Abstract
PURPOSE
To examine the individual-level factors that predict energy intake (EI) following imposed exercise (EX) and sedentary time (SED) in children.
METHODS
Healthy-weight children ages 9-12 years (n = 20) reported to the laboratory for 1 baseline and 2 experimental visits (EX and SED) each separated by 1 week in a randomized crossover design. Percent body fat, weight (kg), and height (m) were used to calculate fat-mass index (FM index) and fat-free mass index (FFM index; kg/m2). On the EX day, children exercised at 70% estimated VO2peak for 30 minutes on a cycle ergometer while cardiovascular responses and ratings of perceived exertion (RPE) were measured. Objective EI (kcal) was measured at identical meals (breakfast, lunch, snack, and dinner) on the EX and SED days.
RESULTS
Total EI was not statistically different between the EX and SED days (t = 1.8, p = 0.09). FFM index was positively associated with EI on the EX day (r = 0.54, p < 0.05). RPE was also positively associated with EI on the EX day (r = 0.82, p < 0.001). Together, FFM index and RPE explained 77% of the variability in EX day EI (F(2,17) = 26.4, p < 0.001). For each unit increase in RPE, children consumed ~270 more calories on the EX day. A similar pattern of associations was observed on the SED day.
CONCLUSION
FFM index was positively associated with EI on the EX day. Despite experiencing the same 70% relative exercise intensity, increased perceived difficulty predicted greater EI on both the EX and SED day. These findings demonstrate a role for both FFM and RPE in explaining EI variability in children.
Keywords: body composition, RPE, pediatric, energy balance, nutrition
Introduction
Previous studies have shown individual differences in the energy intake (EI) response to exercise, but homeostatic or cognitive mechanisms underlying these differences are unclear. In particular, there is limited research in this area in children. In regards to homeostatic mechanisms, there have been studies examining changes in gut peptides, inflammatory markers, and substrate or macronutrient utilization as possible factors that influence post-exercise EI (15, 16, 32). In addition, fat-free mass (FFM) is a known predictor of daily EI in adolescents and adults, predominantly through its effects on resting metabolic rate and total energy expenditure (EE) (4, 8, 9, 27). Furthermore, it has been proposed that cognitive factors also contribute to additional variability in daily EI. For example, studies in adults have shown that increased perceived difficulty of exercise may be associated with caloric compensation and predicts weight regain after successful weight loss (6). This weight regain is assumed to be related to greater EI by participants, but has not been tested.
The majority of studies looking at the effects of exercise on subsequent food intake have focused on homeostatic mechanisms. In particular, there are several pathways by which the body signals information about EE in order to increase EI and properly compensate for the activity-related energy deficit. These include, but are not limited to, gut-brain signaling pathways and markers of glycogen store utilization (15, 16, 30). But homeostatic regulation of energy balance is not limited to metabolic changes associated with acute fluctuations in EE. Body composition, including FFM and fat mass (FM), serves as a longer-term indicator of energy balance. FFM is a large determinant of resting metabolic rate, which contributes greatly to total daily EE (2). In addition, FFM has been shown to be a better predictor of EI than FM in both adolescents and adults (3, 8, 9, 27). However, research in younger children is lacking. Also, the conditions under which FFM is most strongly associated with EI have not been examined. For example, it is not known whether the association between FFM and EI is affected by an exercise-induced energy deficit.
Aside from the homeostatic regulation of EI, there are also cognitive factors that influence eating behaviors. For example, perceived exertion may predict individual responses to a set exercise bout. However, there has been limited research examining whether the perceived difficulty experienced during exercise is associated with subsequent behaviors, above and beyond the effects of the physical stress associated with exercise (26, 37). Of particular interest to the current study, it is unknown whether the perceived difficulty of exercise is associated with post-exercise EI. It is vital to understand the potential consequences of imposed exercise in a sample of children who are at risk for becoming overweight before implementing prescribed exercise on a broader scale.
The purpose of the current paper was to understand individual-level factors that may contribute to differences in daily EI after participation in 30 minutes of 70% estimated VO2max intensity cycling exercise. In particular, we examined the associations between body composition (i.e., FFM and FM), cardiovascular responses to exercise (e.g., heart rate), ratings of perceived exertion, and EI across the experimental day. We hypothesized that children's FFM would be positively associated with total EI, in line with previous research in adolescents and adults. Since the exercise bout was tailored to the same relative intensity of 70% VO2max, we hypothesized that total EI would be positively related to children's ratings of perceived exertion during the exercise, independent of the controlled cardiovascular response to the exercise.
Methods
Study Design
We conducted a within-subjects, crossover design study with a community-based sample of 20 children between the ages of 9-12 years. The overall purpose of the study was to investigate the effects of imposed exercise versus imposed sedentariness on children's total EI over the course of a day. This paper addresses a secondary aim to determine child characteristics that predict individual differences in post-exercise EI. Children completed 1 baseline and 2 experimental visits (EX = exercise day, SED = sedentary day in a pre-assigned randomized order) each separated by 1 week. For all three visits, children arrived to the laboratory after an overnight fast. The baseline visit consisted of a four-hour session in the morning, which served to familiarize children with eating in a laboratory environment (breakfast and lunch) and collect baseline fitness and anthropometric measurements. Outside of meal and exercise testing periods, children had access to a series of toys, books, and games, and could switch freely between activities. The EX and SED days were identical, and consisted of the same four-hour morning session in the laboratory, followed by five hours of free-living time at home, and then an in-laboratory dinner session. The only difference was that the morning sedentary play time was interrupted by the 30-minute exercise bout on the EX day. On the first study visit, a parent signed informed consent for their child and children provided verbal and written assent prior to their participation. Children and their parents received modest financial compensation for their time. This study was approved by the Institutional Review Board (#00578) and the Clinical Research Center Advisory Committee (#330) of The Pennsylvania State University.
Participants
Children were recruited using flyers and online media postings in local schools and businesses located around the university. Interested parents completed a phone screening to determine eligibility. Children were considered eligible if by parental report they were normal weight (< 85th age- and sex-specific body mass index [BMI] percentile) with at least one biological parent who was overweight or obese (BMI > 25 kg/m2), without food allergies, medical conditions or contraindications to exercise testing, and were not participating in competitive sports which could skew test results (year-round or more than 3 practice sessions per week). Children's liking and willingness to eat the test-meal foods was also confirmed at screening, prior to enrollment in the study. Both child and parent weight status were confirmed by measurement at the baseline visit. One male child was retained in the study despite being in the overweight category (89th BMI percentile) after baseline measurements. This participant was not a statistical outlier compared to the group average in regards to body composition (e.g., % body fat) or any of the behavioral measures (e.g., food intake, exercise test performance), and removing him from the analyses did not affect the statistical significance of or conclusions from our findings. All 20 children who were initially enrolled in the study completed all three visits. Sample characteristics for these 20 children are listed in Table 1.
Table 1.
Participant characteristics (n = 20).
| Mean ± SD (Range) | |
|---|---|
| Age (years) | 10.3 ± 1.1 (9 – 12) |
| BMI percentile | 41.6 ± 21.7 (9 – 89) |
| % body fat | 15.6 ± 4.4 (6.7 – 26.6) |
| FM index (kg/m2) | 2.7 ± 0.9 (1.1 – 4.4) |
| FFM index (kg/m2) | 14.3 ± 1.5 (12.1 – 18.3) |
| N (%) | ||
|---|---|---|
| Sex | Male | 12 (60) |
| Female | 8 (40) | |
| Race | White | 20 (100) |
| Non-white | 0 (0) | |
Abbreviations: BMI, body mass index; FM, fat mass; FFM, fat-free mass.
Baseline Measurements
Anthropometrics & body composition
Prior to breakfast on the baseline visit, anthropometrics (height and weight) were measured to the nearest 0.1 cm and 0.1 kg by a trained researcher. Children and their parents were each weighed and measured twice in light clothing, using a standard scale (Detecto model 437, Webb City, MO) and stadiometer (Seca model 202, Chino, CA). Height (m) and weight (kg) were converted to body mass index (BMI; kg/m2) for the parent, and age- and sex-specific BMI z-score and BMI percentile for the child using the Centers for Disease Control and Prevention conversion program (14).
Body composition was measured using bioelectrical impedance analysis (Tanita model BF-350, Arlington Heights, IL, USA). Percent body fat (%BF) was multiplied by body weight (kg) to calculate fat mass (FM; kg). The difference between body weight and FM was taken to calculate fat-free mass (FFM; kg). This method has previously been validated against dual-energy x-ray absorptiometry (DXA) with children in our age range (41). To control for differences in body composition as a function of child height, FFM index and FM index were calculated by dividing the absolute FFM and FM, respectively, by the height squared (kg/m2) (43).
Fitness testing
Two hours into the baseline visit, children completed the YMCA graded submaximal cycle test to estimate cardiorespiratory fitness (28). Children were outfitted with a Polar Heart Rate Transmitter chest strap and wrist unit receiver (Polar Electro Inc. model T31-Coded, Lake Success, NY, USA). Participants remained seated for five minutes while a researcher explained the procedure and instructed the child on the use of the Borg Scale for Ratings of Perceived Exertion (RPE) (5, 13, 23, 34). The Borg RPE Scale (ranging from 6-20) has previously been described as a valid and reliable measure, and is suitable for use with children within this age range (5, 13, 23, 34). At the end of the five minute period, a supervising nurse obtained resting heart rate (HR) and blood pressure (BP) measurements. Children were then familiarized with the cycle ergometer (Lode Corival V2, Lode Holding BV, Groningen, The Netherlands) and completed a three-minute warm-up, followed by the YMCA submaximal cycle test. The YMCA cycle test follows a branching, multi-stage format to determine the relationship between heart rate and work rate in order to estimate the individual's VO2max. Children are required to pedal at a constant rate (50 ± 2 revolutions per minute) while researchers adjust the resistance (i.e., work rate) on the cycle ergometer at each stage. HR values are recorded every minute, while BP and RPE are measured every three minutes. The test ends once a participant has completed two separate workload stages that result in steady-state HR (±5 beats per minute) between 110 and 150 beats per minute. VO2max was estimated using the graph plot and extrapolation technique (28). This estimated VO2max was used to determine the work rate for the 70% intensity cycle test on the EX Day (described below).
70% Intensity Exercise Protocol
Two hours into the EX day session, children completed the cycle ergometer exercise test. Participants were outfitted with the HR monitor, and resting HR and BP measurements were taken. After a three-minute warm-up, children exercised at their individual 70% estimated VO2max for 30 minutes. The starting work rate (in Watts) was determined from the linear association between HR and work rate established during the submaximal exercise test. The work rate was either confirmed or adjusted throughout the test to maintain a target HR between 70-80% age-predicted maximum HR (e.g., 10-year-old: 147-168 beats per minute). HR values were recorded every minute, while BP and RPE were measured every three minutes. Children could request water at any point during the test. Researchers and the attending nurse encouraged children throughout the exercise protocol with positive verbal cues, cheering, and clapping. After completion of the exercise test, participants had a five-minute cool-down period on the bike, followed by ten minutes of light stretching.
Accelerometer Measurements
Children wore an ActiGraph GT3X-BT accelerometer on their non-dominant wrist for 10 hours on each testing day (EX, SED). In addition, children wore a second accelerometer on their non-dominant ankle for the YMCA submaximal cycle test and the 30-minute exercise test (70% individual estimated aerobic capacity) to more accurately measure activity in the seated position on the cycle ergometer. We used this hybrid measure to estimate Activity-related EE, extracted for each child for the entire 10-hour period. All data were validated and scored in ActiLife 6 software (ActiGraph, LLC, Pensacola, FL, USA) using Freedson Combination (1998) to calculate Activity-related EE (40).
Food Intake Measurement
Test-meal procedures
Children arrived to the laboratory after an overnight fast on all three testing days. Objective EI (kcal) was measured at breakfast, lunch, snack, and dinner test meals. The use of laboratory-based test meals for the measurement of food intake, including reliability against other measurement tools, has been discussed previously (12, 22, 33). Meals were identical on the EX and SED days. Fullness ratings were completed before and after each laboratory meal on a vertical 150 millimeter visual analog scale (VAS) referred to as “Freddy Fullness” (29). On the first visit, children conducted taste tests to report liking and wanting for each breakfast, lunch, and snack food on a VAS. On the second visit, children tasted and rated liking and wanting on a VAS for each dinner food.
Breakfast
All children were required to consume a standardized breakfast on all three test days consisting of an English muffin toasted with one tablespoon butter, banana, and orange juice (285 kcal total) (see Table, Supplemental Digital Content 1, Laboratory test-meal menu). Children were considered to have finished the meal if they consumed > 95% of each individual food item within 30 minutes. All 20 children met these requirements at each of the three breakfast meals.
Lunch
Children were offered an identical lunch meal on all three test days. Prior to the first visit, children were given the opportunity to select from a pre-set menu of available items for lunch. Children chose a sandwich (peanut butter & jelly or deli meat & cheese), a vegetable (carrots or tomatoes) with ranch dip, a fruit (apple slices or grapes), and a salty snack (pretzels or baked chips). All children also received brownies and a bottle of water with their lunch. All serving sizes were controlled to ensure that any combination of food items provided approximately the same number of total calories (997-1014 kcal), which provided > 50% of children's caloric needs for the day. Possible food choices and serving sizes are reported in Supplementary Table 1 (see Table, Supplemental Digital Content 1, Laboratory test-meal menu). Children were instructed that they had up to 30 minutes to eat freely from the food items provided. If they were finished before the 30 minutes had elapsed, they notified a researcher.
Snack
The snack (302 kcal) (see Table, Supplemental Digital Content 1, Laboratory test-meal menu) was pre-weighed and packed for children to take home during free-living time on the EX and SED days. Parents were given written and verbal instructions to provide the snack at a set time and to return any packaging and uneaten food items to researchers upon arrival for dinner. Compliance was checked by sending text message reminders to parents at snack time and requesting a response. Children were reminded by the researchers and the parent that they could eat as much or as little as they would like of any of the snack foods. Written and verbal instructions were also given to not eat or drink anything except water for the 2 hours prior to dinner. Upon return, packaging was re-weighed.
Dinner
On the EX and SED days, children reported to the laboratory at least 2 hours fasted for an ad libitum dinner test-meal (1227+ kcal) (see Table, Supplemental Digital Content 1, Laboratory test-meal menu). Children were instructed that they had up to 30 minutes to eat as much or as little as they would like from the available foods. They were also able to request additional servings of the foods at this meal. A researcher was available in the room during the meal and prompted the child if they finished a serving of a particular food. Children could also notify the researcher if they finished eating before the 30 minutes had elapsed.
Nutrient analysis
Pre- and post-meal weights for each food item were measured to the nearest 0.1 gram, and used to calculate intake in grams. This was later converted to EI (kcal) by meal (Breakfast, Lunch, Snack, and Dinner) in SPSS Statistics (Version 22; IBM Corporation, Armonk, NY, USA) using nutrition label information. Total EI (kcal) was computed as the sum of EI from each of the individual meals (Breakfast, Lunch, Snack, and Dinner EI).
Statistical Analysis
Sample size calculations (n = 20) were derived using G*Power software (version 3.1.9.2) for the original aim to compare within-subject differences in EI as a function of condition (EX vs. SED) using paired-samples t-tests (18). The secondary aim of investigating predictors of individual differences in EI was not considered in the sample size calculation. Descriptive statistics for participant characteristics (i.e., means and standard deviations on continuous variables and frequencies on categorical variables) were calculated on the full sample. Paired t-tests were performed to test differences in Total EI between the EX and SED days. Pearson's correlations were computed to determine the associations between body composition (FFMI, FMI), exercise test variables (RPE, HR, fitness), and intake variables. Partial correlations were used to test whether the relationship between RPE and EI was independent of likely covariates (e.g., HR, fitness level). Multiple linear regressions were performed to predict individual variability in post-exercise EI; dependent variable = EX day EI (kcal), independent variables = RPE, HR, FFMI, and/or FMI. A maximum of two independent variables were entered into each model. One male child was a statistical outlier on RPE ratings during the 70% exercise test, reporting more than 3 SD below the group mean on the RPE scale (rating of 9, compared to the group mean 15 ± 2). Results are reported excluding this outlier. Data were analyzed using SPSS Statistics version 22.0 (IBM Corporation, Armonk, NY, USA). Tests were considered significant at p < 0.05.
Results
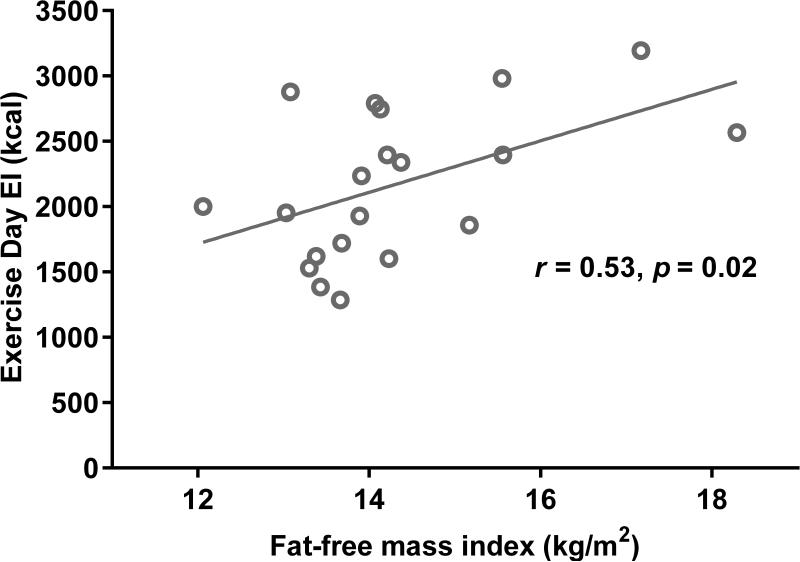
Descriptive statistics for exercise test variables (RPE, HR, fitness), and intake are listed in Table 2. Total EI was not statistically different between the EX and SED days (p > 0.05), and intake on the two days was highly correlated (r = 0.93, p < 0.001). A summary of the correlation results is reported in Table 3. FFMI was positively associated with EI on the EX day (r = 0.53, p < 0.05) (Figure 1), but not the SED day (p > 0.05). FMI was negatively associated with baseline fitness (r = − 0.74, p < 0.01), but was not associated with RPE or HR responses to 70% exercise test. There were no significant associations between FMI and intake.
Table 2.
Descriptive statistics for exercise test variables and intake on the experimental days.
| Mean ± SD (Range) | |
|---|---|
| RPE avg. during 70% test○# | 15 ± 2 (12 – 18) |
| HR avg. during 70% test (bpm) | 159 ± 7 (140 – 169) |
| Baseline fitness (mL/kg/min) | 48 ± 6 (36 – 58) |
| EX Day EI (kcal) | 2171 ± 566 (1285 – 3194) |
| SED Day EI (kcal) | 2088 ± 497 (1401 – 3085) |
Abbreviations: RPE, rating of perceived exertion; HR, heart rate; bpm, beats per minute; EI, energy intake.
Results are reported without the RPE outlier.
Possible range on Borg Scale = 6 – 20.
Table 3.
Correlations between body composition, exercise test variables, and intake.
| FFMI | FMI | RPE○ | HR | Fitness | EX Day EI | SED Day EI | |
|---|---|---|---|---|---|---|---|
| FFM index (kg/m2) | 1 | 0.39 | 0.29 | 0.44 | −0.30 | 0.53* | 0.41 |
| FM index (kg/m2) | 1 | 0.25 | 0.12 | −0.74** | 0.38 | 0.33 | |
| RPE avg. during 70% test○ | 1 | −0.50* | −0.24 | 0.82** | 0.79** | ||
| HR avg. during 70% test (bpm) | 1 | −0.01 | −0.29 | −0.21 | |||
| Baseline fitness (mL/kg/min) | 1 | −0.23 | −0.14 | ||||
| EX Day EI (kcal) | 1 | 0.93** | |||||
| SED Day EI (kcal) | 1 |
Abbreviations: FFM, fat-free mass; FM, fat mass; RPE, rating of perceived exertion; HR, heart rate; bpm, beats per minute; EI, energy intake.
Results are reported without the RPE outlier.
p < 0.05
p < 0.01.
Figure 1.
Correlation between fat-free mass index (FFM index) and energy intake (EI) on the Exercise (EX) Day; r = 0.53, p < 0.05.
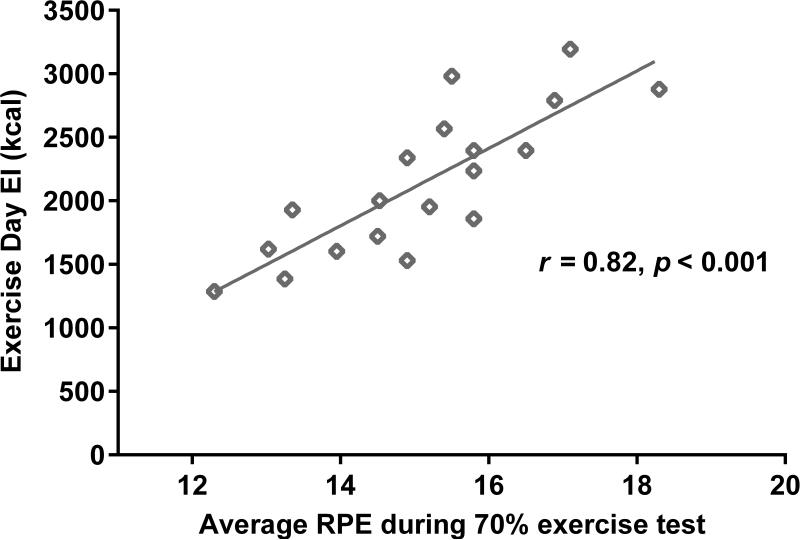
In addition, RPE was positively associated with EI on the EX day (r = 0.82, p < 0.001) (Figure 2). Partial correlations revealed that this finding was not explained by children's baseline fitness level, average HR responses to the 70% exercise test, or Activity-related EE (Mean ± SD = 288 ± 72 kcal). In regression analyses, FFMI and RPE, together, explained 77% of the variability in EI on the EX day (F(2,17) = 26.4, p < 0.001) (Table 4). For each unit increase in FFMI, children's EX Day EI increased by 118 kcal (Table 4). For each unit increase in perceived difficulty of exercise on the RPE scale, children's EX Day EI increased by approximately 270 kcal (Table 4).
Figure 2.
Correlation between ratings of perceived exertion (RPE) and energy intake (EI) on the Exercise (EX) Day; r = 0.82, p < 0.001.
Table 4.
Linear regression prediction of energy intake (EI) on the Exercise (EX) day, using ratings of perceived exertion (RPE) and fat-free mass index (FFM index).
| Model | Beta | Std. Error | t | p |
|---|---|---|---|---|
| (Constant) | −3648.2 | 828.5.0 | −4.4 | 0.001 |
| RPE | 271.1 | 46.7 | 5.8 | 0.001 |
| FFMI (kg/m2) | 118.3 | 47.8 | 2.5 | 0.025 |
Note: F(2,17) = 26.4, p < 0.001, R2 = 0.77.
Similar associations were found between RPE during exercise (r = 0.79, p < 0.001) and FFM index (r = 0.41, p = 0.08) and EI on the SED day, although only the correlation between RPE and EI reached significance (Table 3). Together, RPE and FFM index predicted 66% of the variability in SED Day EI (F(2,17) = 15.7, p < 0.001), but only RPE was a significant predictor in the model (RPE, p < 0.001; FFM index, p = 0.16) (data not shown). For each unit increase in RPE, children's SED Day EI increased by approximately 230 kcal (data not shown).
Discussion
The purpose of the current study was to examine individual differences in ad libitum EI in children at risk for becoming overweight according to family history. Our findings demonstrate a role for both FFM and RPE in EI regulation in children. In line with our hypothesis, FFM was positively associated with daily EI. We also confirmed our hypothesis that total EI would be positively related to children's RPE during the 70% intensity exercise bout, and this was independent of the controlled cardiovascular response to the exercise (e.g., HR). Each unit increase on the Borg Scale was associated with a 270 calorie increase in EI across the EX day. Similar associations were found on the SED day, given that EI was highly correlated between the two testing days.
There is a growing body of literature on the influences of body composition on appetite regulation and energy balance. In particular, FFM has been shown to be positively related to daily EI and meal size (3, 9). FFM may exert its effects on EI through its influence on resting metabolic rate and total EE, as seen in adolescents and adults (3, 8, 9, 27, 44). Our group recently showed in 7- to 10-year-old children that FFM was also positively related to activation in reward areas of the brain (e.g., substantia nigra, amygdala) in response to pictures of high-energy-dense foods (19). These results provide evidence of a potential mechanism for the positive association between FFM and food intake demonstrated previously (3, 8) and in the present cohort. We also found a positive association between FFM and EI on the SED day, but it did not reach significance (p = 0.08) in this small cohort. EI and EE have previously been shown to have a stronger linear association at higher levels of EE (35, 36). One possible explanation for our current findings it that children had lower Activity-related EE on the SED day compared to the EX day.
While the association between FM and EI was in the positive direction on both experimental days, these correlations did not reach significance (both p > 0.10). However, we recruited only healthy weight children, therefore variation in FM was limited. In a cohort with greater diversity in body weight, ethnicity, and socioeconomic status, we recently reported that adiposity was positively related to total EI and intake of savory-fat foods in a laboratory setting (20). However, total EI was also positively related to FFM and estimated resting EE (20). In addition, EI was only measured at a single meal in the previous study, while the current study design allowed for self-regulation over the course of multiple meals, which could explain differences in results between studies. Another recent study in adolescents with obesity demonstrated that even though FM was positively associated with EI assessed by 3-day food records, skeletal muscle mass (lean body tissue) was the strongest predictor of food intake (8). Collectively, these findings suggest that in youth, lean body mass may be a better predictor of EI than fat mass, consistent with studies in adults (3, 27, 44).
While EI is generally under good homeostatic control in healthy populations, psychological factors can also influence eating behavior. Despite experiencing the same 70% relative exercise intensity, children varied in their perceived difficulty of the exercise. Higher average RPE during the exercise bout predicted greater EI on the EX day. These findings suggest that greater perceived difficulty of exercise may result in overcompensation for the energy expended through greater EI at subsequent meals, at least in the short term. It is worth noting that because intake was highly correlated across the two experimental days, RPE was also positively associated with EI on the SED day. RPE was also positively associated with EI at each of the meals (i.e., lunch, snack, dinner) on both the EX and SED days (data not shown). It is possible that individuals with a higher RPE scores during the 70% exercise generally tend to consume more calories day-to-day. Importantly, the association between RPE and intake was significant within the context of a model that controlled for differences in children's body size (i.e., FFMI). In other words, children who think exercise is more difficult may generally have a tendency to eat more, independent of body weight. Based on the cross-sectional design of the current study, the direction of the more general relationship between RPE and EI across multiple eating occasions cannot be determined.
These findings are in line with a previous study that demonstrated that higher perceived difficulty of exercise was associated with greater weight regain following successful weight loss (6). Women in this study were formerly overweight and had completed a weight loss intervention and weight was tracked over the following year. RPE was measured in response to a submaximal walking exercise. In this study, RPE, but not physiological exertion during the submaximal exercise, was positively associated with weight regain (6). The authors suggested that women who have higher RPE during exercise may also have trouble restricting energy intake, which could predispose them towards greater weight regain (6). However, objective intake was not measured and, therefore, conclusions about energy compensation from this study cannot be made. But the hypothesis that weight regain may be attributable to increased EI is in accordance with the results of the current study, wherein we found that RPE was positively associated with short-term EI. The increase in EI was independent of the physiological responses to exercise (i.e., HR), activity-related EE during the exercise bout, or children's baseline fitness levels. We propose that this relationship between RPE and EI is specifically a cognitive phenomenon, and the large effect size warrants further investigation into this particular result. Given the limited sample size and exploratory nature of our study, additional studies should be conducted to confirm this relationship. In addition, studies examining the associations between RPE at multiple exercise intensities and subsequent EI would greatly expand our understanding of this phenomenon.
This is the first study to our knowledge that attributes individual differences in post-exercise energy compensation to the perceived difficulty of the exercise bout. There is a diverse body of evidence on other cognitive factors related to post-exercise eating behavior. One study in adults demonstrated that compensatory eating following 50 minutes of 70% intensity exercise was associated with an enhanced implicit wanting for food (21). Some individuals may not receive the same benefit from imposed exercise due to an increase in the hedonic response to food following exercise-induced energy expenditure. Other research has suggested that RPE and energy compensation following exercise may be related to increased subjective hunger ratings (31), higher levels of disinhibition (7), and greater emotional eating (11), among other cognitive factors. We did not find an effect of rated liking, wanting, or fullness levels on the significance of our main outcomes for the current study (all p < 0.05, data not shown). While we did not measure affect in the current study, previous research in adults has shown that negative affect during exercise can influence subsequent energy intake (42). Researchers have suggested that at submaximal exertion levels, perceived exertion is dominated by cognitive factors and affective responses to exercise, while higher intensity exercise induces heightened sensory attention to the physiological response to the exercise (24, 26, 37). Based on the design of the current study, we cannot determine whether children had reached an intensity level (i.e., ventilatory threshold) at which the physiological responses became more apparent (24, 26). It is important to note that not all behavioral compensatory responses to exercise are deliberate or intentional (30). In the current study, we are unsure whether children with higher RPE actively chose to consume more calories on both days, or whether this effect was passive. Further investigation into the psychological factors associated with post-exercise eating behavior would be extremely valuable.
This study represents a novel examination of individual differences in post-exercise energy compensation that may influence eating behaviors. Some strengths of the study are worth noting. In particular, the design included a controlled, individualized exercise bout to maintain 70% relative intensity, which allowed us to better distinguish physiological effort (e.g., cardiovascular responses) from perceived difficulty. In addition, we obtained objective measures of EI at multiple test-meals, which limits misreporting biases associated with self-reported food intake (10). The study was designed to facilitate familiarity with the laboratory environment (research personnel, test-meals, and exercise testing) during a baseline visit to the Clinical Research Center, which reduced the novelty effect on the outcome variables of interest. We also completed the study with 100% retention of enrolled participants, and full compliance with instructions and protocols across all three testing days.
Despite the strengths of the study, there were some limitations. We had a small sample size and we were underpowered to address secondary outcomes. It is important to note that the correlations between RPE and EI (r = 0.82, r = 0.79) were actually higher than the previously reported test-retest reliability of the instrument (r = 0.78) [21], so interpretations regarding this finding should be made with caution. The study design should be replicated before firm conclusions can be made on the effects of RPE and FFM on daily EI in children. In addition, the homogeneity of the sample limits generalizability of our results to other populations. We used a less sensitive measure of “at risk for becoming overweight” based on a single parental factor, and we did not assess additional genetic, family, or environmental influences on child weight status and risk for obesity. Future studies could include a more diverse sample of children characterized by a well-defined obesity risk phenotype. In regards to the test meals, we used a standard 285 kcal breakfast rather than tailoring the caloric content to children's individual energy needs. Therefore, the breakfast provided a smaller proportion of total energy intake in children with higher body weights. Finally, we did not measure additional homeostatic factors (e.g., gut peptides, inflammatory markers) that might also represent individual differences in appetite regulation.
Pending successful replication of the current findings, future studies looking to incorporate exercise as a strategy for maintaining energy balance may find it valuable to determine strategies to decrease perceived exertion during exercise. A recent study suggested that distraction using virtual reality during treadmill exercise was effective in increasing enjoyment of exercise (1). Other research has shown that listening to music during exercise increases enjoyment (17, 38). Finally, a study in adolescent girls demonstrated that RPE was significantly lower during a self-selected exercise session compared to a prescribed session of matched intensity (72% VO2peak) (25). These are just three examples of many possible strategies (39) to examine in the future to determine whether increasing enjoyment or decreasing perceived exertion of exercise is effective in reducing the likelihood of a compensatory response in post-exercise food intake.
In conclusion, fat-free mass and perceived exertion represent individual-level factors that may contribute to short-term differences in EI and eating behavior, but additional research is needed to confirm these results. These preliminary findings may be useful in future intervention research looking to incorporate exercise.
Supplementary Material
Acknowledgements
This project was supported by Agriculture and Food Research Initiative Grant #2011-67001-30117 from the USDA National Institute of Food and Agriculture, Program A2121. The project described was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR000127. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We would like to thank the Penn State Clinical and Translational Science Institute, including the Clinical Research Center and the Biostatistics, Epidemiology, and Research Design services. Finally, we would like to acknowledge the Metabolic Kitchen and Children's Eating Behavior Laboratory at Penn State.
Footnotes
Conflict of Interest:
The authors have no conflicts of interest to disclose. The results of the present study do not constitute endorsement by the American College of Sports Medicine. All results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Banos RM, Escobar P, Cebolla A, Guixeres J, Alvarez Pitti J, Lison JF, et al. Using Virtual Reality to Distract Overweight Children from Bodily Sensations During Exercise. Cyberpsychology, behavior and social networking. 2016;19(2):115–9. doi: 10.1089/cyber.2015.0283. doi: 10.1089/cyber.2015.0283. PubMed PMID: 26882326. [DOI] [PubMed] [Google Scholar]
- 2.Blundell JE, Caudwell P, Gibbons C, Hopkins M, Naslund E, King N, et al. Role of resting metabolic rate and energy expenditure in hunger and appetite control: a new formulation. Dis Model Mech. 2012;5(5):608–13. doi: 10.1242/dmm.009837. doi: 10.1242/dmm.009837. PubMed PMID: 22915022; PubMed Central PMCID: PMC3424457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blundell JE, Caudwell P, Gibbons C, Hopkins M, Naslund E, King NA, et al. Body composition and appetite: fat-free mass (but not fat mass or BMI) is positively associated with self-determined meal size and daily energy intake in humans. The British journal of nutrition. 2012;107(3):445–9. doi: 10.1017/S0007114511003138. doi: 10.1017/S0007114511003138. PubMed PMID: 21733267. [DOI] [PubMed] [Google Scholar]
- 4.Blundell JE, Finlayson G, Gibbons C, Caudwell P, Hopkins M. The biology of appetite control: Do resting metabolic rate and fat-free mass drive energy intake? Physiology & behavior. 2015 doi: 10.1016/j.physbeh.2015.05.031. doi: 10.1016/j.physbeh.2015.05.031. PubMed PMID: 26037633. [DOI] [PubMed] [Google Scholar]
- 5.Borg GA. Psychophysical bases of perceived exertion. Medicine and science in sports and exercise. 1982;14(5):377–81. PubMed PMID: 7154893. [PubMed] [Google Scholar]
- 6.Brock DW, Chandler-Laney PC, Alvarez JA, Gower BA, Gaesser GA, Hunter GR. Perception of exercise difficulty predicts weight regain in formerly overweight women. Obesity. 2010;18(5):982–6. doi: 10.1038/oby.2009.318. doi: 10.1038/oby.2009.318. PubMed PMID: 19816412; PubMed Central PMCID: PMC2924634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant EJ, Caudwell P, Hopkins ME, King NA, Blundell JE. Psycho-markers of weight loss. The roles of TFEQ Disinhibition and Restraint in exercise-induced weight management. Appetite. 2012;58(1):234–41. doi: 10.1016/j.appet.2011.09.006. doi: 10.1016/j.appet.2011.09.006. PubMed PMID: 21983045. [DOI] [PubMed] [Google Scholar]
- 8.Cameron JD, Sigal RJ, Kenny GP, Alberga AS, Prud'homme D, Phillips P, et al. Body composition and energy intake - skeletal muscle mass is the strongest predictor of food intake in obese adolescents: The HEARTY trial. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2016;41(6):611–7. doi: 10.1139/apnm-2015-0479. doi: 10.1139/apnm-2015-0479. PubMed PMID: 27111402. [DOI] [PubMed] [Google Scholar]
- 9.Caudwell P, Finlayson G, Gibbons C, Hopkins M, King N, Naslund E, et al. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. The American journal of clinical nutrition. 2013;97(1):7–14. doi: 10.3945/ajcn.111.029975. doi: 10.3945/ajcn.111.029975. PubMed PMID: 23193010. [DOI] [PubMed] [Google Scholar]
- 10.Champagne CM, Baker NB, DeLany JP, Harsha DW, Bray GA. Assessment of energy intake underreporting by doubly labeled water and observations on reported nutrient intakes in children. Journal of the American Dietetic Association. 1998;98(4):426–33. doi: 10.1016/S0002-8223(98)00097-2. doi: 10.1016/S0002-8223(98)00097-2. PubMed PMID: 9550166. [DOI] [PubMed] [Google Scholar]
- 11.Chandler-Laney PC, Brock DW, Gower BA, Alvarez JA, Bush NC, Hunter GR. Self-reported low vitality, poor mental health, and low dietary restraint are associated with overperception of physical exertion. Journal of obesity. 2010:2010. doi: 10.1155/2010/207451. doi: 10.1155/2010/207451. PubMed PMID: 20936158; PubMed Central PMCID: PMC2948900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaput JP, Jomphe-Tremblay S, Lafreniere J, Patterson S, McNeil J, Ferraro ZM. Reliability of a food menu to measure energy and macronutrient intake in adolescents. European journal of clinical nutrition. 2016;70(1):104–8. doi: 10.1038/ejcn.2015.116. doi: 10.1038/ejcn.2015.116. PubMed PMID: 26197874. [DOI] [PubMed] [Google Scholar]
- 13.Chen MJ, Fan X, Moe ST. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta-analysis. Journal of sports sciences. 2002;20(11):873–99. doi: 10.1080/026404102320761787. doi: 10.1080/026404102320761787. PubMed PMID: 12430990. [DOI] [PubMed] [Google Scholar]
- 14.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. Bmj. 2000;320(7244):1240–3. doi: 10.1136/bmj.320.7244.1240. PubMed PMID: 10797032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deighton K, Batterham RL, Stensel DJ. Appetite and gut peptide responses to exercise and calorie restriction. The effect of modest energy deficits. Appetite. 2014;81:52–9. doi: 10.1016/j.appet.2014.06.003. doi: 10.1016/j.appet.2014.06.003. PubMed PMID: 24911618. [DOI] [PubMed] [Google Scholar]
- 16.Deighton K, Karra E, Batterham RL, Stensel DJ. Appetite, energy intake, and PYY3-36 responses to energy-matched continuous exercise and submaximal high-intensity exercise. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2013;38(9):947–52. doi: 10.1139/apnm-2012-0484. doi: 10.1139/apnm-2012-0484. PubMed PMID: 23905660. [DOI] [PubMed] [Google Scholar]
- 17.Dyrlund AK, Wininger SR. The effects of music preference and exercise intensity on psychological variables. Journal of music therapy. 2008;45(2):114–34. doi: 10.1093/jmt/45.2.114. PubMed PMID: 18563969. [DOI] [PubMed] [Google Scholar]
- 18.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior research methods. 2007;39(2):175–91. doi: 10.3758/bf03193146. PubMed PMID: 17695343. [DOI] [PubMed] [Google Scholar]
- 19.Fearnbach SN, English LK, Lasschuijt M, Wilson SJ, Savage JS, Fisher JO, et al. Brain response to images of food varying in energy density is associated with body composition in 7-to 10-year-old children: Results of an exploratory study. Physiology & behavior. 2016;162:3–9. doi: 10.1016/j.physbeh.2016.03.007. doi: 10.1016/j.physbeh.2016.03.007. PubMed PMID: 26973134. [DOI] [PubMed] [Google Scholar]
- 20.Fearnbach SN, Thivel D, Meyermann K, Keller KL. Intake at a single, palatable buffet test meal is associated with total body fat and regional fat distribution in children. Appetite. 2015;92:233–9. doi: 10.1016/j.appet.2015.05.036. doi: 10.1016/j.appet.2015.05.036. PubMed PMID: 26049019. [DOI] [PubMed] [Google Scholar]
- 21.Finlayson G, Bryant E, Blundell JE, King NA. Acute compensatory eating following exercise is associated with implicit hedonic wanting for food. Physiology & behavior. 2009;97(1):62–7. doi: 10.1016/j.physbeh.2009.02.002. doi: 10.1016/j.physbeh.2009.02.002. PubMed PMID: 19419671. [DOI] [PubMed] [Google Scholar]
- 22.Goran MI. Measurement issues related to studies of childhood obesity: assessment of body composition, body fat distribution, physical activity, and food intake. Pediatrics. 1998;101(3 Pt 2):505–18. PubMed PMID: 12224657. [PubMed] [Google Scholar]
- 23.Groslambert A, Mahon AD. Perceived exertion : influence of age and cognitive development. Sports medicine. 2006;36(11):911–28. doi: 10.2165/00007256-200636110-00001. PubMed PMID: 17052130. [DOI] [PubMed] [Google Scholar]
- 24.Hall EE, Ekkekakis P, Petruzzello SJ. Is the relationship of RPE to psychological factors intensity-dependent? Medicine and science in sports and exercise. 2005;37(8):1365–73. doi: 10.1249/01.mss.0000174897.25739.3c. PubMed PMID: 16118584. [DOI] [PubMed] [Google Scholar]
- 25.Hamlyn-Williams CC, Freeman P, Parfitt G. Acute affective responses to prescribed and self-selected exercise sessions in adolescent girls: an observational study. BMC sports science, medicine and rehabilitation. 2014;6:35. doi: 10.1186/2052-1847-6-35. doi: 10.1186/2052-1847-6-35. PubMed PMID: 25285215; PubMed Central PMCID: PMC4182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hetzler RK, Seip RL, Boutcher SH, Pierce E, Snead D, Weltman A. Effect of exercise modality on ratings of perceived exertion at various lactate concentrations. Medicine and science in sports and exercise. 1991;23(1):88–92. PubMed PMID: 1997817. [PubMed] [Google Scholar]
- 27.Hopkins M, Finlayson G, Duarte C, Whybrow S, Ritz P, Horgan GW, et al. Modelling the associations between fat-free mass, resting metabolic rate and energy intake in the context of total energy balance. International journal of obesity. 2015 doi: 10.1038/ijo.2015.155. doi: 10.1038/ijo.2015.155. PubMed PMID: 26278004. [DOI] [PubMed] [Google Scholar]
- 28.Kaminsky LA, American College of Sports Medicine., American College of Sports Medicine . ACSM's health-related physical fitness assessment manual. 3rd ed. xiii. Wolters Kluwer Health/Lippincott Williams & Wilkins Health; Philadelphia: 2010. p. 172. [Google Scholar]
- 29.Keller KL, Assur SA, Torres M, Lofink HE, Thornton JC, Faith MS, et al. Potential of an analog scaling device for measuring fullness in children: development and preliminary testing. Appetite. 2006;47(2):233–43. doi: 10.1016/j.appet.2006.04.004. doi: 10.1016/j.appet.2006.04.004. PubMed PMID: 16828929. [DOI] [PubMed] [Google Scholar]
- 30.King NA, Caudwell P, Hopkins M, Byrne NM, Colley R, Hills AP, et al. Metabolic and behavioral compensatory responses to exercise interventions: barriers to weight loss. Obesity. 2007;15(6):1373–83. doi: 10.1038/oby.2007.164. doi: 10.1038/oby.2007.164. PubMed PMID: 17557973. [DOI] [PubMed] [Google Scholar]
- 31.King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. International journal of obesity. 2008;32(1):177–84. doi: 10.1038/sj.ijo.0803712. doi: 10.1038/sj.ijo.0803712. PubMed PMID: 17848941. [DOI] [PubMed] [Google Scholar]
- 32.King NA, Horner K, Hills AP, Byrne NM, Wood RE, Bryant E, et al. Exercise, appetite and weight management: understanding the compensatory responses in eating behaviour and how they contribute to variability in exercise-induced weight loss. British journal of sports medicine. 2012;46(5):315–22. doi: 10.1136/bjsm.2010.082495. doi: 10.1136/bjsm.2010.082495. PubMed PMID: 21596715. [DOI] [PubMed] [Google Scholar]
- 33.Laessle R, Geiermann L. Reliability of laboratory measurement of human food intake. Appetite. 2012;58(1):249–51. doi: 10.1016/j.appet.2011.10.004. doi: 10.1016/j.appet.2011.10.004. PubMed PMID: 22024051. [DOI] [PubMed] [Google Scholar]
- 34.Mahon AD, Marsh ML. Reliability of the rating of perceived exertion at ventilatory threshold in children. International journal of sports medicine. 1992;13(8):567–71. doi: 10.1055/s-2007-1024566. doi: 10.1055/s-2007-1024566. PubMed PMID: 1487338. [DOI] [PubMed] [Google Scholar]
- 35.Mayer J. Regulation of energy intake and the body weight: the glucostatic theory and the lipostatic hypothesis. Annals of the New York Academy of Sciences. 1955;63(1):15–43. doi: 10.1111/j.1749-6632.1955.tb36543.x. PubMed PMID: 13249313. [DOI] [PubMed] [Google Scholar]
- 36.Mayer J, Roy P, Mitra KP. Relation between caloric intake, body weight, and physical work: studies in an industrial male population in West Bengal. The American journal of clinical nutrition. 1956;4(2):169–75. doi: 10.1093/ajcn/4.2.169. PubMed PMID: 13302165. [DOI] [PubMed] [Google Scholar]
- 37.St Clair Gibson A, Baden DA, Lambert MI, Lambert EV, Harley YX, Hampson D, et al. The conscious perception of the sensation of fatigue. Sports medicine. 2003;33(3):167–76. doi: 10.2165/00007256-200333030-00001. PubMed PMID: 12656638. [DOI] [PubMed] [Google Scholar]
- 38.Stork MJ, Kwan MY, Gibala MJ, Martin Ginis KA. Music enhances performance and perceived enjoyment of sprint interval exercise. Medicine and science in sports and exercise. 2015;47(5):1052–60. doi: 10.1249/MSS.0000000000000494. doi: 10.1249/MSS.0000000000000494. PubMed PMID: 25202850. [DOI] [PubMed] [Google Scholar]
- 39.Thivel D, Isacco L, O'Malley G, Duche P. Pediatric Obesity and Perceived Exertion: Difference Between Weight-Bearing and Non-Weight-Bearing Exercises Performed at Different Intensities. Journal of sports sciences. 2016;34(5):389–94. doi: 10.1080/02640414.2015.1061200. doi: 10.1080/02640414.2015.1061200. PubMed PMID: 26090822. [DOI] [PubMed] [Google Scholar]
- 40.Trost SG, Way R, Okely AD. Predictive validity of three ActiGraph energy expenditure equations for children. Medicine and science in sports and exercise. 2006;38(2):380–7. doi: 10.1249/01.mss.0000183848.25845.e0. doi: 10.1249/01.mss.0000183848.25845.e0. PubMed PMID: 16531910. [DOI] [PubMed] [Google Scholar]
- 41.Tyrrell VJ, Richards G, Hofman P, Gillies GF, Robinson E, Cutfield WS. Foot-to-foot bioelectrical impedance analysis: a valuable tool for the measurement of body composition in children. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001;25(2):273–8. doi: 10.1038/sj.ijo.0801531. doi: 10.1038/sj.ijo.0801531. PubMed PMID: 11410831. [DOI] [PubMed] [Google Scholar]
- 42.Unick JL, Michael JC, Jakicic JM. Affective responses to exercise in overweight women: Initial insight and possible influence on energy intake. Psychology of sport and exercise. 2012;13(5):528–32. doi: 10.1016/j.psychsport.2012.02.012. doi: 10.1016/j.psychsport.2012.02.012. PubMed PMID: 24039545; PubMed Central PMCID: PMC3772527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body's fat-free mass and fat mass: potentially useful indicators of nutritional status. The American journal of clinical nutrition. 1990;52(6):953–9. doi: 10.1093/ajcn/52.6.953. PubMed PMID: 2239792. [DOI] [PubMed] [Google Scholar]
- 44.Weise CM, Hohenadel MG, Krakoff J, Votruba SB. Body composition and energy expenditure predict ad-libitum food and macronutrient intake in humans. International journal of obesity. 2014;38(2):243–51. doi: 10.1038/ijo.2013.85. doi: 10.1038/ijo.2013.85. PubMed PMID: 23736368; PubMed Central PMCID: PMC3909024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.