To the Editor
Recent studies examining trends in bisphosphonate (BP) therapy for fracture prevention show declining numbers of prescriptions starting in 2007–2008 through 2012,1, 2 possibly ascribed in part to media reports regarding rare adverse effects.1, 3 This overall decline may reflect both trends in treatment discontinuation and lower rates of BP initiation.3 Treatment paradigms have also shifted towards primary and secondary fracture prevention in higher risk patients, with increasing focus on integrated fracture risk.4 This study examines oral BP initiation patterns during 2004–2012 within an integrated healthcare delivery system.
Methods
This retrospective study identified Kaiser Permanente Northern California (KPNC) female members age ≥45 years old who initiated BP therapy (alendronate, risedronate, oral ibandronate) during 2004–2012. Women were classified by age (≥65 and <65 years), by race/ethnicity (non-Hispanic white, Asian and all others) and by hospital or ambulatory diagnosis of fracture (excluding fingers, toes, face, high energy trauma, pathologic and open fracture codes) within 5 years prior to BP initiation. The percentage of women by age group was examined each year, overall and by prior fracture status. The cohort was also stratified by early (2004–2007) and later (2009–2012) era, bridging 2008, the pivotal year when revised osteoporosis treatment recommendations were introduced.4, 5 Differences between subgroups were compared using the Chi-square test, with trends by year examined using the Cochrane-Armitage test.
Results
Among the 72,026 women who initiated oral BP during 2004–2012 (mean age 70.8±10.7 years; 65% non-Hispanic white, 17% Asian, 18% all others), 50,751 (70%) were 65 and older and 22,299 (31%) received a fracture diagnosis within the prior 5 years, including 17,294 with a fracture diagnosis in the past year. Those without prior fracture were more likely to be under age 65 (34% vs 19%, p<0.001) and of Asian race (21% vs 9%, p<0.001), compared to women with prior fracture.
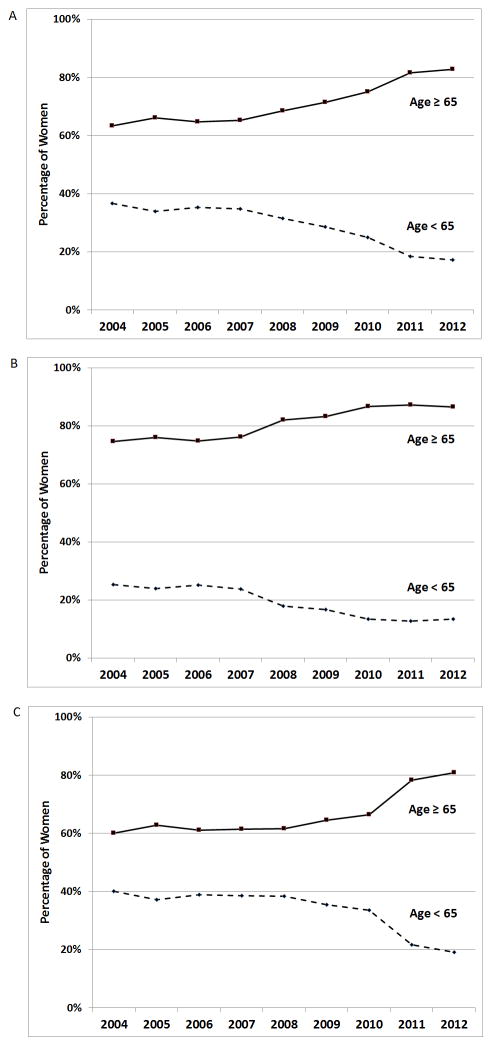
Yearly trends among women initiating oral BP from 2004 to 2012 (Figure 1) showed two distinct time trends. Between 2004 and 2007, the proportions of women under age 65 (34%–37%) and 65 and older (63%–66%) were relatively stable. However, between 2008 and 2012 (Figure 1a), the proportions of younger women progressively declined from 31% to 17% while the proportions of older women increased from 69% to 83% (p<0.001). These age-related trends were similarly observed among those with (Figure 1b) and without (Figure 1c) prior fracture. Among women who initiated BP and also did not have a prior fracture, the percentage under age 65 declined from 38% in 2008 to 19% in 2012 (p<0.001).
Figure 1. The percentage of women initiating bisphosphonate (BP) therapy by age, and by calendar year.
(a) All Women initiating BP therapy
(b) Women initiating BP with prior fracture
(c) Women initiating BP without prior fracture
There were 35,103 women who initiated BP in 2004–2007 (age 69.5±10.8 years) and 29,733 women who initiated BP in 2009–2012 (age 72.4±10.2 years). Comparing these earlier and later time periods, the percentage with prior fracture increased from 25% to 37% (p<0.001), while the number of women who were both under age 65 and without prior fracture fell by half (10,210 to 5,083).
Discussion
During 2004–2012, oral BP initiation within our healthcare system shifted towards older women and those with prior fracture. These trends, apparent beginning in 2008, are consistent with increasing focus on primary and secondary fracture prevention of patients at elevated fracture risk.6 In 2008, the World Health Organization introduced FRAX™, which integrates age, race/ethnicity, clinical risk factors and bone mineral density (BMD) to estimate fracture risk.4, 7 Updated guidelines from the National Osteoporosis Foundation that same year emphasized treatment for postmenopausal women with hip or spine fracture, osteoporosis by BMD criteria, and osteopenia with high estimated fracture risk;4, 8 this was in contrast to prior guidelines that included treatment of all postmenopausal women with T-score −2.0 or below (or −1.5 with risk factors), regardless of age. National quality metrics also emphasized osteoporosis testing and/or treatment of women age 67 and older post-fracture9, 10 and osteoporosis testing for all women age 65 and older.9, 10 In response to these national measures, regional outreach programs were developed within our health plan beginning in 2008, focusing primarily on secondary fracture prevention as well as on BMD screening in older women.
In summary, within Kaiser Permanente Northern California, we observed a substantial shift in BP treatment initiation that reflected changing practice in response to national guidelines and quality metrics. This included emphasizing treatment for older women and those experiencing fracture, with a 50% reduction in treatment of younger women without fracture. Implementation of our focused outreach programs likely prevented the reduction in bisphosphonate prescriptions observed nationally.2 As the U.S. population continues to age, osteoporosis treatment efforts should continue to focus on higher risk older populations for cost-effective care.
Footnotes
Author Contributions:
David Lee, Bruce Ettinger, and Joan Lo contributed to the study concept and design, analysis and interpretation of data, and preparation of the initial draft of the manuscript. Malini Chandra and Rita Hui contributed to the study concept and design, acquisition of data, and analysis and interpretation of data. All authors contributed to revising and editing the manuscript for important intellectual content and approved the final submitted version.
Conflict of Interest Statement
Bruce Ettinger has previously received payments for serving as an expert witness in litigation involving Fosamax. Malini Chandra and Rita Hui have received prior research funding from Amgen Inc. not related to the current study. Joan Lo has received prior research funding from Amgen Inc. and current funding from Sanofi not related to the current study.
Sponsor’s Role:
This project was supported by a grant from the National Institute of Aging at the National Institutes of Health, 1R01AG047230. The sponsor had no role in the design, methods, subject recruitment, data collection, analysis and preparation of the manuscript. The opinions expressed in this publication are solely the responsibility of the authors and do not represent the official views of Kaiser Permanente or the National Institutes of Health.
References
- 1.Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in Media Reports, Oral Bisphosphonate Prescriptions and Hip Fractures 1996–2012: An Ecological Analysis. J Bone Miner Res. 2015;30:2179–2187. doi: 10.1002/jbmr.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wysowski DK, Greene P. Trends in osteoporosis treatment with oral and intravenous bisphosphonates in the United States, 2002–2012. Bone. 2013;57:423–428. doi: 10.1016/j.bone.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Khosla S, Shane E. A Crisis in the Treatment of Osteoporosis. J Bone Miner Res. 2016;31:1485–1487. doi: 10.1002/jbmr.2888. [DOI] [PubMed] [Google Scholar]
- 4.Watts NB, Lewiecki EM, Miller PD, Baim S. National Osteoporosis Foundation 2008 Clinician’s Guide to Prevention and Treatment of Osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): what they mean to the bone densitometrist and bone technologist. J Clin Densitom. 2008;11:473–477. doi: 10.1016/j.jocd.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Qaseem A, Snow V, Shekelle P, Hopkins R, Jr, Forciea MA, Owens DK. Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:404–415. [PubMed] [Google Scholar]
- 6.Improving and measuring osteoporosis management. Oakbrook Terrace, IL: The Joint Commission; 2008. [Google Scholar]
- 7.FRAX WHO Fracture Risk Assessment Tool. World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield; UK: [accessed September 10, 2016]. http://www.shef.ac.uk/FRAX/ [Google Scholar]
- 8.Clinician’s Guide to Prevention and Treatment of Osteoporosis. National Osteoporosis Foundation; Washington D.C: 2008. [Google Scholar]
- 9.Curtis JR, Adachi JD, Saag KG. Bridging the osteoporosis quality chasm. J Bone Miner Res. 2009;24:3–7. doi: 10.1359/jbmr.081116. [DOI] [PubMed] [Google Scholar]
- 10.Appendix A - NCQA Measure Technical Specifications (V.7, April 2008) National Committee for Quality Assurance; 2008. [accessed September 10, 2016]. NQF-endorsed national voluntary concensus standards for physician-focused ambulatory care. http://www.ncqa.org/portals/0/hedisqm/NQF_Posting_Appendix.pdf. [Google Scholar]