Abstract
Purpose
To establish if triple-phase arterial imaging improves the detection of arterial phase hyperintense lesions based on arterial phase capture, motion artifact degradation, and lesion enhancement when compared to single-phase imaging.
Materials and methods
Patients at risk for hepatocellular carcinoma were imaged at 3.0T. Seventy-three consecutive patients with a standard single-phase MRI and eighty-five consecutive patients were imaged using extracellular contrast with triple arterial phase MRI using three sequential accelerated acquisitions of 8 s. Arterial phase capture and image quality were qualitatively categorized. Forty single-phase and forty-four triple-phase studies contained arterially enhancing lesions > 1 cm with washout appearance. The contrast-to-noise ratio (CNR) of the lesions was calculated. We compared the differences in means with Student t-tests and those in arterial phase capture with a Chi squared test with Yates correction.
Results
The triple-phase acquisitions captured the early or late arterial phases more frequently than did the single-phase acquisition (99% vs 86%; P value = 0.006). Triple-phase also provided greater number of patients with early or late arterial phase imaging without motion artifact (92% vs 79%, P-value = 0.05). The lesion analysis revealed increased maximum CNR in the triple-phase imaging (704.4) vs. single-phase imaging (517.2), P-value < 0.001.
Conclusion
Triple-phase acquisition provides more robust arterial phase imaging for hepatic lesions, with increased lesion CNR, compared to standard single-phase arterial phase imaging.
Keywords: Liver MRI, Arterial phase imaging, Respiratory motion, Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is the fifth most common cancer [1], and is the second leading cause of cancer-related deaths [2]. According to the Liver Imaging Reporting and Data System (LI-RADS), arterial phase hyperenhancement is an important major criterion in the characterization of HCC [3]. Without arterial phase hyperenhancement, a lesion cannot be characterized as LI-RADS 5, which will affect a patient’s candidacy for potential liver transplant by the Organ Procurement and Transplantation Network (OPTN) [4]. Therefore, it is pivotal to have an imaging technique, which will help recognize arterial phase hyperintense lesions, and enable us to provide optimal patient care to potential liver transplant candidates.
Magnetic resonance imaging (MRI) is demonstrated to be superior to computed tomography (CT) for detection of HCC [5]. Moreover, several studies have been conducted to capture the ideal arterial phase for lesion enhancement using multiphasic dynamic CT and MRI, and most demonstrated that the late arterial phase imaging exhibits higher sensitivity than the early arterial phase using multi-detector CT [6, 7]. Using fparallel imaging, multiphasic breath-hold MRI has also been evaluated and demonstrated that double arterial phase dynamic MRI demonstrated higher sensitivity than the single arterial phase [8]. Interpretation of these studies is difficult as the definition of arterial phase is based on a fixed time delay and not determined based on current definitions as provided by LI-RADS v2014.
There are two approaches to multiphasic arterial phase MRI. First is to have sequential shortened acquisitions (sequential) in which all k-space associated with each arterial phase is fully acquired separate from adjacent phases. The second approach is to share k-space across all the phases (e.g., view sharing), sampling the center of k-space for each phase and sharing the periphery of k-space across all phases. Using a sequential approach, a recent paper evaluated the utilization of single-breath-hold CAIPIVIBE imaging with three arterial phases and demonstrated reduced motion artifact and provided improved image quality [9]. In this approach, VIBE (volume interpolated breath-hold exam), a 3D spoiled gradient echo, is accelerated using CAIPIRINHA (controlled aliasing in parallel imaging results in higher acceleration), which is a parallel imaging technique that skips lines of k-space along the diagonal, allowing for further acceleration with preserved image quality compared to conventional parallel imaging techniques. View-sharing approaches have also been evaluated for arterially enhancing lesions. For example, a high spatio-temporal resolution multi-phase acquisition (DISCO, or Differential Subsampling with Cartesian Ordering) demonstrated improved arterial phase capture and lesion enhancement [10–12].
Due to frequent poor arterial phase capture and motion artifact that limits conventional single-phase arterial imaging, we decided to evaluate the utility of a sequential triple arterial phase acquisition for imaging of arterial phase hyperintense lesions in patients at risk for HCC. We compared these acquisitions to patients acquired with a single-phase acquisition, in terms of arterial phase capture, respiratory motion artifact, and lesion contrast-to-noise.
Materials and methods
The local institutional review board approved this retrospective study, and informed consent was waived. Seventy-three consecutive patients at risk for HCC were imaged with single arterial phase, and eighty-five with triple-phase technique between December 2013 and July 2014. All imagings were performed on a 3.0T MRI system (Skyra, Siemens Healthcare; Erlangen, Germany), using a 32-channel phased-array coil.
Arterial phase technique
A 1 mL test bolus was used as a timing bolus. In each patient, either 0.1 mmol/kg of gadobutrol (Gadavist, Bayer Healthcare; Wayne, NJ) or gadodiamide (Omniscan, GE Healthcare; Waukesha, WI) was injected at a rate of 2 mL per second. A 20-mL saline flush was used. The single arterial phase technique consisted of a 22 s 3D-spoiled gradient-echo sequence (VIBE, volume interpolated breath-hold examination), using the following parameters: 190 × 320 matrix, 450 kHz bandwidth, 9-degree flip angle, TR/TE 4.5/2.46, and 3.0 mm slice thickness. The single arterial phase acquisition was accelerated using GRAPPA.
The triple arterial phase imaging was performed using three sequential 8-s acquisitions in a single breath-hold [9], using a two-dimensional parallel acceleration technique (CAIPIRINHA, controlled aliasing in parallel imaging results in higher acceleration), with the following parameters: 288 × 169 matrix, 450 kHz bandwidth, 9.0-degree flip angle, TR/TE 3.8/1.7, and 3.0 mm slice thickness.
Arterial phase capture
The arterial phase imaged in each acquisition was categorized into the following groups: angiographic phase refers to hepatic artery enhancement without any contrast seen in the portal venous system; early arterial is defined as hepatic artery enhancement and early filling of the portal venous system; late arterial refers to both hepatic artery and portal venous enhancements without any contrast seen in the hepatic veins; and lastly, portal venous phase is defined as the phase where the hepatic veins are initially opacified. The percentage of patients in which either an early or late arterial phases were seen was calculated for each group.
Respiratory motion analysis
Image quality for respiratory motion artifact was graded on a 4-point scale by a single reader (NI). (0: no artifacts, 1: minimal motion artifact does not limit the diagnostic quality, 2: some motion artifact may affect visualization of smaller lesions, but not that of the larger ones, and 3: non-diagnostic). The grading was characterized into two categories: minimal respiratory motion artifact (grades 0 and 1) and significant artifact (grades 2 and 3).
Lesion enhancement
Arterially enhancing lesions greater than 1 cm with washout appearance on the portal venous or delayed phases were included for lesion analysis. A maximum of three lesions per patient were assessed in order to prevent a clustering bias. The analysis was conducted using OsiriX software [13]. Circular regions of interests (ROIs) were placed over the arterially enhancing component of each lesion being careful not to include the non-enhancing regions of the lesions (N.I). ROIs with roughly 2 cm diameter were also placed over the closest adjacent hepatic parenchyma being careful not to include large vessels, and in background air. The ROIs were propagated between phases to remain consistent in size. However, in many cases the ROIs had to be repositioned to account for patient’s motion among the phases. The ratio of the lesion intensity to adjacent parenchyma in the pre-contrast and in the single and triple-phase acquisitions was calculated using mean intensities within the ROIs. The contrast-to-noise ratio (CNR) was calculated for lesions noted in both single- and triple-phase imaging, where SI refers to signal intensity and SD refers to standard deviation [14].
The maximum CNR of a lesion from the triple-phase acquisition was used for analysis. Additionally, the phase in which the lesion demonstrated the maximum CNR was then correlated with the arterial capture of the image.
Statistical analysis
A Chi squared test with Yates correction was used to compare the percentages of single- vs. triple-phase studies that contained either an early or arterial phase and to compare the number of phases in each group that had significant respiratory motion artifact. A Student t-test was used to compare means of lesion intensity between single- and triple-phase acquisitions and to compare differences in various contrast agents used. Excel 12.1.0 (Microsoft, Redmond, WA) was used for statistical analysis. A P-value of <0.05 was used to assess significance for all results.
Results
Arterial phase capture
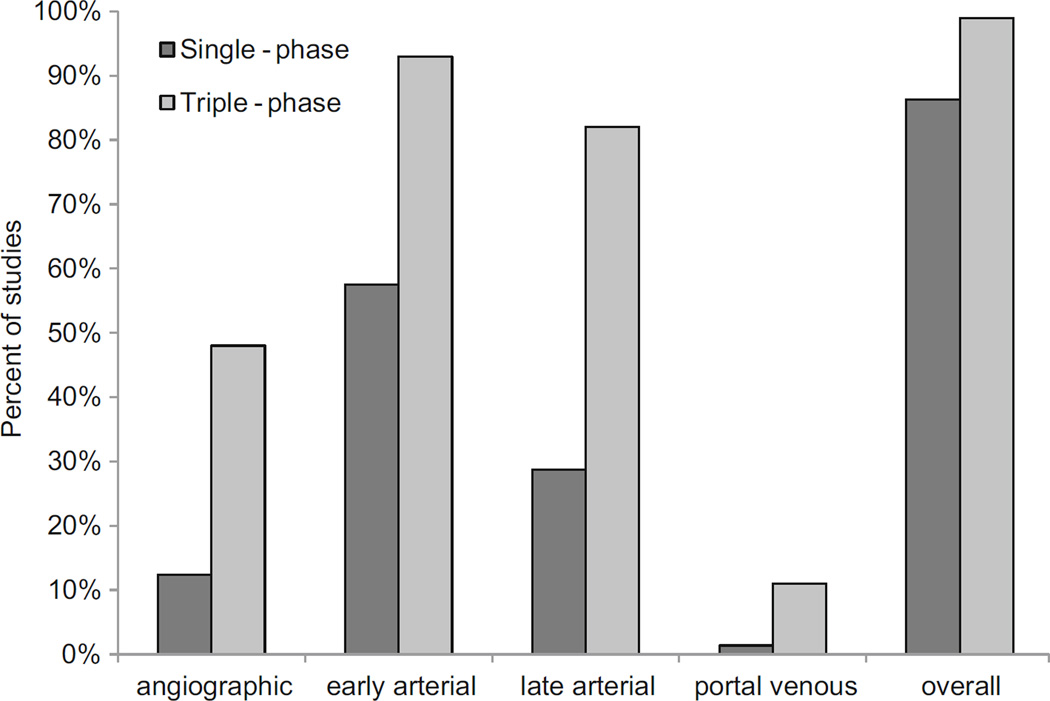
The triple-phase acquisitions resulted in a higher rate of early or late arterial phase capture (Table 1). 63/73 or 86% of patients imaged with single-phase acquisitions had an early or late arterial phase. 84/85 or 99% of patients imaged with multi-phase acquisitions had an early or late arterial phase (Figs. 1 and 2: P-value = 0.006 for comparing single-phase and multi-phase imaging for percent of patients with either early or lateral arterial phase acquisitions).
Table 1.
The triple-phase acquisitions captured the early or late arterial phases more frequently than did the single-phase acquisition (p = 0.006)
| Angiographic phase | Early arterial phase | Late arterial phase | Portal venous phase | Early or late arterial phase | |
|---|---|---|---|---|---|
| Single-phase | 9 (12%) | 42 (58%) | 21 (29%) | 1 (1%) | 63/73 = 86% |
| Triple-phase | 41 (48%) | 79 (93%) | 70 (82%) | 9 (11%) | 84/85 = 99% |
Fig. 1.
Percent of patients in single- and multi-phase acquisitions with each vascular phase. “Overall” refers to the percent of patients with either early or late arterial phase images in both groups; 86% of patients imaged with single-phase imaging and 99% of patients imaged with multi-phase imaging included both early and late arterial phases (P-value = 0.006).
Fig. 2.
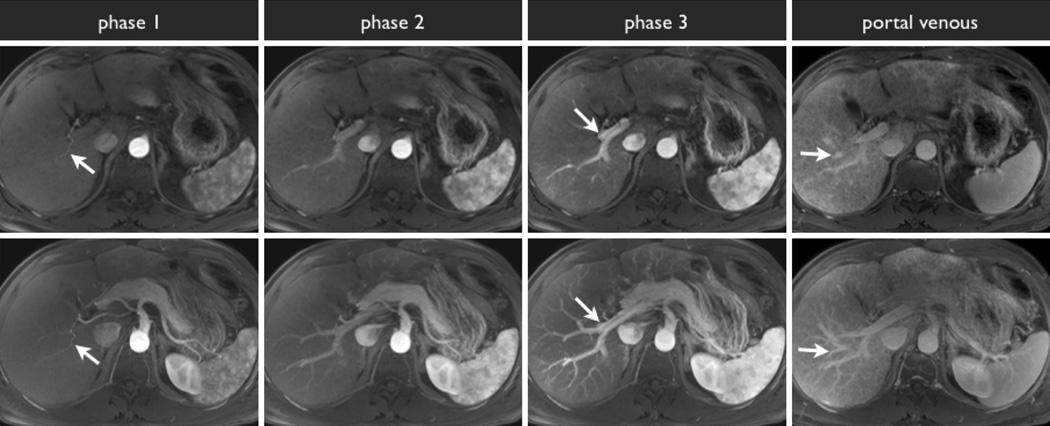
Example of triple-phase MRI acquisition with axial slices through the liver hilum (top row) and axial maximum intensity projection images (bottom row). During the angiographic phase, the arterial system is opacified (phase 1, white arrow), without any opacification of the portal venous system. During the early arterial phase, the portal vein system begins to opacify (phase 2). During the late arterial phase, the portal vein is fully opacified (phase 3, white arrow). Lastly, during the portal venous phase, the hepatic veins are opacified (portal venous, white arrow).
Respiratory motion analysis
The triple-phase acquisition provided a greater number of patients with early or late arterial phase without motion artifact (Table 2 and Fig. 3). Of the 73 patients with single-phase imaging, 66/73 or 900025; of patients were imaged with minimal motion artifact, in which 58/73 or 79% included either early or late arterial phase. Of the 85 patients with triple-phase imaging, 80/85 or 94% of patients with angiographic phase, 72/85 or 85% with early arterial, and 64/85 or 75% with late arterial were imaged with minimal motion artifact. 78/85 or 92% of patients with either early or late arterial phase acquisition have minimal motion artifact (P-value = 0.05).
Table 2.
Triple-phase provided greater number of patients with early or late arterial phase imaging with minimal motion artifact (p = 0.05)
| Patients with one arterial phase with minimal respiratory motion artifact (Grades 0–1) |
Patients with either an early or late arterial phase with minimal respiratory motion artifact (Grades 0–1) |
|
|---|---|---|
| Single-phase | 66/73 = 90% | 58/73 = 79% |
| Triple-phase | 80/85 = 94% | 78/85 = 92% |
Fig. 3.
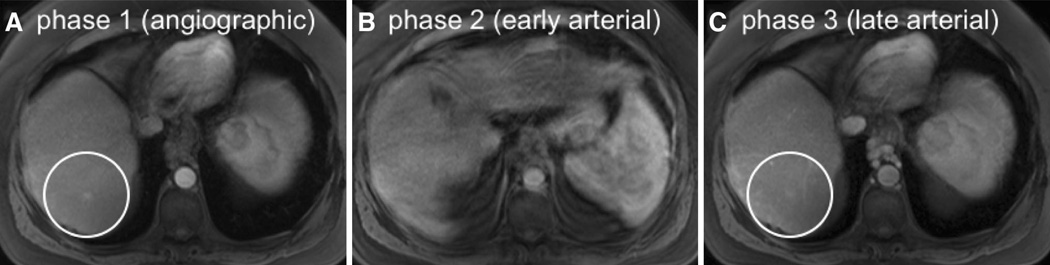
Images from representative triple-phase imaging demonstrating recognition of an arterially enhancing lesion despite motion artifact in one phase. An arterially enhancing lesion seen in both the angiographic (A, circle) and late arterial (C, circle) phases is not well seen in the early arterial phase (B) due to marked motion artifact. Note that the respiratory artifact seen on the second phase of imaging (B) does not propagate to the adjacent phases as view sharing is not performed.
Lesion enhancement
40 lesions using single-phase imaging and 43 lesions using triple-phase imaging were included for analysis. The average size of the lesions in the single-phase imaging is 2.3 cm with a standard deviation (SD) of 1.3 and that in the triple-phase imaging is 2.5 with an SD of 1.8. The maximum relative enhancement of the lesions when compared to the adjacent parenchyma was higher in lesions in multi-phase acquisitions than those in the single phase. The maximum CNR in the triple-phase imaging was 704.4 with an SD of 152.1 vs. that in the single-phase imaging was 517.2 with an SD of 82.8 (P-value < 0.001). There were 3 lesions that demonstrated maximum CNR in the angiographic phase, 21 in the early arterial, 19 in the late arterial, and 1 in portal venous phase in triple-phase imaging (Fig. 4). The CNR is not significantly different in early and late arterial phases (P-value = 0.95).
Fig. 4.
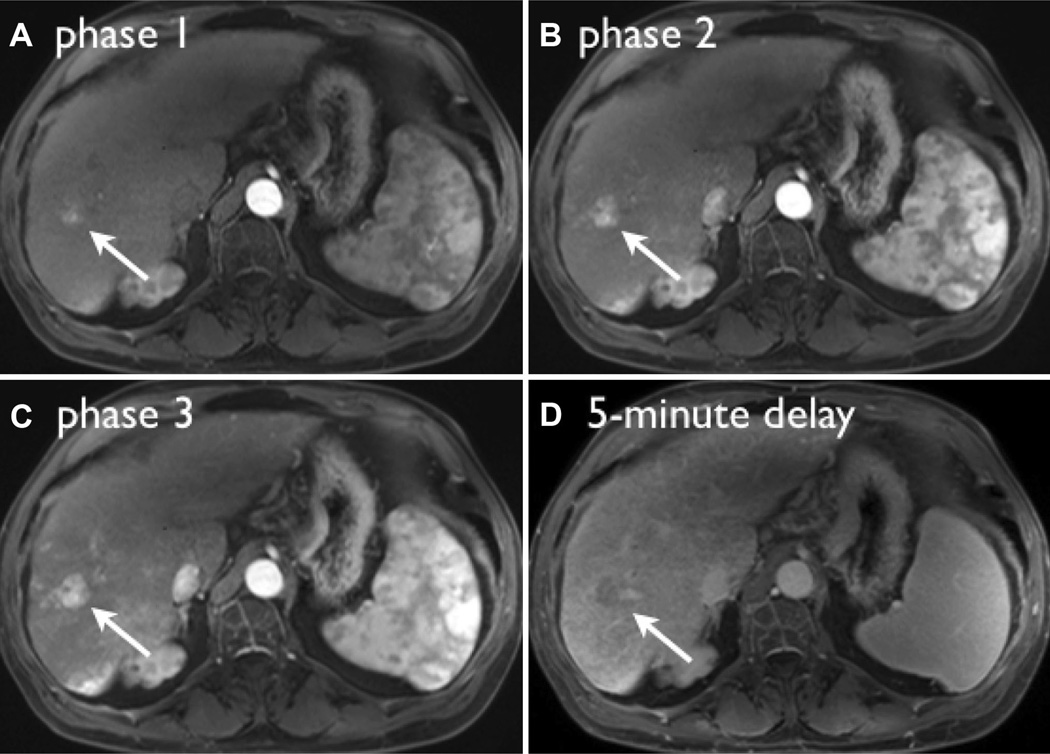
Images from representative triple-phase imaging of a LI-RADS 5 lesion, demonstrating maximum contrast-to-noise ratio (CNR) in the late arterial phase (C). The lesion demonstrates washout appearance on the 5-min delay (D). Note that in phase 1 (A), during the angiographic phase, the lesion is barely visible compared to the late arterial phase where conspicuity and lesion CNR are highest.
There was no statistically significant difference in the degree of lesion enhancement with the two contrast agents, Gadavist and Omniscan, used for arterial phase imaging. In single-phase imaging, 7 patients were imaged with Gadavist and 33 with Omniscan. However, in triple-phase imaging, 18 patients were imaged with Gadavist and 25 with Omniscan. In single-phase imaging, the maximum relative enhancement of lesions with compared to the adjacent parenchyma while utilizing Gadavist was 55.7 with an SD of 22.7 and that while using Omniscan was 517.2 with an SD of 90.2 (P-value = 0.49). For triple-phase imaging, the maximum relative enhancement of lesions with compared to the adjacent parenchyma while utilizing Gadavist was 551.0 with an SD of 149.3 and that while using Omniscan was 704.4 with an SD of 156.4 (P-value = 0.62).
Discussion
This study demonstrates that the use of triple-phase imaging captured the early or late arterial phases more frequently while preserving image quality based on respiratory motion artifact than the single-phase imaging. Additionally, we demonstrated that maximum CNR was higher with the triple-phase technique compared to single-phase imaging. These results demonstrate that triple-phase imaging provides improved imaging for arterially enhancing lesions of the liver.
It should be noted that much of the literature defines arterial phases based on fixed time delays after injection [15, 16]. Since different patients have different rates of enhancement, it is difficult to determine in which vascular phase each arterial phase occurred. Therefore it is difficult to apply their results to current methodologies. For this reason, we used visually graded definitions of arterial phase as described in the LI-RADS atlas [17]. In this study, we found that both early and late arterial phases demonstrate higher lesion conspicuity than angiographic or portal venous phases.
Arterial phase capture has been a focus when using gadoxetate disodium because of the smaller bolus size and the inability to use bolus-timing techniques. View-sharing techniques have been shown to result in increased capture of early and late arterial phases compared to single-phase techniques using gadoxetate [10] while preserving spatial resolution. In addition, capturing the correct arterial phase respiratory motion is a significant issue, particularly with gadoxetate due to transient dyspnea [18].
View-sharing approaches have a limited ability to prevent respiratory artifact from transient dyspnea because respiratory motion can be propagated across phases within a breath-hold due to the sharing of the periphery of k-space across all phases. The sequential lower resolution acquisition was initially suggested to counteract respiratory motion [9]. Since respiratory motion does not propagate between phases, this approach frequently provides at least one non-motion corrupted phase even if patients breathe during the acquisitions. Our results are consistent with these previous results, showing that there were more arterial phases that were not corrupted by respiratory motion using the sequential technique compared to the conventional single-phase acquisition.
Another approach to improving arterial phase timing is bolus tracking. For bolus tracking, a dynamic series is acquired over the abdominal aorta, and when contrast reaches the abdominal aorta, then patient is instructed to hold their breath and the arterial phase is subsequently acquired [19]. This approach has the benefit of not being effected by left ventricular function that can dramatically change the speed at which a bolus reaches the hepatic vasculature. In our experience we have not used bolus-tracking techniques and rather used a timing bolus due to issues with motion associated with the fast breath-hold instructions when using bolus tracking. Like bolus-tracking techniques, the use of a test bolus has also previously been demonstrated to minimize the effect of cardiac function on arterial timing [20].
One of the limitations of the study is that intra-patient analysis could not be conducted in this retrospective analysis. This would require contrast administration in each patient at two separate occasions [21]. One other limitation is that the prior literature predominantly comes from Eastern Asia where patients develop cirrhosis and HCC from hepatitis B infection. However, our patient population mostly acquired the disease process from hepatitis C and alcoholic cirrhosis. Although we show that early and late arterial phase imaging is equivalent in the conspicuity of HCC, it is impossible to determine if the difference from prior literature is due to technique or patient population. Finally the quantification of noise is difficult at best when using parallel imaging, limiting the accuracy of our CNR calculations [22].
In conclusion, triple-phase arterial imaging provides more robust arterial phase imaging for arterial hyper-vascular lesions while providing increased CNR compared to single-phase imaging.
Acknowledgments
Funding No grant funding was used in this study.
Footnotes
Compliance with Ethical Standards
Informed consent The need for individual informed consent was waived by our institutional review board (IRB) for this retrospective study.
Conflict of interest Nabia S. Ikram, Judy Yee, Stefanie Weinstein, Benjamin M. Yeh, Carlos U. Corvera, Alexander Monto and Thomas A. Hope declare that they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47(Suppl):S2–S6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruite I, Tang A, Sirlin CB. Imaging-based diagnostic systems for hepatocellular carcinoma. Amer J Roentgenol. 2013;201:41–55. doi: 10.2214/AJR.13.10570. [DOI] [PubMed] [Google Scholar]
- 4.Wald C, Russo MW, Heimbach JK, et al. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology. 2013;266:376–382. doi: 10.1148/radiol.12121698. [DOI] [PubMed] [Google Scholar]
- 5.Lee YJ, Lee JM, Lee JS, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology. 2015;275(1):97–109. doi: 10.1148/radiol.14140690. [DOI] [PubMed] [Google Scholar]
- 6.Murakami T, Kim T, Takamura M, et al. Hypervascular hepatocellular carcinoma: detection with double arterial phase multi-detector row helical CT. Radiology. 2001;218:763–767. doi: 10.1148/radiology.218.3.r01mr39763. [DOI] [PubMed] [Google Scholar]
- 7.Ichikawa T, Kitamura T, Nakajima H, et al. Hypervascular hepatocellular carcinoma: can double arterial phase imaging with multidetector CT improve tumor depiction in the cirrhotic liver? AJR Am J Roentgenol. 2002;179:751–758. doi: 10.2214/ajr.179.3.1790751. [DOI] [PubMed] [Google Scholar]
- 8.Yoshioka H, Takahashi N, Yamaguchi M, et al. Double arterial phase dynamic MRI with sensitivity encoding (SENSE) for hypervascular hepatocellular carcinomas. J Magn Reson Imaging. 2002;16:259–266. doi: 10.1002/jmri.10146. [DOI] [PubMed] [Google Scholar]
- 9.Pietryga JA, Burke LMB, Marin D, Jaffe TA, Bashir MR. Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology. 2014;271:426–434. doi: 10.1148/radiol.13131988. [DOI] [PubMed] [Google Scholar]
- 10.Hope TA, Saranathan M, Petkovska I, et al. Improvement of gadoxetate arterial phase capture with a high spatio-temporal resolution multiphase three-dimensional SPGR-Dixon sequence. J Magn Reson Imaging. 2013;38:938–945. doi: 10.1002/jmri.24048. [DOI] [PubMed] [Google Scholar]
- 11.Saranathan M, Rettmann DW, Hargreaves BA, Clarke SE, Vasanawala SS. DIfferential Subsampling with Cartesian Ordering (DISCO): a high spatio-temporal resolution Dixon imaging sequence for multiphasic contrast enhanced abdominal imaging. J Magn Reson Imaging. 2012;35:1484–1492. doi: 10.1002/jmri.23602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hope TA, Petkovska I, Saranathan M, Hargreaves BA, Vasanawala SS. Combined parenchymal and vascular imaging: High spatiotemporal resolution arterial evaluation of hepatocellular carcinoma. J Magn Reson Imaging. 2015 doi: 10.1002/jmri.25042. [DOI] [PubMed] [Google Scholar]
- 13.Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17:205–216. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S, Mussi TC, Lee LJ, et al. Effect of flip angle for optimization of image quality of gadoxetate disodium-enhanced biliary imaging at 1.5 T. Am J Roentgenol. 2013;200:90–96. doi: 10.2214/ajr.12.8722. [DOI] [PubMed] [Google Scholar]
- 15.Vogl T, Stupavsky A, Pegios W, et al. Hepatocellular carcinoma: evaluation with dynamic and static gadobenate dimeglumine-enhanced MR imaging and histopathologic correlation. Radiology. 1997;205:721–728. doi: 10.1148/radiology.205.3.9393527. [DOI] [PubMed] [Google Scholar]
- 16.Mori K, Yoshioka H, Takahashi N, et al. Triple arterial phase dynamic MRI with sensitivity encoding for hypervascular hepatocellular carcinoma: comparison of the diagnostic accuracy among the early, middle, late, and whole triple arterial phase imaging. AJR Am J Roentgenol. 2005;184:63–69. doi: 10.2214/ajr.184.1.01840063. [DOI] [PubMed] [Google Scholar]
- 17.Liver Imaging Reporting and Data System (LI-RADS) American college of radiology. [Accessed 18 Feb 2014]; http://www.acr.org/Quality-Safety/Resources/LIRADS. [Google Scholar]
- 18.Davenport MS, Viglianti BL, Al-Hawary MM, et al. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology. 2013;266:452–461. doi: 10.1148/radiol.12120826. [DOI] [PubMed] [Google Scholar]
- 19.Sharma P, Kalb B, Kitajima HD, et al. Optimization of single injection liver arterial phase gadolinium enhanced MRI using bolus track real-time imaging. J Magn Reson Imaging. 2011;33:110–118. doi: 10.1002/jmri.22200. [DOI] [PubMed] [Google Scholar]
- 20.Earls JP, Rofsky NM, DeCorato DR, Krinsky GA, Weinreb JC. Hepatic arterial-phase dynamic gadolinium-enhanced MR imaging: optimization with a test examination and a power injector. Radiology. 1997;202:268–273. doi: 10.1148/radiology.202.1.8988222. [DOI] [PubMed] [Google Scholar]
- 21.Frydrychowicz A, Nagle SK, D’Souza SL, Vigen KK, Reeder SB. Optimized high-resolution contrast-enhanced hepatobiliary imaging at 3 tesla: a cross-over comparison of gadobenate dimeglumine and gadoxetic acid. J Magn Reson Imaging. 2011;34:585–594. doi: 10.1002/jmri.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeder SB, Wintersperger BJ, Dietrich O, et al. Practical approaches to the evaluation of signal-to-noise ratio performance with parallel imaging: application with cardiac imaging and a 32-channel cardiac coil. Magn Reson Med. 2005;54:748–754. doi: 10.1002/mrm.20636. [DOI] [PubMed] [Google Scholar]