Abstract
Recent studies suggest that preferences are conditioned by nutritive (sucrose) but not by non-nutritive (sucralose) sweeteners in mice. Here we compared the effectiveness of nutritive and non-nutritive sweeteners to condition flavor preferences in three mouse strains. Isopreferred sucrose and sucralose solutions both conditioned flavor preferences in C57BL/6J (B6) mice but sucrose was more effective, consistent with its post-oral appetition action. Subsequent experiments compared flavor conditioning by fructose, which has no post-oral appetition effect in B6 mice, and a sucralose + saccharin mixture (SS) which is highly preferred to fructose in 24-h choice tests. Both sweeteners conditioned flavor preferences but fructose induced stronger preferences than SS. Training B6 mice to drink a flavored SS solution paired with intragastric fructose infusions did not enhance the SS-conditioned preference. Thus, the post-oral nutritive actions of fructose do not explain the sugar’s stronger preference conditioning effect. Training B6 mice to drink a flavored fructose solution containing SS did not reduce the sugar-conditioned preference, indicating that SS does not have an off-taste that attenuates conditioning. Although B6 mice strongly preferred flavored SS to flavored fructose in a direct choice test, they preferred the fructose-paired flavor to the SS-paired flavor when these were presented in water. Fructose conditioned a stronger flavor preference than an isopreferred saccharin solution, indicating that sucralose is not responsible for the limited SS conditioning actions. SS is highly preferred by FVB/NJ and CAST/EiJ inbred mice, yet conditioned only weak flavor preferences. It is unclear why highly or equally preferred non-nutritive sweeteners condition weaker preferences than fructose, when all stimulate the same T1r2/T1r3 sweet receptor. Recent findings support the existence of non-T1r2/T1r3 glucose taste sensors; however, there is no evidence for receptors that respond to fructose but not to non-nutritive sweeteners.
Keywords: Sucrose, Fructose, Sucralose, Saccharin, Flavor-preference conditioning, C57BL/6J mice FVB/NJ mice, CAST/EiJ mice
1. Introduction
For many animal species, sugar is a potent reward that motivates ingestion and conditions preferences for associated flavors, e.g, the flavor of cherry [34]. Stimulation of oral sweet receptors is sufficient to stimulate sugar intake and condition flavor preferences in rats. This is demonstrated by three lines of evidence. First, sham-feeding rats with an open esophageal or gastric fistula, which minimizes post-oral nutritive effects, consume substantial amounts of sugar and work avidly for sugar rewards on a progressive ratio (PR) schedule that measures food motivation [22,28,54]. Second, sham-feeding rats learn to prefer a flavor added to a sucrose solution [6,57]. Third, rats learn to prefer neutral flavors added to non-nutritive saccharin solutions, which have a sucrose-like taste [8,12,14,18,19,24,26,53]. In addition to sweet taste, some sugars have post-oral actions that stimulate ingestion and reinforce flavor preferences; this process is referred to as appetition to distinguish it from the post-oral satiation effects of nutrients, which suppress intake [35]. Post-oral sugar appetition is demonstrated by the ability of intragastric (IG) glucose or sucrose infusions to rapidly stimulate the intake of a non-nutritive flavored solution (the conditioned stimulus or CS+) and induce a long-lasting preference for that flavor in rats and mice [38]. IG glucose infusions also increase PR responding for CS+ flavors [36]. Post-oral sugar appetition is indirectly implied by preference shifts from non-nutritive to nutritive sweeteners after separate experience with both sweeteners [49]. Sugars differ substantially in their post-oral appetition effects, however. For instance, IG fructose infusions are much less effective than IG glucose or sucrose infusions in promoting intake of (and preference for) flavored solutions in rodents [37,41,43,60].
In contrast to the many reports of saccharin-conditioned flavor preferences in rats, two studies reported that non-nutritive sweeteners failed to condition preferences in C57BL/6J (B6) mice [3,10]. In these mouse studies, however, the conditioned preference was for the position (i.e., left vs. right side of cage) or color (i.e., black vs. white) of sipper tubes rather than for the flavor of solutions (e.g., cherry vs. grape) in sipper tubes. It may be that preferences are more readily conditioned to flavor than non-flavor cues, as has been reported for conditioned taste aversions [23]. In addition, the mouse studies used sucralose rather than saccharin as the non-nutritive sweetener. It is possible that non-nutritive sweeteners differ in their preference conditioning actions. Alternatively, rats and mice may differ in their ability to acquire sweet taste-based preferences. Also given inbred strain differences in sweetener preferences and flavor conditioning [1,30], some mouse strains may be more susceptible than others to flavor conditioning by sweet taste.
The present study examined the ability of sugars (sucrose, fructose) and non-nutritive sweeteners (sucralose, saccharin) to condition flavor preferences in inbred strains of mice. B6 mice were studied in all experiments except one that involved FVB/NJ and CAST/EiJ mice. These two strains were of interest because they differ from B6 mice in their oral attraction to non-nutritive sweeteners and their post-oral response to fructose [47,48]. As in most prior studies of preference conditioning by non-nutritive sweeteners [8,10,12,14,18,24,26,53], male animals were primarily used in the current study although the conditioning responses of male and female mice were compared in one experiment. The question of whether non-nutritive sweeteners can condition flavor preferences is important because recent studies suggest that sweet taste alone does not reinforce food preferences [3,7,10,11,33,55].
2. Experiment 1. Sucrose and sucralose conditioned flavor preferences
In a study of the neural basis of food reward, de Araujo et al. [10] trained thirsty B6 mice to drink a 0.8 M (27%) sucrose solution from a sipper tube positioned on one side of a cage and plain water from a sipper tube on the other side of the cage. In a subsequent choice test with the two tubes containing water, the mice licked significantly more from the sucrose-paired tube than from the water-paired tube. In contrast, other mice trained with a non-nutritive 0.3 mM (1.2%) sucralose solution failed to acquire a preference for the sucralose-paired tube even though the sucralose and sucrose solutions were isopreferred in brief two-bottle tests. In a subsequent study, Beeler et al. [3] also reported that 0.8 M sucrose but not 0.3 mM sucralose conditioned a sipper tube preference in B6 mice; in their experiment the sipper tubes were distinguished by color (white, black) rather than position. Based on these findings, the authors concluded that only sweeteners that have post-oral nutritive actions reinforce sipper tube preferences in mice. Yet, there are numerous reports that non-nutritive saccharin solutions can condition flavor preferences in rats at various concentrations (0.028 – 0.8%) and with various training procedures [8,12,14,18,19,24,26,53]. Based on these findings, we predicted that both sucrose and sucralose solutions would condition flavor preferences in B6 mice. Sucrose, however, was expected to induce a stronger preference because it has both taste and post-oral conditioning effects. We used concentrations of sucrose (8%, 272 mM) and sucralose (0.8%, 20 mM) that are isopreferred by B6 mice in 1-min and 24-h choice tests but have differential IG conditioning actions [40,44,49].
2.1. Method
2.1.1. Subjects
Experimentally-naive male B6 mice (n=20, 10 weeks old) derived from stock obtained from the Jackson Laboratories (Bar Harbor, ME) were singly housed in plastic tub cages in a room maintained at 22° C with a 12:12 h light-dark cycle (lights on 0900 h). The mice had ad libitum access to chow (5001, PMI Nutrition International, Brentwood, MO) and deionized water throughout this and all subsequent experiments except where noted. The protocols in this and subsequent experiments were approved by the Institutional Animal Care and Use Committee at Brooklyn College and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
2.1.2. Test Solutions
The sweetener solutions were prepared using food-grade sucrose (8% w/w; Domino Foods, Yonkers, NY) or sucralose (0.8% w/w, Tate & Lyle, Dayton, OH) dissolved in deionized water. The CS+ flavor added to the sweetener solution (CS+/Sweetener) was cherry or grape (0.05% w/w Kool-Aid mix, Kraft Foods, Northfield, IL). The CS− solution was the alternate flavor in plain water (CS−/H2O). For half the mice in each group, the CS+ flavor was cherry and the CS− flavor was grape; the CS flavors were reversed for the remaining animals. For the two-bottle tests, the CS+ flavor was presented in plain water (CS+/H2O) as was the CS− flavor. Fluid was available through sipper spouts attached to 50-ml plastic tubes that were placed on top of the home cage. The sipper spouts were inserted through holes positioned 3.7 cm apart in a stainless-steel plate positioned to the right of the food bin, and the drinking tubes were fixed in place with clips. Fluid intakes were measured to the nearest 0.1 g by weighing the drinking bottles on an electronic balance interfaced to a laptop computer. Daily fluid spillage was estimated by recording the change in weight of two bottles that were placed on an empty cage, and intake measures were corrected by this amount.
2.1.3. Procedure
The mice were adapted to their cages with two bottles of water for one week. They were then divided into two groups equated for water intake and body weight. The Sucrose mice (n=10) were subjected to six daily one-bottle training trials. They received the CS− on days 1, 3, and 5 and the CS+ sucrose on days 2, 4, and 6. On day 7, they received water only. On days 8–9, the mice were given a choice test (Test 1) with the CS+ vs. CS− flavors presented in water. On days 10–11, the choice test was repeated (Test 2) to determine the persistence of the CS+ preference. The Sucralose mice (n=10) were given the same sequence of tests, but the CS+ contained sucralose rather than sucrose. In this and all subsequent experiments the daily training and test sessions were nominally 24 h in duration; the drinking bottles were available 23–23.5 h and were then cleaned and refilled. The left-right positions of the CS+ and CS− bottles were alternated during training in an LRRLRL pattern and during testing in an LRLR pattern to minimize the development of side preferences.
Fluid intakes were averaged over 3-day blocks during training and 2-day blocks during testing and evaluated with separate analyses of variance. Percent CS+ intakes (e.g., CS+/Total intake × 100) were calculated for the two-bottle tests and analyzed with analysis of variance.
2.2. Results and Discussion
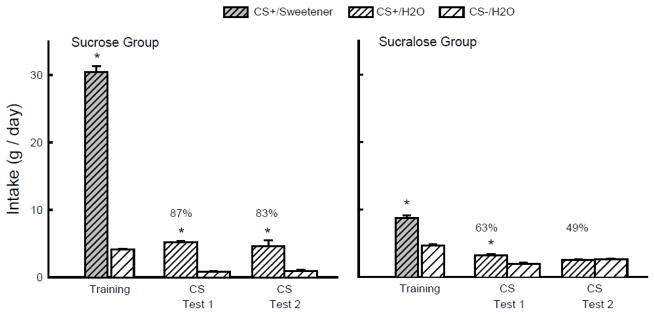
Figure 1 presents the one-bottle training and two-bottle test data. During one-bottle training, the Sucrose and Sucralose groups consumed more CS+/Sweetener than CS−/H2O (F(1,18) = 1200.8, P < 0.001) and the Sucrose mice drank substantially more (P < 0.01) CS+/Sweetener than the Sucralose mice; CS−/H2O intakes did not differ (Group x CS interaction, F(1,18) = 670.8, P < 0.001). Overall, both groups also consumed more (P < 0.01) CS+/H2O than CS−/H2O in the two-bottle tests (F(1,18) = 312.5), but Sucrose mice consumed more CS+/H2O than did the Sucralose (Group x CS interaction, F(1,18) = 170.8, P < 0.001). Furthermore, whereas the Sucrose mice consumed more CS+/H2O than CS−/H2O in both Tests 1 and 2, the Sucralose mice consumed more CS+/H2O only in Test 1. The percent CS+/H2O preferences of the Sucrose group exceeded those of the Sucralose group (F(1,18) = 125.9, P < 0.001) and declined only in the Sucralose group from Test 1 to 2 (Group x Test interaction, F(1,18) = 7.3, P < 0.05) (Fig. 1).
Figure 1.
Experiment 1. Mean intakes (+sem) of CS+ and CS− during one-bottle training and two-bottle Tests 1 and 2 in the Sucrose (left panel) and Sucralose (right panel) B6 groups. (Order of training and testing is from left to right in the panels.) During training the CS+ flavor was added to the sweetener solution (CS+/Sweetener), which was 8% sucrose for the Sucrose group and 0.8% sucralose for the Sucralose group. During testing the CS+ flavor was in water (CS+/H2O); the CS− flavor was always in water (CS−/H2O). Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) intake differences in the training and two-bottle tests are indicated by an asterisk (*).
As predicted, both sucrose and sucralose conditioned flavor preferences in the B6 mice. The sucrose-conditioned preference, however, was stronger and more persistent than the sucralose-based preference, which was expected given the differential post-oral flavor conditioning actions of the two sweeteners [44]. The fact that the Sucrose mice consumed substantially more of the flavored sweetener during one-bottle training than did the Sucralose mice is also consistent with the known post-oral effects of two sweeteners. We previously reported that B6 mice trained to drink a CS+ flavor paired with IG infusion of 16% sucrose (diluted to 8% sucrose by the ingested CS+ solution) consumed more of the CS+ than of a CS− flavor paired with IG water in one-bottle 24-h sessions and strongly preferred the CS+ to the CS− in a subsequent choice test. In marked contrast, B6 mice consumed less of a CS+ paired with IG infusion of 1.6% sucralose (diluted to 0.8% in the stomach) than of the water-paired CS− during training, and preferred the CS− to the CS+ in the subsequent choice test [44]. The post-oral mechanism by which concentrated sucralose inhibits intake is not known but it does not prevent B6 mice from preferring 0.8% sucralose to water [44,49]. However, the post-oral inhibitory actions of 0.8% sucralose may limit its ability to condition a CS+ flavor preference.
3. Experiment 2. Fructose and sucralose + saccharin conditioned flavor preferences
In addition to sucrose, B6 mice learn to prefer CS+ flavors added to glucose and fructose solutions [39], which are the constituent sugars of sucrose. The fructose-conditioned preference, unlike those produced by sucrose or glucose, was attributed primarily to the sweet taste of the sugar because fructose does not have post-oral appetition effects in B6 mice. This is indicated by the failure of IG fructose infusions to stimulate the intake of (or preference for) a flavored CS+ solution [37,60]. In Experiment 2 we compared the flavor conditioning effects of fructose with that of a non-nutritive sweetener mixture containing 0.1% sucralose and 0.1% saccharin (referred to as SS). The 0.1% SS mixture was used instead of 0.8% sucralose because it does not have the post-oral inhibitory actions of the concentrated sucralose solution [49] which, as noted above, might impair flavor conditioning. Furthermore, the 0.1% SS mixture is significantly preferred to 8% fructose in brief-access as well as 24-h choice tests suggesting that it has a sweeter taste [40,49]. Based on these findings, we predicted that the SS mixture would condition a stronger CS+ preference than the 8% fructose solution.
3.1. Method
3.1.1. Test solutions
The sweetener solutions were prepared using food-grade 8% fructose (Tate & Lyle, Honeyville Food Products, Rancho Cucamonga, CA) or 0.1% sucralose + 0.1% sodium saccharin (Sigma, St. Louis, MO) (SS) dissolved in deionized water. The CS+ flavor added to the sweetener (CS+/Sweetener) was 0.05% cherry or grape. The CS− solution was the opposite flavor in plain water. CS+ and CS− flavors were counterbalanced as in Experiment 1. For the two-bottle tests, the CS+ flavor was presented in plain water (CS+/H2O) as was the CS− flavor (CS−/H2O).
3.1.2. Procedure
Naive male B6 mice (n=18, 12 weeks old) were housed and trained as in Experiment 1 except that fructose and SS were the sweeteners. Following the CS+/H2O vs. CS−/H2O choice tests, the Fructose group (n=9) was given a 2-day choice test with unflavored fructose vs. water while the SS group (n=9) was given unflavored SS vs. water.
3.2. Results and Discussion
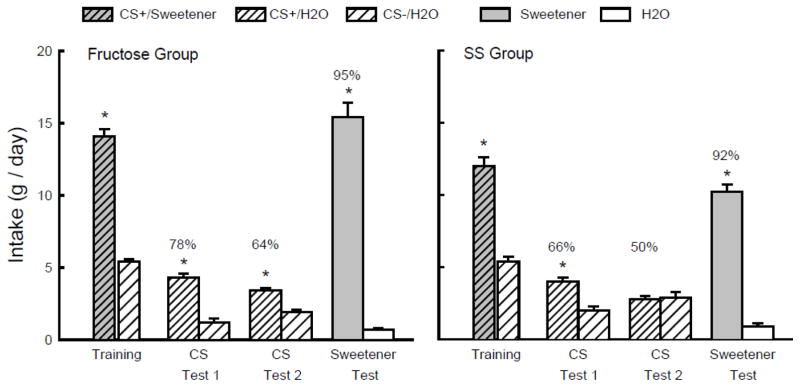
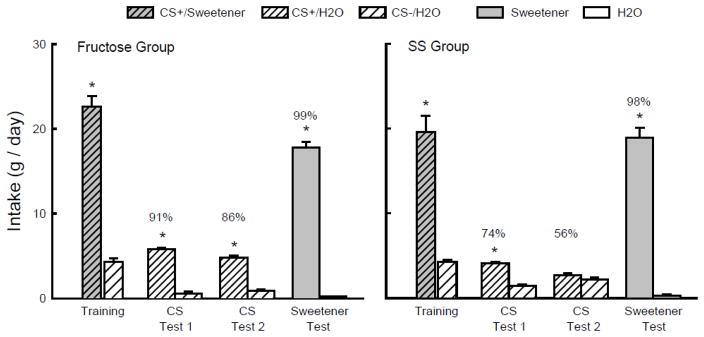
As shown in Figure 2, during one-bottle training, the Fructose and SS groups consumed more CS+/Sweetener than CS−/H2O (F(1,16) = 253.6, P < 0.001). In addition, the Fructose mice drank more (P < 0.01) CS+/Sweetener than the SS mice; CS−/H2O intakes did not differ (Group x CS interaction, F(1,16) = 8.1, P < 0.05). Overall, both groups consumed more (P < 0.01) CS+/H2O than CS−/H2O in the two-bottle tests (F(1,16) = 24.9), but the difference was greater in the Fructose group than the SS group (Group x CS interaction, F(1,16) = 4.4, P = 0.051). Furthermore, whereas the Fructose mice consumed more (P < 0.05) CS+/H2O than CS−/H2O in both Tests 1 and 2, the SS mice consumed more CS+/H2O only in Test 1. Overall, the percent CS+/H2O preferences of the Fructose group exceeded those of the SS group (F(1,16) = 5.5, P < 0.05) although the preferences in both groups declined from the first to the second test (F(1,16) = 27.6, P < 0.005); the Group x Test interaction was not significant (Fig. 2). In the final choice test with unflavored sweeteners, both groups consumed substantially more sweetener than water (F(1,16) = 374.1, P < 0.001) and the Fructose mice consumed more sweetener than did the SS mice (Group x Solution interaction, F(1,16) = 18.6, P < 0.001) (Fig. 2).
Figure 2.
Experiment 2. Mean intakes (+sem) of CS+ and CS− during one-bottle training and two-bottle Tests 1 and 2 in the Fructose (left panel) and SS (right panel) B6 groups. (Order of training and testing is from left to right in the panels.) During training the CS+ flavor was added to the sweetener solution (CS+/Sweetener), which was 8% fructose for the Fructose group and 0.1% sucralose + 0.1% saccharin (SS) for the SS group. During testing the CS+ flavor was in water (CS+/H2O); the CS− flavor was always in water (CS−/H2O). In the Sweetener vs. water Test, the Fructose group was given unflavored 8% fructose and the SS group unflavored SS. Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) intake differences in the training and two-bottle tests are indicated by an asterisk (*).
Contrary to our prediction, the SS sweetener conditioned a weaker CS+ preference than did fructose. The CS+ preferences in the SS group (66 and 50% in Tests 1 and 2, respectively) were similar to those observed in the Sucralose group in Experiment 1 (63 and 49% in Tests 1 and 2, respectively). This suggests that the post-oral inhibitory actions of 0.8% sucralose were not responsible for its relatively weak preference conditioning effect. The next three experiments explored possible reasons for the differential conditioning effects of SS and fructose in B6 mice.
4. Experiment 3: Effect of intragastric fructose on sucralose + saccharin conditioned flavor preferences
A simple explanation for why fructose conditioned a stronger CS+ preference than SS is that the post-oral nutrient actions of the sugar enhanced preference conditioning. Yet, in prior studies of B6 mice, IG fructose infusions failed to induce a preference for a CS+ flavor over a CS− flavor paired with IG water infusions [37,60]. However, in these studies the CS+ and CS− flavors were both presented in isosweet saccharin solutions. In Experiment 2, the CS+ flavor was presented in a sweet fructose solution and the CS− was presented in water. Thus, it is possible that the post-oral actions of fructose, while ineffective in altering the preference for two equally sweet flavors, enhance the preference for a sweetened over an unsweetened flavor. According to this interpretation, the conditioned preference for a CS+ flavor in the SS solution should be significantly enhanced if its intake is paired with IG fructose infusions.
4.1. Method
4.1.1. Subjects
Naive male B6 mice (n=12, 10 weeks old) were surgically fitted with chronic gastric catheters while anesthetized with isoflurane (2%) inhalation as previously described [45,59]. Analgesia was provided by a subcutaneous injection of Buprenorphine SR (1 mg/kg, ZooPharm, Windsor, CO) at the time of surgery.
4.1.2. Apparatus
IG infusion training was conducted in plastic test cages [45,59]. The sipper spouts were connected to electronic lickometers and a computer that operated a syringe pump, which infused liquid into the gastric catheter as the animal licked the sipper spout. The pump rate was nominally 0.5 ml/min, but the overall infusion rate and volume was controlled by the animal’s licking behavior; a 1:1 ratio of oral intake (in ml) to IG infusion (in ml) was maintained throughout training. Daily oral fluid intakes were measured to the nearest 0.1 g, and IG infusions were recorded to the nearest 0.5 ml.
4.1.3. Procedure
Two weeks after surgery, the mice were adapted to the infusion cages with ad libitum access to food and water. The mice were then trained for 6 days. During training days 1, 3 and 5, the CS− flavor was in water (CS−/H2O); and during training days 2, 4 and 6, CS+ flavor was in 0.1% SS (CS+/SS). Intake of the CS−/H2O was paired with IG infusions of water and the CS+/SS was paired with IG infusions of 16% fructose. Note, that the IG infused 16% fructose was diluted in the stomach to 8% fructose by the equal volume of orally consumed CS+/SS solution. Following training, IG infusions ceased. On day 7, the mice were offered water alone. On days 8–11, they were subjected to two consecutive two-bottle tests (2 days each) with the CS+/H2O and CS−/H2O solutions. On days 12–13, the mice received water only. On days 14–15, the mice were subjected to a two-bottle preference test with unflavored SS vs. water.
4.2. Results and Discussion
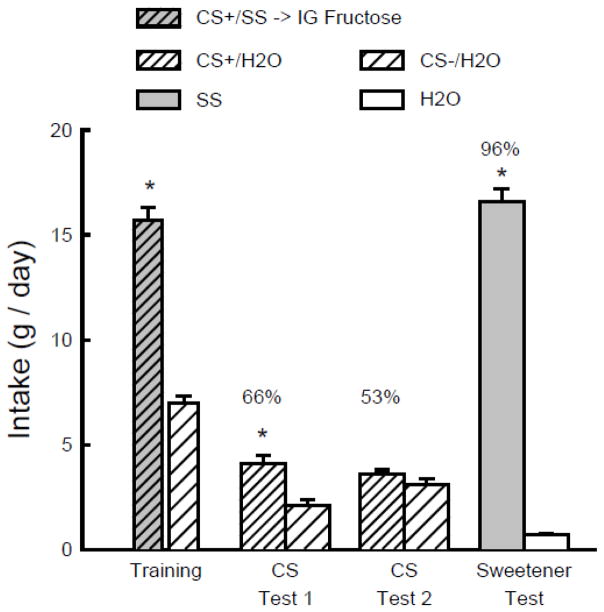
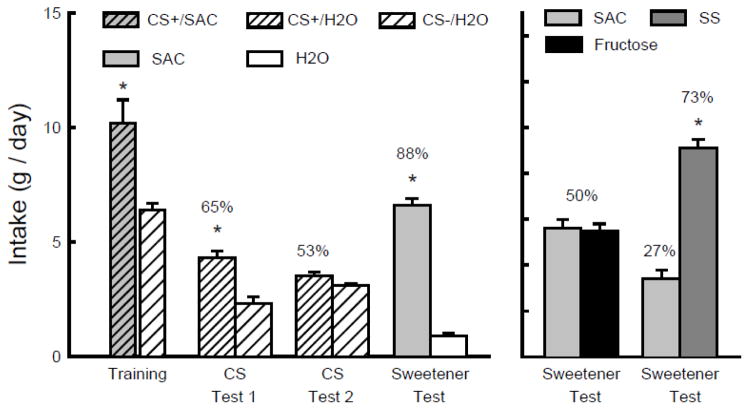
Figure 3 shows that during one-bottle training the total intake of CS+/SS plus IG fructose exceeded that of the CS−/H2O plus IG water (t(1,11) = 11.2, P < 0.001). In the two-bottle tests, the mice consumed significantly more CS+/H2O than CS−/H2O in Test 1 but not in Test 2 (CS x Test interaction F(1,11) = 9.5. P < 0.05). Their percent CS+/H2O intakes declined significantly from Test 1 to 2 (66 to 53%; t(1,11) = 3.0, P < 0.05) and were quite similar to those displayed by the SS group in Experiment 2 (66 to 49%). In the final choice test with unflavored SS and water, the mice consumed substantially more SS than water (t(1,11) = 25.9, P < 0.001) and the percent SS intake was 96%. This was comparable to the 92% preference displayed by the SS mice in Experiment 2.
Figure 3.
Experiment 3. Mean intakes (+sem) of CS+ and CS− during one-bottle training and two-bottle Tests 1 and 2 in B6 mice. (Order of training and testing is from left to right in the panel.) During training, the CS+ flavor was added to 0.1% sucralose + 0.1% saccharin (SS) and was paired with matched IG infusions of 16% fructose (CS+/SS -> IG fructose). During testing the CS+ flavor was in water (CS+/H2O) and was not paired with IG infusions. The CS− flavor was always in water (CS−/H2O) and during training, but not testing it was paired with matched IG water infusions. In the Sweetener Test the mice were given unflavored SS vs. water without IG infusions. Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) intake differences in the training and two-bottle tests are indicated by an asterisk (*).
The present results revealed that the CS+/SS solution with paired IG fructose infusions did not condition a stronger preference for the CS+ flavor than did the CS+/SS solution without paired IG fructose infusions (in Experiment 2). During training, the mice in this experiment co-infused an amount of fructose that was slightly more than that orally consumed by the mice in the Fructose group in Experiment 2 (1.35 vs. 1.13 g sugar/day), yet displayed a weaker CS+ preference than that observed in the Fructose group in the second experiment. These findings contradict the hypothesis that that fructose conditioned a stronger CS+ flavor preference than SS because of its post-oral nutritive actions.
5. Experiment 4: Effect of sucralose + saccharin on fructose conditioned preference
In addition to their sweet taste, non-nutritive sweeteners elicit bitter and metallic off-tastes [20,32]. These off-tastes may interfere with their ability to condition flavor preferences. The present experiment indirectly tested this idea by determining if adding SS to a 8% fructose solution attenuated the CS+ preference conditioned by the sugar.
5.1. Method
Naive male B6 mice (n=10, 9 weeks old) were trained and tested like the Fructose mice in Experiment 2 except that their CS+ flavored 8% fructose solution also contained 0.1% sucralose and 0.1% saccharin (henceforth, CS+/FRU+SS). Following the CS+/H2O vs. CS−/H2O tests, the mice were given a 2-day choice test with unflavored 8% fructose containing SS (FRU+SS) vs. water. This was followed by 2-day choice tests with unflavored FRU+SS vs. fructose alone and unflavored FRU+SS vs. SS alone. These additional tests were run to determine whether adding SS to fructose enhanced or reduced the preference for the sugar relative to fructose alone or SS alone. The mice were given one day of water only prior to each of the tests with unflavored sweeteners.
5.2. Results and Discussion
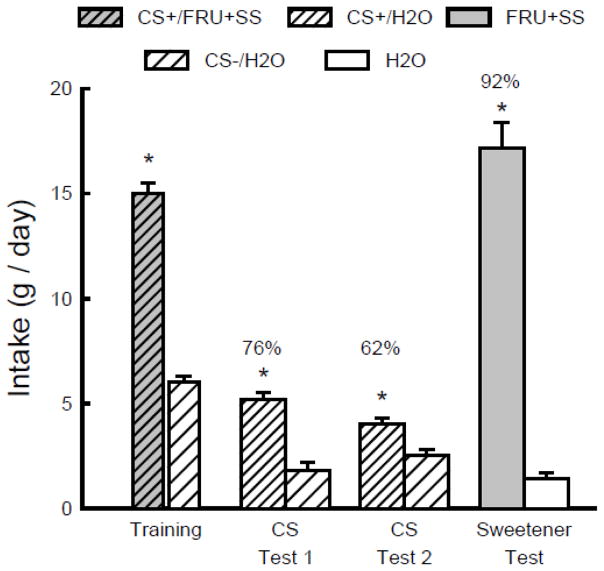
As shown in Figure 4, the mice consumed substantially more CS+/FRU+SS than CS−/H2O during one-bottle training (t(9) = 14.1, P < 0.001). They also drank significantly more CS+/H2O than CS−/H2O in both Tests 1 and 2 (F(1,9) = 72.8, P < 0.001) although the intake differences declined from the first to second test (CS x Test interaction, F(1,9) = 9.1, P < 0.001). The percent CS+/H2O also declined from Test 1 to 2 (t(9) = 2.8, P < 0.05). Nevertheless, the percent CS+/H2O intakes in the two tests (76%, 62%) were comparable to those of the Fructose group mice in Experiment 2 (78%, 64%). The mice consumed substantially more unflavored FRU+SS than water following the CS flavor tests (t(9) = 21.6, P < 0.001), and intakes of the flavored and unflavored FRU+SS solutions were comparable to the 8% fructose intakes observed in Experiment 2.
Figure 4.
Experiment 4. Mean intakes (+sem) of CS+ and CS− during one-bottle training and two-bottle Tests 1 and 2 in B6 mice. (Order of training and testing is from left to right in the panel.) During training the CS+ flavor was added to an 8% fructose solution that also contained 0.1% sucralose + 0.1% saccharin (CS+/FRU+SS) and during testing the CS+ flavor was in water (CS+/H2O). The CS− flavor was always in water (CS−/H2O). In the Sweetener Test the mice were given unflavored 8% fructose mixed with SS (FRU+SS) vs. water. Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) intake differences in the training and two-bottle tests are indicated by an asterisk (*).
In the additional two sweetener choice tests, the mice strongly preferred the FRU+SS to fructose alone (95%, 17.2 vs. 0.9 g/day, t(9) = 12.4, P < 0.001) and to SS alone (92%, 17.2 vs. 1.4 g/day, t(9) = 12.5, P < 0.001).
These results demonstrate that adding SS to 8% fructose did not attenuate the CS+ preference conditioned by the sugar. This suggests that any off-tastes of the sucralose and saccharin sweeteners are not sufficient to inhibit flavor conditioning, although it does not preclude the possibility that off-tastes may contribute to the weaker conditioning response to the SS in the absence of fructose. Adding SS to the fructose solution substantially enhanced its palatability as evidenced by the strong preferences for FRU+SS over fructose or SS alone.
6. Experiment 5: Preference for fructose vs. sucralose + saccharin paired CS+ flavors
B6 mice strongly and persistently prefer unflavored SS to unflavored 8% fructose in 24-h choice tests [40,49]. This observation led to the prediction that SS would condition a stronger CS+ flavor preference than 8% fructose. It is possible, however, that the addition of the cherry and grape flavors, which contain citric and ascorbic acids among other chemicals, alters the relative palatability of the SS and fructose solutions. If so, this could explain why the CS+/SS solution conditioned weaker preferences than did the CS+/fructose solution in Experiment 2. The present experiment therefore compared the preference of naïve B6 mice for cherry or grape flavored SS vs. fructose solutions. A second aim of the experiment was to determine the flavor preference of mice given one-bottle training with the flavored SS and flavored fructose solutions.
6.1. Method
Naive male B6 mice (n=10, 10 weeks old) were given a two-day, two-bottle preference test with solutions containing one flavor in SS (CS+SS/SS) vs. the other flavor in fructose (CS+F/FRU). The flavors were 0.05% grape or cherry Kool-Aid mixes. Half the mice had cherry added to SS and grape added to fructose; while the other half had the flavors reversed. Next, the mice were subjected to six one-bottle training days with the CS+SS/SS (days 1, 3, 5) and CS+F/FRU (days 2, 4, 6). The mice were then given two-bottle choice tests with the CS+F flavor in water (CS+F/H2O) vs. the CS+SS flavor in water (CS+SS/H2O) over 4 days (Tests 1 and 2). The mice were then given another 2-day preference test with CS+SS/SS vs. CS+F/FRU. Finally, they received a 2-day test with unflavored SS vs. fructose. One day of water only preceded the latter two choice tests.
6.2. Results and Discussion
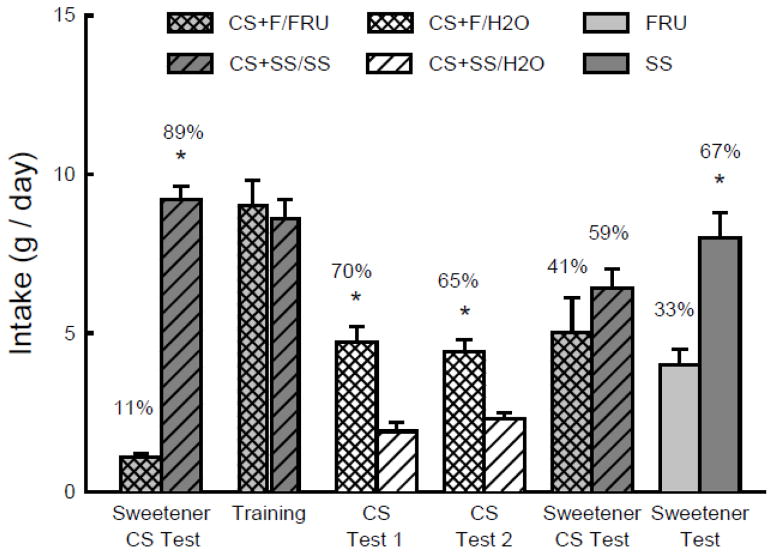
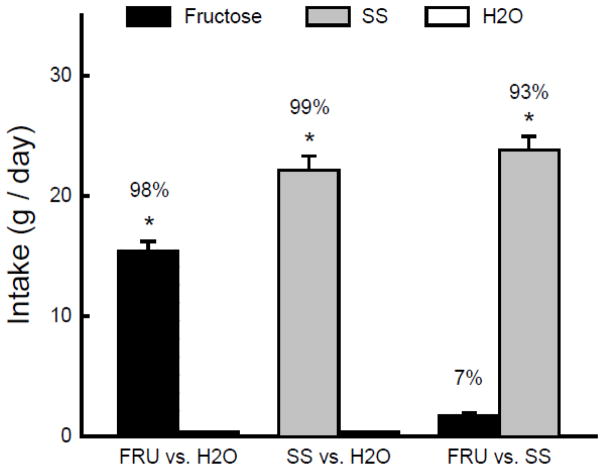
In the initial choice test with the flavored sweetener solutions, the mice consumed substantially more CS+SS/SS than CS+F/FRU (t(9) = 22.1, P < 0.001); their CS+SS/SS preference was 89% (Fig. 5). The mice then consumed comparable amounts of the two flavored sweeteners during the one bottle training sessions (Fig. 5). However, when given the choice between the CS flavors in water only, they consumed significantly more CS+F/H2O than CS+SS/H2O in both Tests 1 and 2 (F(1,9) = 15.5, P < 0.01). The percent CS+F/H2O intake declined slightly but not significantly from the first to second test (70% to 65%). In the subsequent test with the flavored sweeteners, the mice consumed slightly, but not significantly more CS+SS/SS than CS+F/FRU. Their CS+SS/SS preference in this test was significantly less (P < 0.01) than in the initial two-choice test (59% vs. 89%, t(9) = 4.3, P < 0.01). In the final test with unflavored sweeteners, the mice consumed significantly more SS than fructose (t(9) = 6.0, P < 0.001).
Figure 5.
Experiment 5. Mean intakes (+sem) of CS+ solutions during two-bottle tests and one-bottle training in B6 mice. (Order of training and testing is from left to right in the panel.) In the initial and final Sweetener CS Tests and during one-bottle training, the CS+F flavor was added to an 8% fructose solution (CS+F/FRU) and the CS+SS flavor was added to an 0.1% sucralose + 0.1% saccharin (SS) solution (CS+SS/SS). During two-bottle CS tests the CS+F and CS+SS flavors were in water. In the Sweetener Test, the mice were given unflavored 8% fructose vs. unflavored SS. Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) intake differences in the training and two-bottle tests are indicated by an asterisk (*).
The preference for the CS+ flavored SS solution over the CS+ fructose solution displayed by the naïve B6 mice in the initial choice was similar to that previously observed in B6 mice for unflavored SS over 8% fructose [40,49]. Thus, the addition of the CS flavors to the two sweeteners did not attenuate the preference for SS over fructose. Yet, after one bottle training with the flavored sweeteners, the mice preferred CS+F/H2O over CS+SS/H2O in the choice tests. This CS+F/H2O preference, which is consistent with the findings of Experiment 2, indicates that 8% fructose intake conditions a stronger CS+ flavor preference than SS intake. Following training and testing with the CS+ flavors, the mice no longer preferred the CS+SS/SS over CS+F/FRU, although they displayed a significant but moderate preference for unflavored SS to fructose. Thus, the one-bottle exposure to the flavored SS and fructose sweeteners reduced their subsequent preference for SS over fructose. This is not the case, however, for mice given separate 2-day exposure to unflavored SS and fructose. These mice showed only a slight change in their strong SS preference before and after separate exposure to the two sweeteners [40,49]. Thus the reduction in preference for the CS+SS/SS solution (from 89% to 59%) following the one-bottle training indicates that the conditioned preference for the CS+F flavor attenuated the inherent preference of B6 mice for SS over fructose.
The conditioned CS+F flavor preference observed here was replicated in a supplementary experiment in which male and female B6 mice were trained as in the present experiment except that they were not given the initial choice test between CS+SS/SS and CS+F/FRU solutions. After one-bottle training with the flavored sweeteners, the mice significantly preferred the CS+F/H2O to the CS+SS/H2O as in the present experiment (see Supplementary Fig. 1). Yet, in the subsequent sweetener tests they preferred the flavored and unflavored 0.1% SS to the 8% fructose, which is consistent with the present findings (Supplementary Fig. 1). Notably, there were no sex differences in the preference responses which indicates that female mice, like male mice, prefer SS to fructose but acquire a stronger preference for a flavor paired with fructose than one paired with SS.
7. Experiment 6: Flavor preferences conditioned by saccharin
In the preceding experiments sucralose and sucralose + saccharin solutions conditioned weaker CS+ flavor preferences than did sucrose and fructose. The present experiment determined the effectiveness of a saccharin-only solution to condition a CS+ preference in B6 mice. There are many reports of saccharin-conditioned flavor preferences in rats. In some experiments saccharin was as effective as sucrose in conditioning a flavor preference [14,26] whereas in others it was less effective than the sugar [8,12]. Here, we tested the hypothesis that a saccharin-only solution would condition stronger CS+ preferences in B6 mice than a sucralose alone or sucralose + saccharin solution. Parenthetically, because rats (unlike mice) are not generally attracted to sucralose solutions [4,42], sucralose-conditioned flavor preferences have not been studied in this species.
7.1. Method
Adult male B6 mice (n=9, 12 weeks old) were trained and tested like the SS group in Experiment 2 except with a flavored 0.2% saccharin solution (CS+/SAC). Following one-bottle training and two-bottle testing with the CS+/H2O and CS−/H2O, the mice were given a two-day choice test with unflavored 0.2% saccharin vs. water. Then the mice were given two additional 2-day choice tests which compared their preference for 0.2% saccharin vs. 8% fructose and for 0.2% saccharin vs. 0.1% sucralose + 0.1% saccharin. One or more days of water-only preceded the tests with unflavored sweeteners.
7.2. Results and Discussion
During one-bottle training, the mice consumed significantly more CS+/SAC than CS−/H2O (t(8) = 4.7, P < 0.001) (Fig. 6). They also consumed significantly more CS+/H2O than CS−/H2O in Test 1 but not Test 2, resulting in a significant CS x Test interaction (F(1,8) = 11.6, P < 0.01). The percent CS+/H2O intakes declined from 65% to 53% from the first to second test (t(8) = 3.4, P < 0.01). Following the CS tests, the mice consumed significantly more unflavored saccharin than water (t(8) = 16.6, P < 0.01). In the subsequent choice test with unflavored 0.2% saccharin and 8% fructose, the mice consumed similar amounts of the two sweeteners (Fig. 6). In contrast, in the final choice test, the mice consumed significantly more 0.1% sucralose + 0.1% saccharin than 0.2% saccharin (t(8) = 9.4, P < 0.001) (Fig. 6).
Figure 6.
Experiment 6. Mean intakes (+sem) of CS+ and CS− during one-bottle training and two-bottle Tests 1 and 2 in B6 mice. (Order of training and testing is from left to right in two panels.) During training the CS+ flavor was added to 0.2% saccharin (CS+/SAC) and during testing it was in water (CS+/H2O). The CS− flavor was always in water (CS−/H2O). During the first Sweetener Test, the mice were given unflavored 0.2% saccharin (SAC) vs. water (left panel). In two subsequent tests (right panel) the mice were given the choice of unflavored 0.2% saccharin (SAC) vs. 8% fructose and 0.2% saccharin vs. 0.1% sucralose + 0.1% saccharin (SS). Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) intake differences in the training and two-bottle tests are indicated by an asterisk (*).
The saccharin-conditioned preferences obtained in this experiment (65% to 53%) were quite comparable to those obtained with SS in Experiments 2 (66% to 50%) and 3 (66% to 53%). Yet, whereas 0.1% sucralose + 0.1% saccharin is strongly preferred to 8% fructose in B6 mice [49], the 0.2% saccharin and 8% fructose were isopreferred in this experiment. Naive B6 mice without prior sweetener experience also equally preferred saccharin and fructose (6.5 vs. 6.3 g/day) (unpublished findings). Thus, a saccharin-only solution is no more effective than sucralose or sucralose + saccharin solution in conditioning a CS+ flavor preference in B6 mice.
8. Experiment 7. Fructose and Sucralose + Saccharin conditioned flavor preferences in FVB and CAST mice
The preceding experiments found that non-nutritive sweeteners conditioned relatively weak and transient flavor preferences in B6 mice compared to fructose or sucrose. This may not be case with other inbred mouse strains, however, given the strain differences in sweetener preferences [1] as well as in sugar-conditioned preferences [30]. The present experiment investigated this possibility by comparing flavor conditioning by SS and fructose in FVB and CAST strains. These inbred strains were of interest because, like B6 mice, they have “sensitive” forms of the T1r2/T1r3 sweet taste receptor [31]. Yet, both strains appear to be more attracted to SS than are B6 mice. In particular, FVB and CAST mice drink more SS than B6 mice in choice tests vs. water [47,48,49]. However, after separate experience with both sweeteners FVB mice, unlike B6 mice prefer fructose to SS [48]. This is attributed to the post-oral appetition action of fructose in this strain as documented by the ability of IG fructose infusions to condition a CS+ preference in FVB mice [48]. Fructose also has an IG flavor conditioning action in CAST mice but, unlike FVB mice, CAST mice do not reverse their strong preference for SS over fructose after separate experience with the two sweeteners [47]. Furthermore, unlike B6 and FVB mice, CAST mice do not develop a preference for 8% glucose over SS. It appears that the strong oral attraction of CAST mice to SS overrides the post-oral appetition effects of fructose and glucose. Based on these findings, we predicted that SS should condition stronger flavor preferences in FVB and even more so in CAST mice than in B6 mice. We also predicted that these strains would develop stronger preferences than B6 mice for a fructose-paired CS+ given that IG fructose conditions flavor preferences in FVB and CAST, but not B6 mice.
8.1. Method
Adult male FVB/NJ mice (FVB; n=20, 10 weeks of age) and CAST/EiJ (CAST; n=18, 9 weeks of age) were obtained from the Jackson Laboratories. Note that characteristic of the CAST strain, the CAST mice were small and weighed significantly (P < 0.01) less than did the adult FVB (15.3 vs. 27.2 g) and B6 mice in Experiment 2 (27.4 g). The mice were housed and tested as in Experiment 2. After adaptation to the lab for 1–2 weeks, the FVB and CAST mice were each divided into two groups of equal size equated for body weight and water intake. One group in each strain was trained with fructose-paired flavors while the other group was trained and tested with SS-paired flavors. Following the CS+/H2O vs. CS−/H2O and unflavored sweetener vs. water choice tests, the CAST mice were given additional 2-day tests with 0.1% SS and 8% fructose as described below.
8.2. Results and Discussion
FVB mice
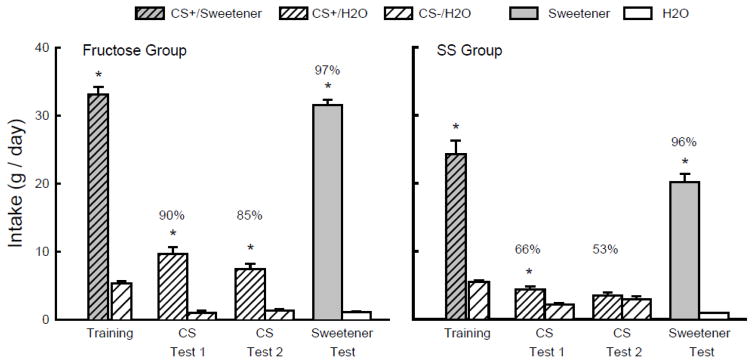
On one-bottle training days, the Fructose and SS groups consumed more CS+/Sweetener than CS−/H2O (F(1,18) = 442.8, P < 0.001), and mice in the Fructose group drank significantly more (P < 0.01) CS+/Sweetener than the SS mice; CS−/H2O intakes did not differ (Group x CS interaction, F(1,18) = 16.7, P < 0.001) (Fig. 7). In the two-bottle tests both groups consumed more CS+/H2O than CS−/H2O (F(1,16) = 76.9, P < 0.001), but the difference was greater in the Fructose group than the SS group (Group x CS interaction, F(1,16) = 36.2, P = 0.051) (Fig. 7). Furthermore, whereas the mice in the Fructose group consumed more (P < 0.01) CS+/H2O than CS−/H2O in Tests 1 and 2, the mice in the SS group consumed more CS+/H2O only in Test 1. Overall, the percent CS+/H2O preferences of the Fructose group exceeded those of the SS group (F(1,16) = 30.7, P < 0.001) and the preferences in both groups declined from the first to second test (F(1,16) = 11.9, P < 0.01). In the final choice test with unflavored sweeteners, both groups consumed substantially more sweetener than water (F(1,16) = 1228.1, P < 0.001) and the Fructose mice consumed more sweetener than did the SS mice (Group x Solution interaction, F(1,16) = 63.1, P < 0.001).
Figure 7.
Experiment 7. Mean intakes (+sem) of CS+ and CS− during one-bottle training and two-bottle Tests 1 and 2 in the Fructose (left panel) and SS (right panel) FVB groups. (Order of training and testing is from left to right in the panels.) During training the CS+ flavor was added to the sweetener solution (CS+/Sweetener) which was 8% fructose for the Fructose group and 0.1% sucralose + 0.1% saccharin (SS) for the SS group. During testing the CS+ flavor was in water (CS+/H2O); the CS− flavor was always in water (CS−/H2O). In the Sweetener vs. water test the Fructose group was tested with unflavored 8% fructose and the SS group with unflavored SS. Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) intake differences in the training and two-bottle tests are indicated by an asterisk (*).
CAST mice
The Fructose and SS groups consumed substantially more CS+/Sweetener than CS−/H2O during one-bottle training (F(1,16) = 262.2, P < 0.001) and the two groups did not differ in their CS+/Sweetener or CS−/H2O intakes (Fig. 8). In the two-bottle tests, both groups consumed more CS+/H2O than CS−/H2O (F(1,16) = 460.1, P < 0.001), although the differences were greater in the Fructose than SS group (Group x CS interaction, F(1,16) = 110.3, P < 0.001). While both groups consumed more CS+/H2O than CS−/H2O in Test 1, only the Fructose group consumed more in Test 2. The percent CS+/H2O intakes of mice in the Fructose group exceeded those of mice in the SS group (F(1,16) = 68.8, P < 0.001) and percent intakes significantly declined from Test 1 to 2 in the SS group but not in the Fructose group (Group x Test interaction, F(1,16) = 7.4, P < 0.05). The Fructose and SS groups consumed substantially more unflavored sweetener than water (F(1,16) = 673.9, P < 0.001) and they did not differ in their sweetener intakes or preferences.
Figure 8.
Experiment 7. Mean intakes (+sem) of CS+ and CS− during one-bottle training and two-bottle Tests 1 and 2 in the Fructose (left panel) and SS (right panel) CAST groups. (Order of training and testing is from left to right in the panels.) During training the CS+ flavor was added to the sweetener solution (CS+/Sweetener) which was 8% fructose for the Fructose group and 0.1% sucralose + 0.1% saccharin (SS) for the SS group. During testing the CS+ flavor was in water (CS+/H2O); the CS− flavor was always in water (CS−/H2O). In the Sweetener vs. water test the Fructose group was tested with unflavored 8% fructose and the SS group with unflavored SS. Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) intake differences in the training and two-bottle tests are indicated by an asterisk (*).
In the final test, the two CAST groups were evaluated for their SS vs. fructose preference. First, the Fructose group was given a SS vs. water test and the SS group was given a fructose vs. water test so that all mice had experience with both sweeteners. Then all mice were given the choice of SS vs. fructose; all tests were 2 days in duration and were preceded by water-only days. Since the two groups did not differ in their final test results, only the combined group data are presented in Figure 9. The mice consumed considerably more fructose and SS than water (F(1,16) = 503.7, P < 0.001) and more SS than fructose (F(1,16) = 89.6, P < 0.001) in the sweetener vs. water tests. The mice then consumed substantially more SS than fructose in the direct choice test with the two sweeteners (t(17) = 18.9, P < 0.001) and their SS preference was 93%.
Figure 9.
Experiment 7. Mean intakes (+sem) of 8% fructose (FRU) vs. water, 0.1% sucralose + 0.1% saccharin (SS) vs. water, and fructose vs. SS. The Fructose group was tested with fructose vs. water followed by SS vs. water; the SS group was tested in the reverse order. All mice were given fructose vs. SS in the final test. Numbers atop bars represent mean percent preference for that solution. Significant (P < 0.05) intake differences in the training and two-bottle tests are indicated by an asterisk (*).
The FVB and CAST mice acquired stronger preferences for the fructose-paired CS+ flavor than did the B6 mice (~90% vs. 78%, Test 1 data). This was expected because fructose has post-oral appetition effects in FVB and CAST mice unlike in B6 mice [47,48]. Consequently, their preference for the fructose-paired CS+ flavor was reinforced by both the sweet taste and post-oral actions of the sugar. However, contrary to our prediction, the SS sweetener did not condition stronger or more persistent CS+ preferences in FVB and CAST mice than in B6 mice. In particular, the Test 1 and 2 CS+ preferences of the FVB mice (66% - 53%) were quite similar to those of the B6 mice (66% - 50%). The CAST mice displayed a somewhat stronger Test 1 preference (74%) than did the FVB and B6 mice (66%), but this difference was not significant and the Test 2 preferences of the three strains were similar (50 – 56%). Although the strains did not differ in their SS-conditioned CS+ flavor preferences, the FVB and CAST mice consumed considerably more CS+/SS during training than did the B6 mice (24.2 and 19.6 vs. 12.0 g/day, F(2,25) = 14.3, P < 0.001) and more unflavored SS in the sweetener vs. water test (20.1 and 18.9 vs. 10.2 g/day, F(2,25) = 26.7, P < 0.001), which confirms prior results [47,48,49]. The elevated SS intakes of the CAST mice are remarkable given their low body weights. When expressed on a body weight basis, the CAST mice consumed more SS than did the FVB and B6 mice (24.7 vs. 14.7 vs. 7.5 g/20 g bw/day, F(2,24) = 55.1, P < 0.001). Another notable feature of the CAST mice is that, while they displayed a greater preference for the fructose-paired CS+ than the SS-paired CS+ (91% vs. 74%), they strongly preferred (93%) SS to fructose in the final choice test with the unflavored sweeteners. This confirms the prior finding that CAST mice prefer SS to fructose even after experience with both sweeteners [47].
9. General Discussion
The present study compared flavor preference conditioning by nutritive and non-nutritive sweeteners in mice. Consistent with many reports of saccharin-conditioned flavor preferences in rats [8,12,14,18,19,24,26,53], we observed that saccharin, sucralose and sucralose+saccharin solutions each conditioned flavor preferences in mice. This contrasts with recent reports that sucrose but not sucralose conditions sipper tube preferences in B6 mice. The discrepant results may be related to differential effectiveness of flavor vs. sipper tube cues to support preference conditioning or to other procedural differences (see below). The non-nutritive sweeteners conditioned weaker and less persistent preferences than did sucrose and fructose, which would appear to support the idea that post-oral nutritive feedback enhances the preference conditioning effects of sugars. However, in the case of fructose conditioning in B6 mice, the findings indicate that oral rather than post-oral factors are responsible for the enhanced sugar-conditioned preferences.
In Experiment 1, 8% sucrose conditioned a stronger flavor preference than did 0.8% sucralose. At these concentrations, the sweeteners are isopreferred by B6 mice in 1-min and initial 24-h choice tests, although the mice develop a strong sucrose preference in subsequent 24-h tests [44,49]. This acquired sucrose preference is attributed to the differential post-oral effects of the sweeteners. That is, whereas 8% sucrose has a post-oral appetition action that stimulates intake and preference, 0.8% sucralose has a post-oral inhibitory effect that limits intake and preference [44]. Nevertheless, the mice preferred orally consumed 0.8% sucralose to water, indicating that the sweet taste of sucralose overrode the post-oral actions of sweetener [44]. The CS+ preference displayed by the sucralose group in Experiment 1 further indicates that the sweet taste of sucralose is sufficient to condition a flavor preference although it was weaker than that produced by sucrose. Using higher concentrations of sucrose (27%) and sucralose (1.2%), other investigators reported that only sucrose conditioned a sipper tube preference [3,10]. As noted above, it may be that flavor cues are more effective than sipper tube cues in supporting taste-induced preferences. It is also possible, however, that post-oral actions of the more concentrated sucralose solution used in the sipper tube studies interfered with preference conditioning. Other procedural differences (training and test duration, deprivation state) may also contribute to the discrepant results. Future studies could resolve this issue by comparing flavor and sipper tube conditioning using the same sweetener concentrations, test durations, and deprivation states.
To reduce post-oral differences between nutritive and non-nutritive sweeteners, in subsequent experiments we compared flavor conditioning using (a) fructose, which has minimal, if any, post-oral positive reinforcing action in B6 mice, and (b) a 0.1% sucralose + saccharin mixture, which has minimal post-oral inhibitory actions in this strain [37,49,60]. Furthermore, B6 mice significantly prefer 0.1% SS to 8% fructose in brief-access and 24-h two-bottle tests and lick more SS than fructose in PR lick tests [49]. Consequently, we predicted that SS would condition a stronger CS+ preference than fructose in Experiment 2. However, the opposite result was obtained; namely, the mice in the Fructose group displayed a greater CS+ preference than did the SS group. Experiment 3 revealed that the stronger fructose conditioned preference cannot be attributed to the post-oral actions of the sugar. B6 mice trained to drink a flavored SS solution paired with IG fructose infusions displayed CS+ preferences comparable to those obtained with the SS mice in Experiment 2. The fourth experiment revealed that adding SS to 8% fructose did not reduce the sugar-conditioned CS+ preference, indicating that the off-taste or post-oral actions of SS do not attenuate flavor conditioning.
In Experiment 5 we considered the possibility that the addition of the CS flavor mix to the sweeteners altered the relative palatability of fructose and SS. This proved not to be the case: naive B6 mice strongly preferred (by 89%) flavored SS to flavored fructose in the initial two-bottle test. Yet, after one-bottle training with the flavored sweeteners, the same mice preferred the CS+F/H2O to the CS+SS/H2O. The one-bottle training also reduced their subsequent preference for the CS+SS/SS solution over the CS+F/FRU solution to 59% and resulted in a relatively weak preference (67%) for unflavored SS over fructose compared to the 78% preference displayed by B6 mice given experience with unflavored SS and fructose [49]. Thus, it appears that fructose taste is more potent than sucralose taste in reinforcing flavor preferences and that acquiring a fructose-based flavor preference reduces the inherent preference of B6 mice for SS over fructose [49].
Consistent with many rat studies [8,12,14,18,19,24,26,53], Experiment 6 showed that B6 mice acquired a preference for a CS+ flavor added to a 0.2% saccharin solution. However, the saccharin-conditioned CS+ preference, like those conditioned by 0.8% sucralose and 0.1% SS, was weaker than that produced by 8% fructose. Yet, naïve and experienced B6 mice equally preferred 0.2% saccharin and 8% fructose in a 2-day choice test. The equal preference for these two sweeteners provides an argument against one potential explanation for the weak CS+ preferences induced by the SS solution. A feature of the training and test procedure used in the current and prior studies is that the animals were trained with a sweetened CS+ flavored solution and then tested with an unsweetened CS+ solution. The reduction in the palatability of the CS+ flavored solutions from training to testing may reduce its attractiveness via a negative contrast effect. (For example, animals switched from 32% sucrose to 4% sucrose show less attraction to the 4% sugar than do animals without prior experience with the concentrated sugar [13].) Since B6 mice strongly prefer 0.1% SS to 8% fructose, mice trained with flavored SS solutions may experience more negative contrast when switched to the unsweetened CS+ solution than do mice switched from flavored fructose to unsweetened CS+ solution. In theory this could explain the weaker preferences conditioned by SS compared to fructose. This explanation is refuted, however, by the finding that 0.2% saccharin is isopreferred to 8% fructose but conditions CS+ preferences as weak as those produced by 0.1% SS. Note that in Experiment 4, adding 0.1% SS to 8% fructose enhanced the palatability of the sugar solution such that it was strongly preferred to plain fructose and SS. Yet, the mice trained with fructose+SS displayed CS+ preferences comparable to mice trained with fructose only. Taken together, these findings demonstrate that the magnitude of the conditioned CS+ preference is independent of the palatability of the sweetened CS+ training solution.
The last experiment determined if the non-nutritive SS solution would condition stronger preferences in FVB and CAST mice than those observed in B6 mice. This proved not to be the case; the FVB and CAST mice did not differ from B6 mice in displaying significant but relatively weak preferences for a SS-paired CS+ flavor that dissipated by the second two-bottle choice test. On the other hand, FVB and CAST mice acquired stronger fructose-based CS+ preferences than B6 mice, which was expected based on the post-oral appetition actions of fructose in these two strains. But even though CAST mice in the Fructose group acquired a robust preference for the sugar-paired flavor following training, they still strongly preferred SS to fructose in the final choice test. Thus, in all three mouse strains, preferences for SS over fructose did not result in strong SS-conditioned CS+ preferences. Conceivably there might be other mouse strains that acquire strong preferences for flavors paired with non-nutritive sweeteners but this remains to be determined. It is also possible that the conditioning results obtained with fructose and SS might differ if different test procedures were used, e.g., short-term instead of 24-h test sessions, oromotor taste reactivity or lick microstructure analyses rather than intake measures.
The oral attractiveness of fructose, sucrose and non-nutritive sweeteners is largely, if not exclusively mediated by the T1r2/T1r3 sweet taste receptor. This is demonstrated by the failure of these sweeteners to stimulate brief access licking in knockout mice missing one or both of these receptor units [17,58,61]. In long-term tests, however, KO mice acquire preferences for sucrose and glucose based on the post-oral appetition actions of these sugars. T1r3 KO mice, however, do not acquire preferences for fructose, consistent with the failure of IG fructose infusions to condition flavor preferences in B6 mice. Given that sucralose, saccharin and fructose act on the same T1r2/T1r3 sweet receptors, it is surprising that saccharin and SS solutions that are equally or more preferred than fructose condition weaker flavor preferences than do fructose. Recent studies have revealed the existence of T1r2/T1r3-independent oral sugar sensors in mice including a glucose transporter (GLUT2), a sodium-glucose transporter (SGLT1) and an ATP-gated K+ glucose sensor [25,51,56]. Furthermore, one or more of these sensors are implicated in the cephalic-phase insulin response (CPIR) to sugars because T1r3 KO and B6 wildtype mice display comparable CPIRs to oral glucose and sucrose [17]. However, fructose, saccharin and sucralose do not elicit a CPIR in B6 mice indicating that the sugar-sensing CPIR pathway cannot account for the differential preference conditioning effects of these three sweeteners [16,17].
Some investigators have proposed the existence of oral “calorie” or “carbohydrate” sensors that are distinct from oral sweet receptors based on the differential exercise enhancement or fMRI responses in humans to carbohydrates (glucose, maltodextrin) vs. non-nutritive sweeteners (sucralose) [9,15,50]. In fact, there is strong evidence that rodents have a maltodextrin taste receptor independent of their T1r2/T1r3 sweet receptor: T1r2 and/or T1r3 KO mice are strongly attracted to maltodextrin but not sucrose in short-term lick tests [52,61]. Recent findings suggest that humans also have a maltodextrin taste receptor [21]. The putative maltodextrin receptor remains to be identified, but it could not mediate the differential flavor conditioning response to fructose vs. non-nutritive sweeteners. In rats a conditioned aversion to maltodextrin does not generalize to fructose or saccharin. This indicates that these two sweeteners do not bind to the maltodextrin receptor [29]. Furthermore and most interestingly, maltodextrin taste, unlike sucrose taste does not support flavor preference conditioning in rats. This is demonstrated by the finding that sham-feeding rats learn to prefer a CS+ flavor added to a sucrose solution but not to a CS+ flavor added to an isopreferred maltodextrin solution [6]. Why sucrose but not maltodextrin taste conditions flavor preferences in rats is not known, although some data suggest that these tastants differentially activate brain dopamine reward systems involved in flavor preference learning [6].
The dopamine reward system is also implicated in fructose-conditioned flavor preferences [2,5] and the differential sipper tube conditioning response to sucrose and non-nutritive sweeteners (sucralose, saccharin) [3,10]. Conceivably, fructose may activate the dopamine reward system more than does the highly preferred SS or equally preferred saccharin solutions used in the present study. How this differential activation might occur is uncertain given that fructose, sucralose and saccharin all stimulate the same T1r2/T1r3 sweet receptor. Fructose-selective taste receptors have been identified in flies [27] but there is as yet no evidence for mammalian taste receptors that respond to fructose but not to sucralose or saccharin.
While the present study revealed that orally consumed non-nutritive sweeteners condition weaker flavor preferences than do sucrose and fructose, they are effective in potentiating the post-oral appetition actions of sucrose in B6 mice. This is demonstrated by the findings that B6 mice trained to drink a CS+ flavor paired with IG 16% sucrose infusions consumed more of a saccharin-sweetened CS+ flavor than of an unsweetened CS+ flavor (18.2 vs. 6.8 g/day) during training and displayed a stronger preference in choice tests for the sweetened than for the unsweetened CS+ relative to the CS− (98% vs. 83%) [46]. These results indicate that the potent appetite-stimulating effects of orally consumed sugars in mice are due to the combined actions of the sugars on oral T1r2/T1r3 sweet receptors and post-oral sugar sensors (glucose sensors in B6 mice and glucose and fructose sensors in FVB and CAST mice) [37,46].
Supplementary Material
Highlights.
Flavor preferences are conditioned by sugars and non-nutritive sweeteners in mice.
A sucralose-saccharin (SS) mixture is strongly preferred to fructose in mice.
Yet, fructose conditioned a stronger flavor preference than the SS mixture.
The post-oral actions of fructose do not account for its conditioning effects.
Rather, orosensory factors are implicated in the differential conditioning actions.
Acknowledgments
This research was supported by grant DK-31135 from the National Institute of Diabetes and Digestive and Kidney Diseases. We thank Martin Zartarian and Mohammed Riad for their technical assistance and John I. Glendinning for his helpful comments on this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bachmanov AA, Bosak NP, Floriano WB, Inoue M, Li X, Lin C, Murovets VO, Reed DR, Zolotarev VA, Beauchamp GK. Genetics of sweet taste preferences. Flavour Fragr J. 2011;26:286–294. doi: 10.1002/ffj.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker RM, Shah MJ, Sclafani A, Bodnar RJ. Dopamine D1 and D2 antagonists reduce the acquisition and expression of flavor-preferences conditioned by fructose in rats. Pharmacol Biochem Behav. 2003;75:55–65. doi: 10.1016/s0091-3057(03)00039-x. [DOI] [PubMed] [Google Scholar]
- 3.Beeler JA, McCutcheon JE, Cao ZF, Murakami M, Alexander E, Roitman MF, Zhuang X. Taste uncoupled from nutrition fails to sustain the reinforcing properties of food. Eur J Neurosci. 2012;36:2533–2546. doi: 10.1111/j.1460-9568.2012.08167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bello NT, Hajnal A. Male rats show an indifference-avoidance response for increasing concentrations of the artificial sweetener sucralose. Nutr Res. 2005;25:693–699. doi: 10.1016/j.nutres.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernal SY, Dostova I, Kest A, Abayev Y, Kandova E, Touzani K, Sclafani A, Bodnar RJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens shell on the acquisition and expression of fructose-conditioned flavor-flavor preferences in rats. Behav Brain Res. 2008;190:59–66. doi: 10.1016/j.bbr.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonacchi KB, Ackroff K, Sclafani A. Sucrose taste but not Polycose taste conditions flavor preferences in rats. Physiol Behav. 2008;95:235–244. doi: 10.1016/j.physbeh.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke MV, Small DM. Effects of the modern food environment on striatal function, cognition and regulation of ingestive behavior. Curr Opin Behav Sci. 2016;9:97–105. doi: 10.1016/j.cobeha.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capaldi ED, Owens J, Palmer KA. Effects of food deprivation on learning and expression of flavor preferences conditioned by saccharin or sucrose. Anim Learn Behav. 1994;22:173–180. [Google Scholar]
- 9.Carter JM, Jeukendrup AE, Jones DA. The effect of carbohydrate mouth rinse on 1-h cycle time trial performance. Med Sci Sports Exerc. 2004;36:2107–2111. doi: 10.1249/01.mss.0000147585.65709.6f. [DOI] [PubMed] [Google Scholar]
- 10.de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MAL, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 11.Domingos AI, Vaynshteyn J, Sordillo A, Friedman JM. The reward value of sucrose in leptin-deficient obese mice. Mol Metab. 2014;3:73–80. doi: 10.1016/j.molmet.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedorchak PM, Bolles RC. Hunger enhances the expression of calorie- but not taste-mediated conditioned flavor preferences. J Exp Psychol Anim Behav Process. 1987;13:73–79. [PubMed] [Google Scholar]
- 13.Flaherty CF. Incentive Relativity. Cambridge University; New York: 1996. [Google Scholar]
- 14.Forestell CA, LoLordo VM. Palatability shifts in taste and flavour preference conditioning. Q J Exp Psychol B. 2003;56B:140–160. doi: 10.1080/02724990244000232. [DOI] [PubMed] [Google Scholar]
- 15.Frank GKW, Oberndorfer TA, Simmons AN, Paulus MP, Fudge JL, Yang TT, Kaye WH. Sucrose activates human taste pathways differently from artificial sweetener. Neuroimage. 2008;39:1559–1569. doi: 10.1016/j.neuroimage.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 16.Glendinning JI, Frim YG, Hochman A, Basile A, Sclafani A. Glucose elicits cephalic-phase insulin release in mice by activating K(ATP) channels in taste cells. Am J Physiol Regul Integr Comp Physiol. 2017 doi: 10.1152/ajpregu.00433.2016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glendinning JI, Stano S, Holter M, Azenkot T, Goldman O, Margolskee RF, Vasselli JR, Sclafani A. Sugar-induced cephalic phase insulin release is mediated by a T1r2/T1r3-independent taste pathway in mice. Am J Physiol Regul Integr Comp Physiol. 2015;309:R552–R560. doi: 10.1152/ajpregu.00056.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grigson PS, Reilly S, Shimura T, Norgren R. Ibotenic acid lesions of the parabrachial nucleus and conditioned taste aversion: Further evidence for an associative deficit in rats. Behav Neurosci. 1998;112:160–171. [PubMed] [Google Scholar]
- 19.Holman EW. Immediate and delayed reinforcers for flavor preferences in the rat. Learn Motiv. 1975;6:91–100. [Google Scholar]
- 20.Kuhn C, Bufe B, Winnig M, Hofmann T, Frank O, Behrens M, Lewtschenko T, Slack JP, Ward CD, Meyerhof W. Bitter taste receptors for saccharin and acesulfame K. J Neurosci. 2004;24:10260–10265. doi: 10.1523/JNEUROSCI.1225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lapis TJ, Penner MH, Lim J. Humans can taste glucose oligomers independent of the hT1R2/hT1R3 sweet taste receptor. Chem Senses. 2016;41:755–762. doi: 10.1093/chemse/bjw088. [DOI] [PubMed] [Google Scholar]
- 22.Liang NC, Freet CS, Grigson PS, Norgren R. Pontine and thalamic influences on fluid rewards: I. Operant responding for sucrose and corn oil. Physiol Behav. 2012;105:576–588. doi: 10.1016/j.physbeh.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin JY, Arthurs J, Reilly S. Conditioned taste aversions: From poisons to pain to drugs of abuse. Psychon Bull Rev. 2016 doi: 10.3758/s13423-016-1092-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mediavilla C, Martin-Signes M, Risco S. Role of anterior piriform cortex in the acquisition of conditioned flavour preference. Sci Rep. 2016;6:33365. doi: 10.1038/srep33365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merigo F, Benati D, Cristofoletti M, Osculati F, Sbarbati A. Glucose transporters are expressed in taste receptor cells. J Anat. 2011;219:243–252. doi: 10.1111/j.1469-7580.2011.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messier C, White NM. Contingent and non-contingent actions of sucrose and saccharin reinforcers: Effects on taste preference and memory. Physiol Behav. 1984;32:195–203. doi: 10.1016/0031-9384(84)90129-x. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151:1113–1125. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mook DG. Oral and postingestional determinants of the intake of various solutions in rats with esophageal fistulas. J Comp Physiol Psychol. 1963;56:645–659. [Google Scholar]
- 29.Nissenbaum JW, Sclafani A. Qualitative differences in polysaccharide and sugar tastes in the rat: A two-carbohydrate taste model. Neurosci Biobehav Rev. 1987;11:187–196. doi: 10.1016/s0149-7634(87)80025-8. [DOI] [PubMed] [Google Scholar]
- 30.Pinhas A, Aviel M, Koen M, Gurgov S, Acosta V, Israel M, Kakuriev L, Guskova L, Fuzailov I, Touzani K, Sclafani A, Bodnar RJ. Strain differences in sucrose- and fructose-conditioned flavor preferences in mice. Physiol Behav. 2012;105:451–459. doi: 10.1016/j.physbeh.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci. 2004;24:938–946. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riera CE, Vogel H, Simon SA, Damak S, le Coutre J. The capsaicin receptor participates in artificial sweetener aversion. Biochem Biophys Res Commun. 2008;376:653–657. doi: 10.1016/j.bbrc.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 33.Scheggi S, Secci ME, Marchese G, De Montis MG, Gambarana C. Influence of palatability on motivation to operate for caloric and non-caloric food in non food-deprived and food-deprived rats. Neuroscience. 2013;236:320–331. doi: 10.1016/j.neuroscience.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Sclafani A. Carbohydrate taste, appetite, and obesity: An overview. Neurosci Biobehav Rev. 1987;11:131–153. [PubMed] [Google Scholar]
- 35.Sclafani A. Gut-brain nutrient signaling: appetition vs. satiation. Appetite. 2013;71:454–458. doi: 10.1016/j.appet.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sclafani A, Ackroff K. Nutrient-conditioned flavor preference and incentive value measured by progressive ratio licking in rats. Physiol Behav. 2006;88:88–94. doi: 10.1016/j.physbeh.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Sclafani A, Ackroff K. Flavor preferences conditioned by intragastric glucose but not fructose or galactose in C57BL/6J mice. Physiol Behav. 2012;106:457–461. doi: 10.1016/j.physbeh.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sclafani A, Ackroff K. The role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1119–R1133. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sclafani A, Ackroff K. Flavor preference conditioning by different sugars in sweet ageusic Trpm5 knockout mice. Physiol Behav. 2015;140:156–163. doi: 10.1016/j.physbeh.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sclafani A, Adamantidis A, Ackroff K. MCH receptor deletion does not impair glucose-conditioned flavor preferences in mice. Physiol Behav. 2016;163:239–244. doi: 10.1016/j.physbeh.2016.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sclafani A, Cardieri C, Tucker K, Blusk D, Ackroff K. Intragastric glucose but not fructose conditions robust flavor preferences in rats. Am J Physiol Regul Integr Comp Physiol. 1993;265:R320–R325. doi: 10.1152/ajpregu.1993.265.2.R320. [DOI] [PubMed] [Google Scholar]
- 42.Sclafani A, Clare R. Female rats show a bimodal preference response to the artificial sweetener sucralose. Chem Senses. 2004;29:523–528. doi: 10.1093/chemse/bjh055. [DOI] [PubMed] [Google Scholar]
- 43.Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference and indifference produced by intragastric infusions of galactose, glucose and fructose in rats. Physiol Behav. 1999;67:227–234. doi: 10.1016/s0031-9384(99)00053-0. [DOI] [PubMed] [Google Scholar]
- 44.Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1643–R1650. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sclafani A, Glendinning JI. Flavor preferences conditioned in C57BL/6 mice by intragastric carbohydrate self-infusion. Physiol Behav. 2003;79:783–788. doi: 10.1016/s0031-9384(03)00174-4. [DOI] [PubMed] [Google Scholar]
- 46.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: Oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol. 2005;289:R712–R720. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- 47.Sclafani A, Vural AS, Ackroff K. CAST/Ei and C57BL/6J mice differ in their oral and post-oral attraction to glucose and fructose. Chem Senses. 2017 doi: 10.1093/chemse/bjx003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sclafani A, Zukerman S, Ackroff K. Fructose and glucose conditioned preferences in FVB mice: Strain differences in post-oral sugar appetition. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1448–R1457. doi: 10.1152/ajpregu.00312.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sclafani A, Zukerman S, Ackroff K. Post-oral glucose sensing, not caloric content, determines sugar reward in C57BL/6J mice. Chem Senses. 2015;40:245–258. doi: 10.1093/chemse/bjv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smeets PAM, de Graaf C, Stafleu A, van Osch MJP, van der Grond J. Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am J Clin Nutr. 2005;82:1011–1016. doi: 10.1093/ajcn/82.5.1011. [DOI] [PubMed] [Google Scholar]
- 51.Toyono T, Seta Y, Kataoka S, Oda M, Toyoshima K. Differential expression of the glucose transporters in mouse gustatory papillae. Cell Tissue Res. 2011;345:243–252. doi: 10.1007/s00441-011-1210-x. [DOI] [PubMed] [Google Scholar]
- 52.Treesukosol Y, Blonde G, Spector AC. The T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: Implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R855–R865. doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ueji K, Minematsu Y, Takeshita D, Yamamoto T. Saccharin taste conditions flavor preference in weanling rats. Chem Senses. 2016;41:135–141. doi: 10.1093/chemse/bjv064. [DOI] [PubMed] [Google Scholar]
- 54.Weingarten HP, Watson SD. Sham feeding as a procedure for assessing the influence of diet palatability on food intake. Physiol Behav. 1982;28:401–407. doi: 10.1016/0031-9384(82)90131-7. [DOI] [PubMed] [Google Scholar]
- 55.Wright GA. Appetitive learning: memories need calories. Curr Biol. 2011;21:R301–R302. doi: 10.1016/j.cub.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 56.Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci U S A. 2011;108:5431–5436. doi: 10.1073/pnas.1100495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu W-Z, Sclafani A, Delamater AR, Bodnar RJ. Pharmacology of flavor preference conditioning in sham-feeding rats: Effects of naltrexone. Pharmacol Biochem Behav. 1999;64:573–584. doi: 10.1016/s0091-3057(99)00124-0. [DOI] [PubMed] [Google Scholar]
- 58.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJP, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 59.Zukerman S, Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1635–R1647. doi: 10.1152/ajpregu.00425.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zukerman S, Ackroff K, Sclafani A. Post-oral appetite stimulation by sugars and non-metabolizable sugar analogs. Am J Physiol Regul Integr Comp Physiol. 2013;305:R840–R853. doi: 10.1152/ajpregu.00297.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol Regul Integr Comp Physiol. 2009;296:R866–R876. doi: 10.1152/ajpregu.90870.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.