upper arm prevents post-exercise impairment of brachial artery FMD in sedentary individuals.
Methods
Nine sedentary individuals who exercise ≤1 time/week and six exercise-trained individuals who exercise ≥3 times/week performed leg press exercise to maximal exertion on two separate occasions. During one visit a blood pressure cuff, proximal to the site of brachial artery measurement, was inflated to 100 mmHg to protect the distal vasculature from the rise in intraluminal pressure which occurs during resistance exercise. Brachial artery FMD was determined using ultrasonography before and 30 minutes after weight lifting.
Results
Without the protective cuff, brachial artery FMD in sedentary individuals was reduced after weight lifting (9.0 ± 1.2% pre-lift vs. 6.6 ± 0.8% post-lift; p=0.005) while in exercise-trained individuals FMD was unchanged (7.4 ± 0.7% pre-lift vs. 8.0 ± 0.9% post-lift; p=0.543). With the protective cuff, FMD no longer decreased, but rather increased in sedentary individuals (8.7 ± 1.2% pre-lift vs. 10.5 ± 1.0% post-lift, p=0.025). An increase in FMD was also seen in exercise-trained subjects when the cuff was present (6.6 ±0.7% pre-lift vs. 10.9 ±1.5% post-lift, p<0.001).
Conclusion
Protecting the brachial artery from exercise-induced hypertension enhances FMD in sedentary and exercise-trained individuals. These results indicate that increased intraluminal pressure in the artery contributes to the reduced FMD following heavy resistance exercise in sedentary individuals.
Keywords: Exercise, Endothelium, Vasodilation, Brachial Artery
Introduction
The negative effects of chronic hypertension on vascular endothelial function are well documented; however, less is understood about the effects of transient elevations in blood pressure on vascular reactivity. Fluctuations in blood pressure occur on a minute-to-minute basis throughout daily living, and more dramatically, blood pressure substantially increases during resistance exercise.(19) In a landmark study by Lamping and Dole, brief exposure to hypertension (1-5 minutes) was sufficient to potentiate vasoconstriction in the coronary vasculature, and this potentiation could last for at least 2 ½ hours.(18) This raises the question whether elevations in blood pressure that occur with isometric exercise also impair endothelial function in human conduit and resistance arteries. This is important since systolic blood pressure can surpass 400 mmHg during high intensity isometric exercise.(19) Consistent with this observation, when young, healthy, sedentary (SED) subjects perform resistance exercise at maximal effort, significant increases in systolic blood pressure are observed along with impairment of both endothelium-dependent brachial artery flow mediated dilation (FMD)(9, 10, 16, 23) and microvascular vasodilation in response to acetylcholine.(8)
In a review of the effects of acute exercise on FMD in healthy humans by Dawson et al. there appears to be a biphasic FMD pattern in response to exercise that varies according to fitness level, exercise duration, intensity and mode.(5) Previous work from our group and others would support the idea that fitness level predicts the FMD response after maximal-exertion exercise, as exercise trained (ET) individuals are protected from reductions in conduit and resistance artery function post-exercise whereas young, healthy, sedentary subjects are not.(8, 16) Although the specific mechanism responsible for the protective effect of exercise training on endothelial function is not known, it is well established that exercise increases endothelial nitric oxide synthase (12) and superoxide dismutase enzyme expression (24), both of which would increase nitric oxide bioavailability.
The reduction in FMD in SED subjects could be explained by either barotrauma to the brachial artery from the rise in arterial pressure during exercise,(8, 16, 23) or possibly due to the exercise-induced release of neurohumoral agents and catecholamines (1, 7) that reduce nitric oxide bioavailability. Additionally, exercise acutely increases numerous factors which can also have direct vasoconstrictor properties, including angiotensin II (21), endothelin-1 (20) and norepinephrine.(7) The purpose of this study is to determine if the rise in intraluminal pressure contributes to the impaired FMD after resistance exercise in healthy, sedentary subjects. We aim to measure brachial artery FMD in young, sedentary subjects who have a cuff around the upper arm inflated to 100 mmHg during a leg bench press exercise to protect the distal brachial artery from the exercise-induced increase in intra-arterial pressure. Because the inflation pressure of the cuff is never greater than systolic pressure, neither at rest nor during exercise, the distal vasculature of the cuffed arm will still be exposed to the same circulating factors. Our hypothesis is that increased intraluminal pressure within the brachial artery is responsible for impaired FMD after resistance exercise in healthy, sedentary subjects.
Methods
All subjects issued written informed consent prior to any study procedures, and all methods were approved by the Institutional Review Board at the Medical College of Wisconsin.
Subjects
Nine SED and six ET healthy male and female subjects between the ages of 18-38 years were recruited by posting flyers at local universities and through Craigslist advertisement. All subjects acted as their own controls. Our study defined healthy individuals as having no known history of cardiovascular disease and a BMI ≤25 kg/m2. SED subjects included individuals who self-reported regular strength and resistance exercises ≤1 time/week for at least the past 6 months. ET subjects included individuals who self-reported concurrent strength and resistance exercises ≥3 times/week for at least the past 6 months. Exclusion criteria for all subjects included hypercholesterolemia (total cholesterol >200 mg/dl), hypertension (resting blood pressure >140/90 mmHg), diabetes (blood glucose >200 mg/dl), tobacco use in the previous six months, subjects who were currently abusing alcohol or drugs, a history of lower extremity injury, and female subjects who were pregnant or nursing. All subjects had a skinfold test performed by the same licensed nutritionist to determine percent body fat. The average of three measurements at selected anatomical skinfold sites based on sex were used to estimate body density. Each body density measurement was then used in a population specific equation for either men (14) or women (15) to estimate body fat percent. All skinfold measurements were taken with a Lange Skinfold Caliper (Beta Technology Inc., Santa Cruz, CA).
Weight lifting protocol
All subjects were instructed to fast for 12 hours prior to each study visit, and asked to abstain from exercise for 48 hours prior to their visit. Each subject performed the same weight lifting protocol during both study visits, and the visits were separated by at least 7 days to allow for recovery. Prior to the lifting protocol, subjects were instructed to stretch their leg muscles for approximately 5 minutes to avoid injury.
The weight lifting protocol was performed on a recumbent leg press machine. Subjects performed two sets each of ten repetitions of leg press exercise at 35%, 50% and 90% of their approximate one-repetition maximum or until fatigue as previously described.(16) Briefly, after each set of ten lifts, subjects were asked to rate their perceived level of exertion on a ten-point scale. Weight was then added to the next set of lifts at the discretion of the study team. All subjects performed repetitions until failure on the last two sets of lifts. On the last repetition of each set, subjects were instructed to continue to breathe regularly and perform an isometric hold with the knees bent at approximately 45° while blood pressure was measured in the left arm using a sphygmomanometer and stethoscope.
During one of the visits, a second blood pressure cuff was placed proximal to the site of brachial artery measurement and inflated to 100 mmHg to protect the distal vasculature from the acute rise in blood pressure observed during weight lifting exercise. A cuff inflation pressure of 100 mmHg was chosen based on studies which demonstrated that upper arm cuff inflation pressures of 60 and 100 mmHg blunted increased shear stress during handgrip exercise (27) and reactive hyperemia in response to forearm heating, (11) respectively. The placement of the “protective” cuff on visit one or two was randomized for all participants. The cuff remained inflated for one minute after the last repetition of each set of ten lifts to allow adequate time for blood pressure to return to baseline values. After one minute, the cuff was then released for a period of one minute prior to the next set of weight lifting to avoid venous engorgement in the test arm. To confirm that a cuff inflation pressure of 100 mmHg on the upper arm blunted the rise in systolic pressure in the distal vasculature during maximal exertion, in a separate group of five healthy individuals blood pressure was measured distal to the protective cuff by determining the pressure at which the radial pulse returned after forearm occlusion with a blood pressure cuff.
Brachial artery FMD protocol
All FMD procedures were performed in a temperature controlled room between the hours of 7:00 AM and 9:00 AM, and all subjects had been fasting for ≥12 hours. Subjects reported to the study site and were instructed to lie quietly in a supine position for 15 minutes prior to beginning FMD assessment. In the supine state, imaging of the brachial artery on the right arm was performed by the same person each visit using a Sonosite MicroMaxx (Bothell, Washington, USA) portable ultrasound machine. The brachial artery was visualized in a longitudinal plane at a site proximal to the antecubital fossa of the supinated right arm abducted ~80°. The ultrasound probe (10.5 mHz) was positioned at 90° to the vessel to visualize anterior and posterior lumen-intimal interfaces. Three, 6-second video clips of the brachial artery were recorded to determine resting artery diameter and one Doppler image of arterial blood flow was captured to compare baseline and peak hyperemic flow responses in the artery. The clips and images were stored on the ultrasound machine for off-line analysis. After recording baseline images, a forearm blood pressure cuff was inflated to 250 mmHg for five minutes. After releasing the cuff, brachial artery flow velocity was measured during peak hyperemia and 6-second clips of brachial artery diameter were recorded every minute for 5 minutes after cuff release. The probe position was marked with a surgical pen to ensure transducer placement over the brachial artery was consistent between measurements. The probe position in relation to the antecubital fossa was also recorded to ensure the same segment of the brachial artery was visualized during the second visit.
The post-lift FMD was assessed approximately 30 minutes after the weight lifting session. After the second FMD assessment, endothelium-independent vasodilation was determined by administering 0.4 mg sublingual nitroglycerin (NTG) and recording the change in brachial artery diameter after three, four, and five minutes.
Brachial artery diameter was measured using the automatic edge detection feature of Brachial Analyzer (Medical Imaging Applications LLC, Coralville, IA, USA) at a sampling rate of 10 frames/second. The resting diameter of the brachial artery was determined by averaging the values of the three resting measurements. Peak FMD was determined by reporting the largest value from the average resting diameter. The brachial artery was re-measured 10 minutes post cuff release to determine average resting baseline diameter prior to NTG administration. NTG-mediated dilation was determined by reporting the largest value from the new series of baseline measurements. Percent change in FMD was defined as 100 × (maximum diameter during reactive hyperemia - resting diameter) / resting diameter. Peak brachial artery shear stress was calculated using the following equation:(2, 6, 28, 29) Shear rate (s−1) = 4 × blood velocity (cm/s) / vessel diameter (cm).
Statistical Analysis
All data are presented as mean ± standard error of the mean. Differences between subjects were determined using an unpaired Students t-test. Differences in pre- and post-lift FMD and NTG-mediated dilation, as well as pre- and post-lift hemodynamic values were determined using a two-way repeated measures analysis of variance (ANOVA) with the presence of the cuff and weight lifting as independent variables. A post hoc Student Newman Keuls test was used to determine differences between individual means. To determine the effect size of either weight lifting or the presence of the cuff during weight lifting, Cohen’s d was calculated using the means and standard deviations of FMD before and after the lifting exercise. An analysis of covariance (ANCOVA) was performed to test if covariates had an impact on the FMD response after weight lifting. P<0.05 was considered statistically significant.
Power Analysis
Previous studies by Phillips (23) and Jurva (16) have shown that relative brachial FMD is reduced between 29-65% from pre-lift values in SED subjects with similar inclusion criteria who undergo leg press exercise to maximal exertion (absolute FMD was reduced in those studies by 2.3% and 4.7%, respectively). Based on these effect sizes, and an inter-observer variation of 1.3 ± 0.7% for repeated measures from our group using the FMD method described above,(25) our sample size of SED subjects (n=9) could detect a relative difference in FMD of 20% (or an absolute difference of 1.6% assuming a pre-lift FMD of 8.0%) with 80% power and α=0.05 using paired analysis.
Results
Nine SED (2 male, 7 female) and six ET subjects (3 male, 3 female), ages 18-38, were consented and completed the study protocol (Table 1). Two SED subjects (1 male, 1 female) were screened but not enrolled in the study because they had total cholesterol levels >200 mg/dl.
Table 1.
Cardio-Metabolic Characteristics of Study Participants
| Characteristic | SED (n=9) | ET (n=6) | P-value |
|---|---|---|---|
| Sex, male | 2 | 3 | NA |
| Age | 26 ± 2.4 | 25 ± 0.3 | 0.726 |
| Height, cm | 168 ± 3.5 | 171 ± 2.7 | 0.491 |
| Weight, kg | 64 ± 2.5 | 69 ± 6.3 | 0.441 |
| Body Mass Index, kg/m2 | 23 ± 0.5 | 23 ± 1.5 | 0.710 |
| Body Fat, % | 27 ± 2.8 | 17 ± 2.7 | 0.039* |
| Waist Circumference, cm | 80 ± 1.8 | 77 ± 3.5 | 0.452 |
| Hip Circumference, cm | 98 ± 1.5 | 97 ± 2.7 | 0.766 |
| Waist/Hip Ratio | 0.81 ± 0.01 | 0.80 ± 0.03 | 0.662 |
| Blood Glucose, mg/dl | 81 ± 2.6 | 88 ± 8.8 | 0.400 |
| Hematocrit, % | 41 ± 0.6 | 42 ± 1.2 | 0.194 |
| Total Cholesterol, mg/dl | 158 ± 8 | 163 ± 8 | 0.661 |
| LDL Cholesterol, mg/dl | 76 ± 6 | 78 ± 5 | 0.837 |
| HDL Cholesterol, mg/dl | 65 ± 4 | 70 ± 8 | 0.572 |
| Resting SBP, mmHg | 115 ± 2 | 120 ± 4 | 0.243 |
| Resting DBP, mmHg | 77 ± 3 | 73 ± 3 | 0.351 |
| Resting Heart Rate, bpm | 62 ± 3 | 59 ± 5 | 0.577 |
| Max Weight Lifted, kg. | |||
| No Cuff | 119 ± 8.8 | 142 ± 10.8 | 0.132 |
| Cuff | 122 ± 5.9 | 140 ± 12.2 | 0.175 |
| Max SBP, mmHg | |||
| No Cuff | 194 ± 8 | 235 ± 11 | 0.010* |
| Cuff | 198 ± 10 | 235 ± 7 | 0.021* |
| Max DBP, mmHg | |||
| No Cuff | 89 ± 2 | 97 ± 3 | 0.047* |
| Cuff | 91 ± 3 | 92 ± 1 | 0.771 |
All values are expressed as mean ± SEM. n=number of participants. DBP, Diastolic Blood Pressure; ET, Exercise Trained Subjects; LDL, Low Density Lipoprotein; HDL, High Density Lipoprotein; SBP, Systolic Blood Pressure; SED, Sedentary Subjects.
Significant difference (P<0.05) ET vs. SED, t-test.
The maximum weight lifted during the session with the protective upper arm blood pressure cuff was 122 ± 5.9 kg for the SED group and 140 ± 12.2 kg for the ET group (p=0.175) (Table 1). Similarly, the maximum weight lifted during the session without the protective cuff was 119 ± 8.8 kg for SED group and 142 ± 10.8 kg for the ET group (p=0.132).
The maximum systolic blood pressure measured during the isometric hold during the session without the protective cuff was 194 ± 8 mmHg and 235 ± 11 mmHg for the SED and ET subjects, respectively (p=0.010) (Table 1). The presence of the protective cuff had no effect on maximum systemic systolic blood pressure measured in the opposite, uncuffed arm in either group (SED 194 ± 8 mmHg without cuff vs. 198 ± 10 mmHg with cuff, p=0.350; ET 235 ± 11 mmHg without cuff vs. 235 ± 7 mmHg with cuff; p=0.964). We confirmed that inflation of the upper arm cuff to 100 mmHg reduced distal systolic blood pressure in the cuffed arm in a subset of 5 healthy subjects (2 male, 3 female; aged 27±2.0 years; BMI = 24±1.5; resting SBP = 110±4 mmHg) during consecutive maximal exertion lifts (maximum weight lifted = 123±9.0 kg). The upper arm cuff reduced distal systolic blood pressure by 64±12 mmHg in these subjects (159 ± 4 mmHg without cuff vs. 94±13 mmHg with cuff, p = 0.005).
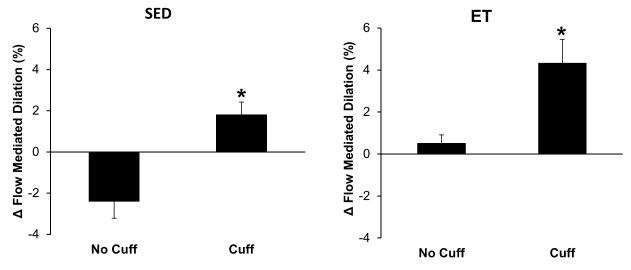
Without the protective cuff, brachial artery FMD in SED subjects was reduced after weight lifting (9.0 ± 1.2% pre-lift vs. 6.6 ± 0.8% post-lift; p=0.005) whereas in ET individuals FMD was unchanged (7.4 ± 0.7% pre-lift vs. 8.0 ± 0.9% post-lift; p=0.543) (Figure 1). With the protective cuff, FMD increased in both SED (8.7 ± 1.2% pre-lift vs. 10.5 ± 1.0% post-lift; p=0.025) and ET (6.6 ± 0.7% pre-lift vs. 10.9 ± 1.5% post-lift; p<0.001) subjects (Figure 1). Of the cardiometabolic factors presented in Table 1, percent body fat was found to be the only covariate that had an influence on the FMD response after weight lifting. The absolute change in brachial artery FMD in SED and ET individuals after weight lifting ± the protective cuff is shown in Figure 2. When the protective cuff was present, FMD increased after weight lifting in both groups compared to pre-lift values.
Figure 1.
Individual and mean brachial artery FMD measurements for all individuals before and after performing leg bench press exercise to maximal exertion. FMD was reduced in sedentary subjects (SED) following the lifting exercise (top, left; p=0.005 vs. pre-lift). When a blood pressure cuff inflated to 100 mmHg was placed on the upper arm proximal to the site of FMD measurement, FMD increased after weight lifting compared to pre-lift values (top, right; p=0.025). Exercise-trained (ET) individuals showed no change in brachial artery FMD following resistance exercise to maximal effort (bottom, right; p=0.543 vs. pre-lift); however brachial artery FMD increased when a protective cuff was placed around the upper arm (bottom, left; p<0.001 vs. pre-lift).
Figure 2.
Change in brachial artery FMD in sedentary (SED) and exercise trained (ET) individuals following leg press exercise to maximal exertion with and without a blood pressure cuff inflated to 100 mmHg on the upper arm. Both SED (left; n=9) and ET subjects (right; n=6) showed an increase in brachial artery FMD following the lifting session with the cuff on the upper arm.
The presence of the protective cuff during the lifting protocol had no effect on peak hyperemic shear stress (immediately post-cuff release) during the FMD protocol performed 30 minutes after lifting (Table 2). When FMD was normalized to peak shear stress, both ET and SED subjects still demonstrated augmented FMD when the protective cuff was present during the lifting protocol. Endothelium-independent vasodilation was unaffected by either weight lifting or the blood pressure cuff as all subjects demonstrated appropriate and similar endothelial-independent vasodilation (≥20% in all groups) following administration of nitroglycerin (Table 2). There were also no differences in NTG-mediated dilation between SED and ET subjects. Peak hyperemic shear stress following release of the cuff was also not different between SED and ET subjects, or between cuff- and no-cuff sessions.
Table 2.
Vascular Characteristics of Brachial Artery Before and After Weight Lifting
| SED (n=9) |
ET (n=6) |
|||||||
|---|---|---|---|---|---|---|---|---|
| No Cuff |
Cuff |
No Cuff |
Cuff |
|||||
| Characteristic | Pre WL | Post WL | Pre WL | Post WL | Pre WL | Post WL | Pre WL | Post WL |
| Resting BA Diameter, mm | 3.22 ± 0.15 | 3.27 ± 0.13 | 3.13 ± 0.16 | 3.21 ± 0.15 | 4.03 ± 0.36 | 4.07 ± 0.35 | 3.90 ± 0.32 | 3.92 ± 0.33 |
| Max BA Diameter, mm | 3.50 ± 0.15 | 3.48 ± 0.13 | 3.39 ± 0.15 | 3.54 ± 0.15 | 4.33 ± 0.38 | 4.40 ± 0.39 | 4.15 ± 0.35 | 4.36 ± 0.39 |
| FMD, % | 8.96 ± 1.19 | 6.55 ± 0.83* | 8.65 ± 1.21 | 10.47 ± 0.98* | 7.44 ± 0.74 | 7.96 ± 0.86 | 6.55 ± 0.70 | 10.89 ± 1.53* |
| Peak SS – Resting, s | 11.6 ± 0.9 | 12.7 ± 1.8 | 11.6 ± 1.3 | 11.8 ± 1.1 | 7.0 ± 0.5 | 7.5 ± 0.5 | 7.5 ± 0.6 | 8.3 ± 0.8 |
| Peak SS – Hyperemic, s | 18.4 ± 1.8 | 16.6 ± 1.3 | 18.5 ± 1.5 | 18.5 ± 1.3 | 12.6 ± 1.4 | 13.0 ± 1.2 | 14.3 ± 1.6 | 14.5 ± 1.2 |
| Normalized FMD (FMD/Peak Hyperemic SS) |
0.51 ± 0.09 | 0.40 ± 0.03* | 0.46 ± 0.04 | 0.57 ± 0.04* | 0.60± 0.10 | 0.63 ± 0.07 | 0.48 ± 0.06 | 0.77 ± 0.12* |
| Diameter after NTG, mm | NA | 3.94 ± 0.15 | NA | 3.93 ± 0.15 | NA | 4.98 ± 0.38 | NA | 4.86 ± 0.37 |
| NTG Dilation, % | NA | 22.21 ± 1.49 | NA | 21.67 ± 1.48 | NA | 21.40 ± 1.52 | NA | 22.88 ± 2.48 |
All values are expressed as mean ± SEM. n=number of participants. BA, Brachial Artery; ET, Exercise Trained Subjects; FMD, Flow Mediated Dilation; NTG, Nitroglycerin; SED, Sedentary Subjects; SS, Shear Stress; WL, Weight Lifting.
Significant difference (P<0.05) Pre WL vs. Post WL within same condition (Cuff or No Cuff), 2-Way Repeated Measures ANOVA.
Table 3 shows the effect size of weight lifting and the presence of the protective cuff on FMD in SED and ET subjects as estimated by the Cohen method. This analysis indicates that resistance exercise (without the protective cuff) had a larger effect on FMD in SED subjects (d=0.78) compared to ET subjects (d=0.27). Conversely, while the lifting session had a positive, and medium-to-large effect on FMD in both groups when the cuff was present, the larger effect was observed in ET subjects compared to SED subjects (d=1.49 and 0.55, respectively). This is in agreement with the larger increase in FMD which was observed in ET subjects when the protective cuff was present (Figure 2). When comparing post-lift FMD in SED and ET subjects with and without the protective cuff, a large-to-very large effect of the cuff was seen in both groups (d=1.43 and 0.96, respectively).
Table 3.
Effect of Weight Lifting and Protective Cuff on FMD (Cohen's d)
| Effect of Weight Lifting without Cuff (Pre- vs. Post-Lift FMD) |
Effect of Weight Lifting with Cuff (Pre- vs. Post-Lift FMD) |
Effect of Cuff (No Cuff Post-Lift FMD vs. Cuff Post-Lift FMD) |
|
|---|---|---|---|
| SED | 0.78 | 0.55 | 1.43 |
| ET | 0.27 | 1.49 | 0.96 |
Effect Size Threshold
d = 0.20: Small Effect
d = 0.50: Medium Effect
d = 0.80: Large Effect
d = 1.30: Very Large Effect
Discussion
Previously we have shown that systemic endothelial-dependent vasodilation is impaired in healthy, SED subjects, but not ET subjects, after they perform isometric exercise to maximal exertion.(8, 16, 23) The major finding of this study is that the impairment in SED subjects is abrogated when the brachial artery is protected from the marked rise in blood pressure that occurs with weight lifting, indicating that the barostress on the vessel is responsible for the reduced brachial FMD observed in SED subjects after maximal exertion.
This is the first study to our knowledge to control systolic blood pressure to a limb during high-intensity isometric exercise. Interestingly, when the rise in blood pressure within the brachial artery during exercise was blunted, an increase in brachial artery FMD was observed in both athletes and non-athletes, indicating that in the absence of increased intra-arterial pressure within the arm during exertion, vascular endothelial function is augmented in both groups. This observation is consistent with numerous studies (as reviewed by Dawson et al.) which demonstrate post-exercise FMD is augmented following medium- to light-intensity aerobic exercise (where the pressor response is low), but reduced in high-intensity aerobic and resistance exercise (where the pressor response is high). The increased FMD seen in both ET and SED groups could be explained by a systemically circulating dilatory factor whose effect dominates in the vasculature which was not exposed to the high intraluminal pressures during maximal exertion resistance exercise. Alternatively, it has been proposed that acute, high shear levels during acute bouts of hypertension directly reduce NO release from the endothelium, (3, 5) although this hypothesis has not been explored in terms of exercise-induced FMD changes.
Atkinson et al. demonstrated that alpha-one adrenoreceptor blockade with prazosin during cycle ergometer exercise prevented exercise-induced reductions in FMD, suggesting a competing mechanism between the sympathetic constriction and endothelium-dependent dilator capacity.(1) In that study it should be noted that mean arterial pressure during exercise was lower in prazosin-treated subjects; however this finding was not statistically significant, possibly due to the relatively small sample size of ten individuals. The results from that study, in addition to our data, would indicate a possible combined mechanism of increased sympathetic activity with elevated intraluminal pressure as the critical factors for reducing FMD following maximum effort resistance exercise. Our data would however suggest it is primarily the increased intra-arterial pressure which is responsible for reducing FMD as the brachial artery was exposed to the same levels of circulating factors in both the cuff and no-cuff sessions.
Our findings suggest a gradual approach should be undertaken (from the standpoint of systemic vascular endothelial function) when initiating resistance exercise training in an untrained individual. ET individuals, in contrast, are protected from the negative effects of exercise-induced hypertension on the vasculature (8, 16, 23). This begs the question of whether repeated exposures to high arterial pressure are necessary to condition the vasculature to maintain vasodilation following maximal exertion. Because the time necessary for this conditioning process to occur has yet to be determined, more gradual resistance training programs should be recommended for previously sedentary subjects to limit the potential for damage which occurs to the vascular endothelium during the initial training period.
Study limitations
We recognize several limitations in our study. First, the sample size of nine SED and six ET subjects is small. Previous studies by Jurva (16) and Phillips (23) have demonstrated that FMD is reduced in SED subjects following leg press exercise to maximal exertion, whereas FMD is maintained in ET subjects. The goal of this study was to determine if protecting the distal vasculature from barostress during the same lifting protocol using a protective cuff would result in maintained or augmented FMD in SED and ET subjects. As shown by the individual responses plotted in Figure 1, FMD increased in 8/9 SED, and 6/6 ET subjects; therefore it is unlikely that increasing enrollment numbers would change the interpretation of our findings. Our study was also not designed to examine gender-specific differences in FMD or blood pressure, so our relatively small sample size does preclude this analysis.
A second limitation was that brachial artery diameter and Doppler flow were not continuously measured post-cuff release per established guidelines, (26) thus time to peak dilation and the total shear rate (area under the curve; AUC) were not calculated. As shown in Table 2, peak hyperemic shear stress immediately following cuff release did not change either after weight lifting or when the protective cuff was present during the lifting protocol. When FMD was normalized to peak shear stress, normalized FMD was still greater in the cuff vs. no-cuff session in both ET and SED subjects. While continuous measurement of arterial diameter may give a more robust measure of FMD,(26) measurements of diameter at 1, 2 and 3 minutes post-cuff release have been shown to have high levels of agreement with traditional QRS-gated continuous measurements, thus it is unlikely that the peak FMD response was underestimated.(17)
We did not control the stage in the menstrual cycle when female subjects participated. Because subjects served as their own controls, and FMD responses were only compared with pre- and post-lift values taken on the same day, the effects of hormone changes during the different stages of menses are expected to be minimal.
A final limitation of the study is that NTG-mediated dilation was not tested following the first FMD procedure and prior to weight lifting to avoid lingering effects of the drug on the second FMD measurement, and potentially the pressor response during lifting. All subjects showed robust dilation to NTG (>20%) after weight lifting, so it is unlikely that weight lifting reduced endothelium-independent vasodilation compared to normal pre-lift values.
Conclusion
This is the first study to our knowledge which directly examines the effects of increased arterial pressure on endothelium-dependent dilation following maximal exertion resistance exercise. It is well established by our group (8, 16, 23) and by others (9, 10) that high intensity resistance exercise causes both increases in systolic blood pressure and a post-exercise decline in endothelial function in untrained subjects. As the results of this study indicate, when the blood pressure increase is prevented in a controlled limb, FMD in that limb increases in both SED and ET subjects. This response is similar to what is observed in subjects following low- to moderate-intensity aerobic exercise (4, 13, 22), during which systolic blood pressure does not significantly increase. Together, these findings suggest that a circulating, pro-dilatory factor may be present, and its effects are dampened by the increased barostress on the blood vessels which occurs during weight lifting.
Acknowledgments
The authors would like to thank the staff at the Sports Medicine Center at the Medical College of Wisconsin for allowing use of their facilities and leg press machine. We would also like to thank the nursing and bionutritional staff at the Translational Research Unit of the Medical College of Wisconsin for performing anthropomorphic measurements of all subjects and administrating nitroglycerin. This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 8UL1TR000055 and NIH Training Grant T35 HL072483. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose. The results of the present study do not constitute endorsement by the American College of Sports Medicine. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Atkinson CL, Lewis NC, Carter HH, Thijssen DH, Ainslie PN, Green DJ. Impact of sympathetic nervous system activity on post-exercise flow-mediated dilatation in humans. J. Physiol. 2015;593(23):5145–56. doi: 10.1113/JP270946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betik AC, Luckham VB, Hughson RL. Flow-mediated dilation in human brachial artery after different circulatory occlusion conditions. Am J Physiol Heart Circ Physiol. 2004;286(1):H442–8. doi: 10.1152/ajpheart.00314.2003. [DOI] [PubMed] [Google Scholar]
- 3.Bilfinger TV, Stefano GB. Human aortocoronary grafts and nitric oxide release: relationship to pulsatile pressure. Ann. Thorac. Surg. 2000;69(2):480–5. doi: 10.1016/s0003-4975(99)01083-8. [DOI] [PubMed] [Google Scholar]
- 4.Cosio-Lima LM, Thompson PD, Reynolds KL, et al. The acute effect of aerobic exercise on brachial artery endothelial function in renal transplant recipients. Prev. Cardiol. 2006;9(4):211–4. doi: 10.1111/j.1520-037x.2006.05408.x. [DOI] [PubMed] [Google Scholar]
- 5.Dawson EA, Green DJ, Cable NT, Thijssen DH. Effects of acute exercise on flow-mediated dilatation in healthy humans. J Appl Physiol (1985) 2013;115(11):1589–98. doi: 10.1152/japplphysiol.00450.2013. [DOI] [PubMed] [Google Scholar]
- 6.de Groot PC, Poelkens F, Kooijman M, Hopman MT. Preserved flow-mediated dilation in the inactive legs of spinal cord-injured individuals. Am J Physiol Heart Circ Physiol. 2004;287(1):H374–80. doi: 10.1152/ajpheart.00958.2003. [DOI] [PubMed] [Google Scholar]
- 7.Dimsdale JE, Moss J. Plasma catecholamines in stress and exercise. JAMA. 1980;243(4):340–2. [PubMed] [Google Scholar]
- 8.Durand MJ, Dharmashankar K, Bian JT, et al. Acute exertion elicits a H2O2-dependent vasodilator mechanism in the microvasculature of exercise-trained but not sedentary adults. Hypertension. 2015;65(1):140–5. doi: 10.1161/HYPERTENSIONAHA.114.04540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzales JU, Thompson BC, Thistlethwaite JR, Scheuermann BW. Association between exercise hemodynamics and changes in local vascular function following acute exercise. Appl. Physiol. Nutr. Metab. 2011;36(1):137–44. doi: 10.1139/H10-097. [DOI] [PubMed] [Google Scholar]
- 10.Gori T, Grotti S, Dragoni S, et al. Assessment of vascular function: flow-mediated constriction complements the information of flow-mediated dilatation. Heart. 2010;96(2):141–7. doi: 10.1136/hrt.2009.167213. [DOI] [PubMed] [Google Scholar]
- 11.Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DH, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J. Physiol. 2010;588(Pt 9):1571–7. doi: 10.1113/jphysiol.2010.186965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hambrecht R, Adams V, Erbs S, et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107(25):3152–8. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 13.Harris RA, Padilla J, Hanlon KP, Rink LD, Wallace JP. The flow-mediated dilation response to acute exercise in overweight active and inactive men. Obesity (Silver Spring) 2008;16(3):578–84. doi: 10.1038/oby.2007.87. [DOI] [PubMed] [Google Scholar]
- 14.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br. J. Nutr. 1978;40(3):497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- 15.Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med. Sci. Sports Exerc. 1980;12(3):175–81. [PubMed] [Google Scholar]
- 16.Jurva JW, Phillips SA, Syed AQ, et al. The effect of exertional hypertension evoked by weight lifting on vascular endothelial function. J. Am. Coll. Cardiol. 2006;48(3):588–9. doi: 10.1016/j.jacc.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Kizhakekuttu TJ, Gutterman DD, Phillips SA, et al. Measuring FMD in the brachial artery: how important is QRS gating? J Appl Physiol (1985) 2010;109(4):959–65. doi: 10.1152/japplphysiol.00532.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamping KG, Dole WP. Acute hypertension selectively potentiates constrictor responses of large coronary arteries to serotonin by altering endothelial function in vivo. Circ. Res. 1987;61(6):904–13. doi: 10.1161/01.res.61.6.904. [DOI] [PubMed] [Google Scholar]
- 19.MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol (1985) 1985;58(3):785–90. doi: 10.1152/jappl.1985.58.3.785. [DOI] [PubMed] [Google Scholar]
- 20.Maeda S, Miyauchi T, Sakane M, et al. Does endothelin-1 participate in the exercise-induced changes of blood flow distribution of muscles in humans? J Appl Physiol (1985) 1997;82(4):1107–11. doi: 10.1152/jappl.1997.82.4.1107. [DOI] [PubMed] [Google Scholar]
- 21.Miura S, Ideishi M, Sakai T, et al. Angiotensin II formation by an alternative pathway during exercise in humans. J. Hypertens. 1994;12(10):1177–81. [PubMed] [Google Scholar]
- 22.Padilla J, Harris RA, Fly AD, Rink LD, Wallace JP. The effect of acute exercise on endothelial function following a high-fat meal. Eur. J. Appl. Physiol. 2006;98(3):256–62. doi: 10.1007/s00421-006-0272-z. [DOI] [PubMed] [Google Scholar]
- 23.Phillips SA, Das E, Wang J, Pritchard K, Gutterman DD. Resistance and aerobic exercise protects against acute endothelial impairment induced by a single exposure to hypertension during exertion. J Appl Physiol (1985) 2011;110(4):1013–20. doi: 10.1152/japplphysiol.00438.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rush JW, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am. J. Physiol. Heart Circ. Physiol. 2003;284(4):H1378–87. doi: 10.1152/ajpheart.00190.2002. [DOI] [PubMed] [Google Scholar]
- 25.Suboc TB, Strath SJ, Dharmashankar K, et al. Relative importance of step count, intensity, and duration on physical activity's impact on vascular structure and function in previously sedentary older adults. J Am Heart Assoc. 2014;3(1):e000702. doi: 10.1161/JAHA.113.000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am. J. Physiol. Heart Circ. Physiol. 2011;300(1):H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55(2):312–8. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- 28.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Heterogeneous limb vascular responsiveness to shear stimuli during dynamic exercise in humans. J Appl Physiol. 2005;99(1):81–6. doi: 10.1152/japplphysiol.01285.2004. [DOI] [PubMed] [Google Scholar]
- 29.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. Am J Physiol Heart Circ Physiol. 2006;290(3):H1271–7. doi: 10.1152/ajpheart.00883.2005. [DOI] [PubMed] [Google Scholar]