Abstract
The deterioration in arterial and cardiac function with aging impairs arterial ventricular coupling, an important determinant of cardiovascular performance. However, exercise training improves arterial ventricular coupling especially during exercise during the age and disease process. This review examines the concept of arterial-ventricular coupling, and how age, and disease uncouples but exercise training recouples the heart and arterial system.
Keywords: arterial ventricular coupling, Aging, Cardiovascular Disease, Exercise training
Introduction
By 2050, it is anticipated that approximately 89 million people in the U.S. will be over 65 years of age, more than double the number of older Americans in 2010. This is of particular concern as the process of aging significantly increases cardiovascular (CV) morbidity even in the absence of other risk factors (e.g., hypertension, obesity, diabetes, hypercholesterolemia). As such, the risk of death from heart disease is approximately 60-fold greater in the 8th decade compared to the 4th decade of life. Furthermore, aging is also associated with a drastic increase in subclinical/occult CV diseases (i.e., silent coronary atherosclerosis). Therefore, aging of the U.S. population is one of the major public health challenges that we face.
Compelling evidence has shown that with healthy aging (absence of CV disease), the heart and vasculature undergo considerable remodeling and a deterioration in function, especially in response to stress (such as acute exercise). Although such changes are considered to be representative of ‘normative’ aging, the CV adaptations to aging lower the threshold for the development of CV disease. Indeed, aging in the presence of CV diseases (hypertension, diabetes, atherosclerosis) or the clustering of CV risk factors (i.e., metabolic syndrome) accelerates the arterial and cardiac dysfunction. As such, these age and disease interactions impair the coupling between the heart and arterial system, termed arterial-ventricular coupling (EA/ELV), a key determinant of CV performance and efficiency. The CV system is modulated to provide sufficient pressure and flow to the tissues. Understanding the performance (pressure and flow output) of the heart requires examination of the properties of the left ventricle (LV) itself (power and stroke capacity), and the modulating effects of the arterial system (capacitance and inertial properties of the aorta, and the resistance capacity of the micro-circulation) on LV performance. EA/ELV allows the examination of the crosstalk between the heart and arterial system.
Importantly, individuals who maintain a physically active lifestyle, or who partake in exercise training later in life, can either ameliorate or delay some, but not all, of the CV alterations that accompany advancing age. This review examines the underlying concept of EA/ELV, and examines how age and CV disease result in a mismatch in the coupling between the LV and arterial systems, and how exercise training “recouples” the interaction.
What is Arterial-Ventricular Coupling?
LV performance is influenced by the arterial load, and arterial properties are, in turn, influenced by LV performance. Such interactions influence the magnitude and efficiency of transfer of cardiac stroke work to the circulation. By characterizing the arterial system and LV in terms of elastance, we are directly able to examine the crosstalk between the LV and arterial system in the same measurement domain. Typically, arterial load is characterized in the frequency domain as impedance spectra, while LV performance is characterized in the time domain by indexes of pressure and volume. This hinders the ability to directly examine the interaction between the heart and arterial system. Sunagawa and colleagues (51) conceived a measure of arterial load (EA) that could be directly compared to a measure of LV contraction (ELV; albeit ELV is also influenced by non-contractile aspects; see below for more detail) in the same units (elastance; change in pressure for a given change in volume). Through characterizing the arterial and cardiac systems in terms of pressure and volume, we are able to examine their direct interaction with one another.
Arterial Elastance
Arteries are simply not pipes through which the heart ejects blood to the vital organs and tissues. Rather they act to dampen the pulsatile blood flow from the heart to a steadier flow within the peripheral circulation. The stiffness of the arterial wall affects the blood vessel's ability to ‘store’ stroke volume (SV) from the heart without imposing an excessive afterload, to later recoil during diastole resulting is a progressive reduction in pulsatile blood flow across the arterial tree. As such, the arterial system plays a key role in LV function and determining LV mechanical efficiency (the ratio of energy transferred to the arterial system to the energy consumed for this action). For example, arterial end-systolic pressure (ESP) changes with SV in a roughly linear manner, provided that heart rate (HR) and the diastolic-systolic time intervals remain constant. Thus, the greater the SV ejected into the arterial system, the greater the generated ESP.
In the pressure-volume plane, we can characterize the arterial load on the LV as EA. Rather than specific arterial properties or location on the arterial tree, EA simplifies the arterial load into an integrative index that incorporates the principal elements of arterial load (peripheral vascular resistance, total arterial compliance, characteristic impedance, and systolic and diastolic time intervals) (51). As such, EA can be considered a measure of the net arterial load that is imposed on the LV. Invasively EA is determined from the pressure-volume loop as the negative slope of the line joining the end-diastolic volume and ESP points (Figure 1). An increase in afterload is depicted by an increase in the slope of the end-systolic pressure to end-diastolic volume relationship to the right, resulting in a higher LV pressure for a given end-diastolic volume. Non-invasively EA can be calculated from ESP and SV (Equation 1).
Figure 1.
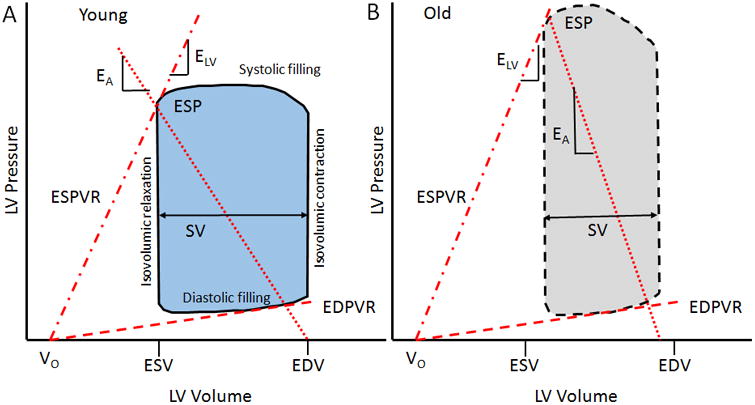
Pressure volume loop. A representative depiction of the pressure-volume relationship comparing the slope of the end-systolic pressure-volume relationship (ESPVR) and the slope of the line joining the ESP and EDV points (EA) from a healthy young control (A) and an older individual with hypertension (B). End-systolic elastance (ELV) reflects a load-independent measure of left ventricular (LV) contraction, and is steeper in an older hypertensive individual. Effective arterial elastance (EA), a measure of net arterial load on the LV, is increased (thereby reducing SV and increasing ESP) in an older individual compared to a young healthy individual. Vo represents the theoretical volume intercept of the ESPVR; ESP, end systolic pressure; SV, stroke volume; ESV, end systolic volume; EDV, end diastolic volume; and EDPVR, end diastolic pressure volume relationship.
| Equation 1 |
ESP can be estimated from the formula [2 × (systolic BP + diastolic BP)]/3, or from ESP = 0.9 × brachial systolic BP. The latter was found to approximate ESP measured invasively (14). However, with recent advances in the imaging field, ESP can also be derived from the arterial waveform, obtained from applanation tonometry, and this method of obtaining ESP was shown to be more reliable than using a calculation (30).
It is important to state that EA has disadvantages as a measure of arterial load, in that EA is sensitive to HR, which is a cardiac rather than arterial property. Further, the incorporation of the ‘stiffness’ component of arterial load within EA has been questioned. Theoretically, an increase in arterial stiffness would lead to an increase in blood pressure pulsatility and a higher ESP. Because EA is calculated from ESP/SV, a widening of ESP from mean pressure (due to arterial stiffening), would lead to a higher EA relative to mean resistance load (systemic vascular resistance). Further, it has also been shown that EA is directly related to arterial resistance, and inversely related to arterial compliance (13). During exercise, the contribution of compliance and resistance to EA are nearly equal (45). Despite this, evidence collected in human subjects suggested that EA is mostly dependent on resistance, and is negligibly affect by changes in pulsatile afterload (17). This is important given that the pulsatile component of arterial load is highly relevant with aging and CV disease. As such, it is imperative that EA not be considered a measure of arterial ‘stiffness’. Although arterial stiffness may not significantly affect EA, the pulsatile component of arterial load is incorporated in the EA/ELV paradigm due to the effects of arterial stiffness on LV fibrosis, dysfunction, and failure. Thus, EA can be considered as a surrogate measure of net arterial load whose advantage is that it can be related to measures of ELV, thus allowing the study of arterial and ventricular interactions.
End-Systolic Elastance
Invasively, the contractile function of the LV is expressed from the slope of the end-systolic pressure-volume relationship (ESPVR; Figure 1), which can be obtained from a series of pressure-volume loops recorded while the preload of the heart is altered. Historically, ELV reflects a relatively (within normal physiological limits) load-independent measure of LV contraction (chamber stiffness at end systole). An increase in contractility is depicted by an increase in the slope and a shift in the ESPVR to the left, which allows the ventricle to generate more pressure for a given LV volume. In Figure 1, V0 represents the theoretical volume intercept of the ESPVR. The calculation of ELV assumes that the ESPVR is independent of load, that its slope is linear, and that V0 is insensitive to inotropic influences. However, ESPVR is generally nonlinear and Vo is not totally independent of the inotropic state. Thus caution is warranted when comparing between groups with different loading conditions. Although ELV reflects the inotropic state of the myocardium, ELV also reflects the geometric (structural remodeling) and biochemical properties (i.e., stiffness or compliance of myocytes, composition of muscle, fibrosis, collagen, etc.) in the LV wall (8). For example, a ‘stiffer’ LV due to remodeling leads to a higher ELV. ELV should, therefore, be considered an integrated measure of LV chamber performance that can be related to an integrated measure of arterial load (i.e., EA). Traditionally ELV is assessed from multiple cardiac cycles (acquiring pressure and volume data), however, algorithms have been generated and validated to derive ELV noninvasively from single steady-state data. See Chantler et al. (8) for a more detailed review of the methods to measure EA and ELV. None-invasively ELV can be calculated from ESP and SV (Equation 2).
| Equation 2 |
Arterial-Ventricular Coupling & Clinical Relevance
At rest and in healthy individuals, the properties of the heart and arteries are closely matched so that near maximal cardiac work, power, and chamber efficiency are achieved. Effective coupling between the heart and arteries reflects an optimal transfer of SV without excessive changes in blood pressure, and to provide optimal CV flow reserve without compromising arterial pressures. It is suggested that the mechanical energy (i.e., stroke work) transferred from the ventricular to the arterial system is maximal when the slopes of EA and ELV are ∼equal (52). Further exploration of optimal matching between EA and ELV revealed, in isolated canine hearts, that stroke work was maximal at EA/ELV ~ 0.8, while cardiac efficiency was maximal at EA/ELV ~ 0.7 (20). In healthy humans the optimal range of EA/ELV to cardiac efficiency and stroke work range from 0.7 to 1.0. None-invasively the coupling ratio can be calculated from EA and ELV (Equation 3).
| Equation 3 |
How EA and ELV interact with each other has significant implications on the transfer of blood from the LV through the circulatory system and the preservation of CV reserve. For example, at rest, a stiffer LV (increased ELV), or an increase in arterial load (increased EA) means that systolic blood pressures are much more sensitive to changes in cardiac volumes (32). Thus, situations that alter cardiac volumes acutely, such as a change in posture, overeating, or use of medications would result in an exaggerated change in pressures and increase the amount of myocardial oxygen consumption required to deliver a given SV. In turn, such a response can negatively impact systolic and diastolic LV function and worsen regional coronary ischemia (32). The clinical importance of EA/ELV was reflected by its ability to predict outcome (all-cause mortality, stroke, and myocardial infarction) in patients with heart failure (33), acute coronary syndrome (38), and myocardial infarction (1). EA/ELV was also shown to correlate with B-type natriuretic peptide levels, which is released from the myocardium in response to myocyte stretch and transmural pressure load in patients with a history of myocardial infarction (1). Finally, recent data suggests the application of EA/ELV for risk stratification of patients undergoing stress echocardiography, whereby an small EA/ELV reserve capacity was associated with a higher prevalence of adverse outcomes (5).
Aging and Arterial-Ventricular Coupling
At Rest
Age is the dominant risk factor for CV disease, and is linked to the age-associated changes to the structure and function of the heart and arteries. The CV changes with age occur in everyone but not necessarily at the same rate or to the same extent, and this may account for the difference noted in the development of CV disease between individuals of the same chronological age. In the resting state, invasive assessments of EA have reported an increase in EA of 44%-73% between 20 to 79 years of age (15, 19), indicating a deterioration in arterial health. However, these data provided limited insight into the influence of healthy aging on EA, as patients with coronary artery disease, and on chronic CV medications were included. Non-invasive cross-sectional studies in healthy individuals have reported an increase in EA with age (45-95 years) between 7-12%, and that inclusion of individuals with existing CV disease further increased the age-associated change in EA by 17-20% (47). The increase in EA with age reflects the age-associated alterations in conduit and microvascular structure and function, including an increase in conduit arterial lumen diameter, wall remodeling and stiffness (increases in collagen deposition and decreases in elastin), and endothelial dysfunction (in both conduits and microvessel) via reduced bioavailability of nitric oxide, which likely reflect the age-associated accumulation of oxidative stress and inflammation (34, 35). However, the gradual increase in EA with healthy aging was not confirmed in a longitudinal study over 4 years, and in individuals with CV disease, EA decreased slightly (2%) (7), highlighting the importance of performing longitudinal studies.
In healthy individuals, cross-sectional studies have shown that resting ELV increased between 10-28% with age (20-79 years) (15, 19, 47). Further, unlike EA, longitudinal examination of the change in ELV with healthy aging confirmed the cross-sectional reports of a gradual increase in ELV (11%) and that inclusion of people with CV disease did not alter the age-associated increase in ELV (8% increase) (7). It is important to note that the increase in ELV with age is unlikely to be a reflection of an increase in LV contractility, but rather reflects passive stiffening and LV remodeling, reflective of a reduction in the number of cardiac myocytes, an increase LV wall thickness and collagen deposition, with non-enzymatic cross-linking within the heart (34, 35). An important implication of this finding is that a significant component of LV stiffening seems to be mediated by processes that are independent of elevations in arterial load.
A consequence of the age-associated increase in ELV, with a minimal change in EA, ensured that the EA/ELV ratio gradually decreased with increasing age. However, EA/ELV values remained in a narrow range, allowing for optimal energetic efficiency at the expense of mechanical efficacy (7, 15, 19, 47). Although, having a lower resting EA/ELV would mean that older individuals would have less reserve capacity to call upon during times of stress.
With certain disease states, the resting EA/ELV ratio remained stable in people with hypertension, obesity and metabolic syndrome (MetS). We have previously shown that in patients with the MetS who have a clustering of metabolic risk factors (mild hypertension, obesity, elevated glucose and hyperlipidemia) that at rest EA, ELV, and therefore EA/ELV, were similar to healthy controls. However, other groups have shown that with hypertension and obesity, EA and ELV were increased between 15-60% and 16-95% respectively, compared to controls (9, 16, 18, 36, 49). Such elevations in EA and ELV at rest reflect further increases in wall stiffness, wall thickness, with increased reflected pressure waves, (augmenting central systolic pressure).
Although the age-associated increase in EA and ELV maintains EA/ELV within a normal range, the absolute magnitude of both the numerator (EA) and denominator (ELV) is equally important, and has detrimental effects on hemodynamic stability and CV reserve. In young healthy individuals, the low resting EA and ELV, with optimal coupling, maintains an optimal transfer of blood from the LV to periphery without excessive changes in blood pressure, and provides optimal CV flow reserve without compromising arterial pressures (31). However, with increasing age or the presence of CV diseases, the increased resting EA and ELV results in a large change in LV pressures for a given change in LV volume (15, 32). Consequently, the stroke work (myocardial demand) required to perform this task is increased and can potentially have negative consequences on systolic and diastolic function, including coronary flow (i.e., greater dependence upon systolic pressure for coronary flow) (32). Thus, older individuals are working at a higher set point regarding changes in pressure for a given change in loading conditions and this disadvantage is further exaggerated in CV disease. Because the absolute level of any given hemodynamic variable during exercise is determined in part by the resting value for that variable, any elevation in resting EA and ELV (despite matched coupling) would likely reduce the coupling reserve capacity, i.e., rest minus peak values (see next section for more detail).
During Exercise
Exercise provides a powerful tool to examine the response of the CV system to stress, to assess its functional reserve capacity, and to reveal pathophysiological changes that are often hidden from sight at rest. The CV system meets the demands of the exercising tissues by modifying a complex combination of alterations in HR, LV contractility, preload, and afterload. During exercise, the goal of the CV system is to prioritize cardiac efficacy over energetic efficiency, and this is manifested by a decrease in the coupling ratio (i.e., a greater relative increase in ELV than EA). In other words, during exercise the reduction in the coupling ratio reflects a suboptimal set point from the standpoint of LV performance and metabolic efficiency, however, it does reflect an optimization of LV stroke work. The decrease in EA/ELV during exercise is supported from data collected in both animal and human models. In adult dogs, Little and Cheng (43) reported a 25% decrease in EA/ELV from rest to submaximal exercise. In healthy human subjects undergoing supine cycle ergometry, Asanoi et al. (3) observed that EA/ELV decreased by 35% and 54% at workloads corresponding to 30% below and 30% above the anaerobic threshold, respectively; and Chantler et al. (15) found that EA/ELV decreased by approximately 65% (from an average of 0.58 to 0.34, and 0.52 to 0.27 in men and women, respectively) from rest to peak exercise. In these scenarios, the reduction in EA/ELV with exercise is due to an acute increase in ELV which reflects an increase in LV contractility. The response of EA during exercise is dependent on the changes in its components. EA is linearly related to HR and peripheral resistance, and inversely related to compliance (10, 13, 44). Both resistance and compliance usually decrease during exercise (reflecting less resistance to blood flow in the microcirculation, but increased stiffness of the conduit arteries) and the relative contribution of the pulsatile component (compliance) to EA increases, so that by 80% of peak exercise the resistive and the pulsatile components provide nearly equal contributions to EA (44).
With aging, the ability to increase HR, and lower resistance, during exercise is blunted (34, 35), however, the change in EA during exercise was not affected by age (40). Perhaps the blunted changes in resistance, compliance, and HR with age are compensated for by the greater increase in blood pressure during exercise in older vs. younger healthy individuals (43). We have shown that some of the components of the changes in EA seem to be related to each other (11). That is, greater preservation of compliance during exercise is associated with a greater reduction in systemic vascular resistance. This suggests that the tandem changes in vascular resistance and compliance appear to be linked. Further, the change in EA during exercise is also linked to a specific pattern of change in ventricular volumes and function, whereby the change in EA is inversely related to the recruitment of end-diastolic volume, and the enhancement of SV and cardiac output with exercise. Indeed individuals who expressed a large increase in EA during exercise demonstrate a reduced ability to augment SV via the Frank-Starling mechanism (11).
In contrast to EA, there is a clear limitation in the increase in ELV during exercise with age, with noticeable deficits at submaximal workloads, and an approximately 40-55% smaller maximal ELV in individuals 60 vs. 40 years of age (40). This would suggest a decrease in LV contraction during exercise with increasing age are evident at lower exercise workloads, which is clinically significant given that most individuals spend more time during low levels of physical activity. As such, older individuals are working harder at the same submaximal workload than younger individuals. It is known that with aging that LV emptying is substantially impaired during maximal exercise, likely reflecting a stiffer heart, impaired intrinsic myocardial contraction, and reduced cardiac response to β-adrenergic receptor stimulation (26). As a consequence of the impaired ELV response during exercise there is a corresponding blunted reduction in EA/ELV with increasing age (40). Importantly, we have shown that acute pharmacologic reduction in both cardiac and vascular components of LV afterload by sodium nitroprusside (nitric oxide donor) in older, healthy individuals lowered maximal EA/ELV, and therefore represents an enhanced peak exercise CV response similar to levels noted in younger individuals (12, 42). The improved CV response was due to an increase in maximal ELV as the effects of sodium nitroprusside on EA waned at higher levels of exercise, such that peak EA was similar during both sodium nitroprusside and placebo infusions. Further examination of the components of EA reveal that sodium nitroprusside did not alter arterial resistance or compliance at peak exercise. However, as discussed above, EA is less sensitive to changes in compliance, and given the known effects of sodium nitroprusside on reducing arterial wave reflection (and thus pulsatile afterload), the increased peak exercise ELV (and decreased EA/ELV) may have been also due to a reduction in peak exercise arterial wave reflection, especially given the reduction in systolic blood pressure at rest that persisted throughout exercise. Thus, at peak exercise in the old heart, sodium nitroprusside was able to augment the CV performance predominately through increasing LV contractility directly and via a reduction in LV wall stress, and possibly via a reduction in pulsatile component of arterial load.
To what extent the age-associated deterioration in peak exercise EA/ELV is further blunted in the presence of CV disease is limited to a handful of studies, and those studies suggest that the alteration in peak EA/ELV is dependent on the type of disease state. Borlaug et al. (6) showed that hypertensive individuals expressed a similar increase in EA, and ELV, and a decrease in EA/ELV at submaximal and maximal exercise compared to normotensive individuals matched by age and sex. Although a closer look at these data would suggest that the decrease in EA/ELV at peak exercise had started to become blunted with hypertension, and significant differences may have been obtained if the hypertensive response was compared to a healthy control group without obesity or elevated cholesterol. In contrast, we have previously shown that in individuals with systolic hypertensive, but otherwise healthy people, that EA/ELV at 50% of peak exercise, and at peak exercise did not differ compared to healthy age-and sex matched controls, i.e., both groups had a similar decrease in EA/ELV (9). This would suggest that EA/ELV performance at peak exercise is not affected by the presence of hypertension in persons without overt cardiac disease. However, due to the lower resting EA/ELV (i.e., less optimal coupling) in systolic hypertensive women, the EA/ELV reserve (peak-rest EA/ELV) was reduced, which was not evident in men (9). Further, we found that the EA reserve was also reduced in systolic hypertensive men and women, which may a reflect a compensatory response to prevent an excessive increase in arterial load, and therefore permit an adequate ELV increase during exercise. However, what is clear is that more research is needed to tease out how the various forms of hypertension affect the sex-specific coupling response during exercise.
Another modern CV risk factor is obesity, which is at epidemic levels. Similar to hypertension, peak EA/ELV values (and EA/ELV reserve) were similar between obese and none-obese individuals (22). However, this was despite a smaller increase in peak exercise EA (by 27%) and ELV (by 39%) in obese compared to none-obese individuals. Recently, we have shown that when multiple CV/metabolic risk factors cluster together in a given individual, namely MetS, that the reduction in EA/ELV during exercise is blunted compared to healthy age- and sex-matched controls. This limited EA/ELV response was due to a smaller increase in ELV (but not EA) from rest to peak exercise in MetS (28), suggesting that peak exercise LV contractility is reduced in MetS. The discrepancy between the impaired peak coupling response with obesity vs. MetS (for which obesity is a key determinant) suggests that the added CV risk factors that determine MetS (elevated blood pressure and glucose, hyperlipidemia) have a critical role in uncoupling peak arterial and LV interactions. Further, our data in MetS demonstrate that pathophysiological CV alterations occur in the earliest stages of MetS development, prior to any evidence of chronic disease such as diabetes and/or overt CV disease, and that impaired LV systolic function during exercise occurs prior to evidence of LV systolic dysfunction at rest.
So what is the clinical significance of a reduced ability to lower EA/ELV during exercise with age or with certain CV disease states? The reduced EA/ELV reserve with age that is further exacerbated in MetS suggests an inability to attain maximal efficacy, manifested by a limited increase in LV contractility during exercise. Given that EA/ELV is a determinant of CV performance, it is not surprising that the EA/ELV reserve is inversely correlated with peak aerobic capacity in various populations (6, 25, 27). A reduction in peak aerobic capacity represents one of the most important age-and disease-associated physiological changes with regard to quality of life and functional independence. Indeed, peak aerobic capacity is a predictor of all-cause and CV-specific mortality (39). The alterations in resting and exercise EA and ELV likely contribute to this reduced peak aerobic capacity. Whereby, an increased ELV at rest would translate into a less effective increase in ELV during exercise thereby limiting CV performance (6). For example, individuals who start at a higher ELV at rest likely have a limited capacity to further increase SV, and the limited SV response is further exacerbated when a stiff heart is connected to a stiff artery (15, 32). Further, acute infusion of verapamil improved EA/ELV and corresponded with an improved exercise capacity (11). These data suggest a direct link between EA/ELV and aerobic capacity, in that a blunted coupling likely results in a reduced effective transfer of blood from the heart to periphery, thereby reducing functional reserve capacity.
Can Exercise Re-Couple the Heart and Vasculature?
People are living longer, and with an increase prevalence of CV diseases, it is therefore imperative to identify and implement strategies/interventions that will reverse or at the very least delay the development of CV disease. Habitual exercise has been shown to be an effective intervention to improve various aspects of arterial and cardiac systems that impact EA and ELV, although the effects of exercise training on CV performance are in certain circumstances dependent on sex and exercise activity/intensity (aerobic, resistance, aquatics etc.). In general, moderate aerobic exercise interventions reduce blood pressure and aortic arterial stiffness in healthy middle/older men/women (46) and in patients with MetS (23), but do not improve arterail stiffness in the more muscular femoral arteries (41). We have also shown that exercise training can improve central blood pressure, augmentation pressure, and arterial wave reflection in MetS (23). Similarly, brachial artery endothelial function has also been shown to be affected by habitual exercise training. In older men, brisk walking restored brachial artery endothelial function to levels noted in young healthy individuals (21). The improved endothelial function was likely due to an increase in nitric oxide bioavailability and an upregulation in endothelial nitric oxide synthase protein expression and phosphorylation (21). In contrast, the influence of regular aerobic exercise on macrovascular endothelial function with aging in women is far less clear with either no improvements (46) or an increase in endothelial function was noted (53).
Important exercise-induced adaptations also occur to the aged heart including physiological eccentric LV remodeling (3), lower resting HR, improved peak exercise cardiac function (increased SV and reduced end-systolic volume) and aerobic capacity (29, 50). However, in terms of resting LV function (SV, end-diastolic volume, ejection fraction, or LV contractility), moderate/high intensity exercise training for 8-48 weeks in previously healthy sedentary older persons had limited effects (4, 24, 50). Thus, regular exercise training seems to be an effective strategy, for the most part, for combating several adverse cardiac and arterial changes associated with aging. We have previously shown that EA/ELV is moderately correlated with peak aerobic capacity again suggesting a relationship between EA/ELV and fitness (27). However only a handful of studies have examined how exercise training affects EA/ELV at rest and during exercise. We hypothesize that exercise training is an effective therapeutic intervention to “recouple” the interactions between the LV and vasculature.
In young healthy men and women, 8 weeks of aerobic exercise training increased ELV and lowered EA/ELV at rest, but only in women (37). This increase in ELV likely reflected an increase in LV contractility rather than an increase in passive LV stiffness (as evidence with aging or the presence of CV disease), or an attempt to offset an increase in EA, which was not evident in this study. Indeed, irrespective of sex, aerobic exercise did not alter resting EA. This was despite both groups showing lower central pulse wave velocities (a measure of arterial stiffness) (37). Further, the increase in ELV was accompanied by a reduction in ESP which likely allowed for a more efficient CV system, i.e., improved transfer of SV without an excessive increase in pressure. To what extent exercise training in young healthy individuals only showed beneficial effects on resting EA/ELV in women is unknown and highlights the importance of examining the sex-specific changes in CV function after exercise training.
In an older population free of CV disease, Schulman et al. (50) explored the role of exercise training and detraining on CV performance. Although the focus of the study was not on EA/ELV, one can obtain a rough idea as to how EA/ELV was impacted in this study by calculating EA/ELV and its components from reported average data. In healthy older men, 6 months of aerobic exercise training (3-4 times/week) slightly lowered resting EA and ELV. As such, no change in EA/ELV was noted (Figure 2). This is important given that EA and ELV are elevated at rest with age and have important consequences on resting CV efficiency. Thus a decrease in EA and ELV likely improve mechanical efficiency at rest. Whereas at peak exercise, exercise training lowered EA (∼8%) and increased ELV (∼46%), resulting in a lower EA/ELV (∼37%) (Figure 2). The greater reduction in EA/ELV during exercise would have allowed for a greater stroke work and an improved peak aerobic capacity. In this case, the improved EA/ELV was due to a combined effect of EA and ELV. Whereby, the reduction in peak exercise EA after exercise training likely, in part, contributed to the improved ELV (i.e., the LV has less arterial load to contract against). In the same study, 8 master athletes stopped their endurance training for 12 weeks. Exercise detraining had minimal effects on EA and ELV with a slight increase in EA/ELV at rest. However, significant deficits were noted at peak exercise with a decreased ELV (∼12%) and a blunted reduction in EA/ELV (∼32%). This would suggest that highly trained individuals, who have undergone decades of training, are to some extent protected from brief periods of physical inactivity, at least at rest. In contrast, the coupling response to acute exercise is most impacted by deconditioning in highly trained master athletes, and the most significant deconditioning response is a reduced LV contractility.
Figure 2.
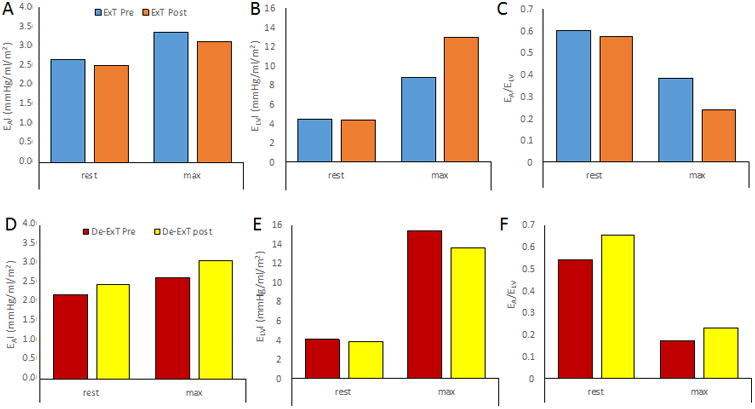
The change in arterial ventricular coupling after aerobic exercise training or detraining. Aerobic exercise training in healthy older individuals had minimal effects on (A) resting arterial elastance indexed to body surface area (EAI) and (B) end-systolic elastance indexed to body surface area (ELVI), but a slight increase in EA/ELV (C) at rest. However, at peak exercise aerobic exercise improved ELVI and lowered EA/ELV in previously sedentary older individuals. In contrast, in master athletes who stopped their endurance training, resting EAI (D), ELVI (E), and EA/ELV (F) was minimally affected by this detraining, whereas exercise detraining decreased ELV (∼12%) and blunted EA/ELV (∼32%) at peak exercise. Created from previously published data Schulman et al. (50).
Exercise training also has beneficial effects on arterial-ventricular coupling in diseased states. We have previously shown that in patients with MetS, who are associated with a three-fold increase risk of CV morbidity and mortality, that aerobic exercise training did not alter resting EA/ELV, EA, and ELV but resulted in a significant increase in peak exercise ELV and consequently a decrease in peak exercise EA/ELV which also corresponded with an improved aerobic capacity in patients with the MetS (Figure 3) (27). Of note, exercise training in these patients also improved lifetime risk score (a predictor for future CV mortality), which was also correlated with EA/ELV (r = 0.50, p<0.05) and ELV (r = - 0.50, p<0.05) reserve, suggesting that the higher the CV risk, the smaller the reduction in EA/ELV and increase in ELV during exercise. We also have unpublished data in older women with MetS (n=9, mean age = 58 years) that 8 weeks of deep water aerobic exercise training did not alter resting EA, ELV, or EA/ELV, however at peak exercise, a 19% increase and a 22% decrease in ELV and EA/ELV was noted respectively (Chantler, PD, unpublished manuscript/observations, October 2016). These data suggest that aquatic-based exercise training can improve LV contractility at peak exercise, resulting in a larger EA/ELV reserve and a greater optimization of CV performance. Such data highlight that various forms of aerobic exercise training are beneficial in improving EA/ELV, in particular exercising in an aquatic environment could benefit obese people who find exercising on a treadmill difficult, or for people with arthritis, etc. These findings have clinical importance as they provide insight that some of the pathophysiological changes associated with MetS can be improved and lower the risk of CV disease.
Figure 3.
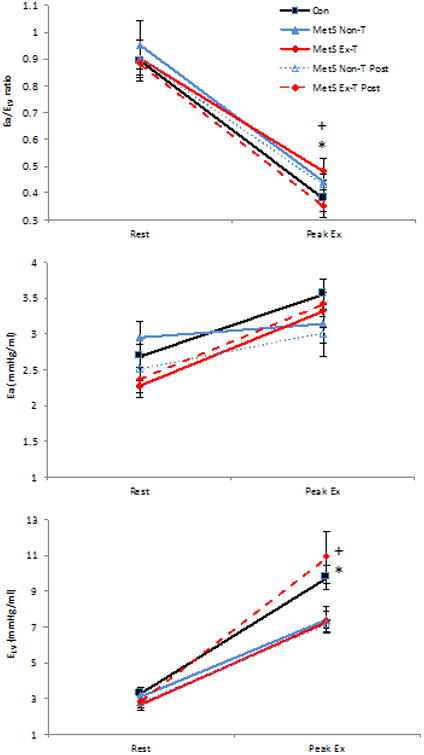
The effects of exercise training on resting and peak exercise arterial-ventricular coupling in patients with the metabolic syndrome (MetS). Change in arterial-ventricular coupling (Ea/ELV), LV end-systolic elastance (ELV), and arterial elastance (Ea) from rest to peak exercise in Mets patients who underwent exercise training (MetS-ExT, diamond) and in MetS who remained inactive (MetS-NonT, triangles). Healthy age- and sex-matched controls are depicted by the solid black line and closed squares. Post intervention for both MetS groups are depicted by a dashed line. Control healthy age-matched individuals are depicted by black squares with a solid line. Exercise training significantly reduced peak Ea/ELV, and increased peak ELV in MetS, and there was a significant time (pre- and post-intervention) by group (MetS-ExT vs. MetS-NonT) for Ea/ELV and ELV. *P<0.05 illustrates significant differences pre and post intervention in MetS Ex-T; +P<0.05 time by group interaction. Data presented as means ± SEM. Created from previously published data, Fournier et al. (27).
Improvements in arterial-ventricular coupling have also been noted in other disease populations. In patients with coronary artery disease, 12 months of aerobic exercise training did not alter resting EA/ELV, or ELV (albeit a slight reduction in EA was evident) (48). However, the major effects of exercise training in this population was noted during hand grip exercise performed at 30% of maximal voluntary contraction, whereby after exercise training a 37% increase in ELV and a 23% reduction in EA/ELV was evident. Similarly, 20 sessions of exercise-based cardiac rehabilitation in heart failure with reduced ejection fraction increased resting EA/ELV from 0.56+0.18 to 0.67+0.21 via a slight (9%) reduction in EA. As such, resting cardiac energetic efficiency was improved, i.e., the ratio of energy transferred to the arterial system (external work) to the energy consumed for this action (2). Collectively, exercise training induces significant CV adaptations during exercise in various populations with different severities of CV disease. Figure 4 summarizes the change in EA/ELV with age and CV disease.
Figure 4.
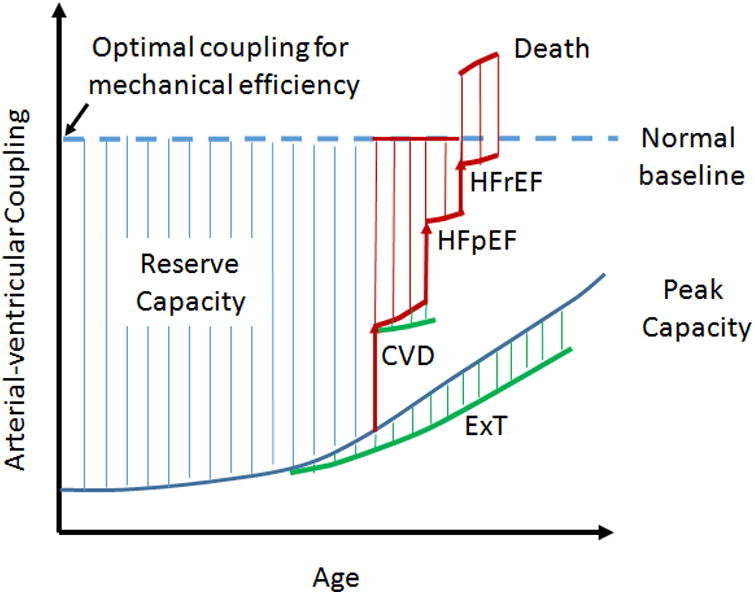
Schematic representation of the changes in Arterial-ventricular coupling as a consequent of age, and cardiovascular disease (CVD: hypertension, obesity, metabolic syndrome). The dashed blue line represents resting EA/ELV and the solid blue line maximal EA/ELV. The hatched area between represents the reserve capacity of EA/ELV. This can be affected by age or cardiovascular disease, and is further acerbated in the presence of heart failure either with preserved ejection fraction (HFpEF) or reduced ejection fraction (HFrEF). However, we hypothesize that exercise training (ExT) improves the EA/ELV reserve capacity with age, and the presence of CVD.
Conclusion
Analyses of the LV and arterial system in the pressure-volume plane, and characterizing these data in a simplified, intuitive and useful approach (EA/ELV), permits the global evaluation of the specific contributions of the arterial system and LV in determining SV and mechanical energetics (external mechanical energy, potential energy, and energetic efficiency). Importantly, to gain further insights into the role of the vasculature and heart on determining CV performance, EA/ELV should be complemented by more specific parameters of arterial (pule wave velocity, pulse wave analysis, characteristic impedance, conduit remodeling, etc.) and cardiac (myocardial contractility, wall stress, chamber size, and thickness) load that provide additional physiological insights into the role of aging and disease.
With advancing age, silent changes occur within the CV systems that uncouple the crosstalk between the heart and arteries, affecting CV efficiency that is further exacerbated in the presence of CV disease (Figure 5). Such age-disease changes limit the CV response to exercise, decreasing ELV and EA/ELV reserve capacity. Importantly, aerobic exercise training has the capacity, at least in part, to “recouple” the interaction between the heart and arterial systems irrespective of disease state (Figure 4).
Figure 5.
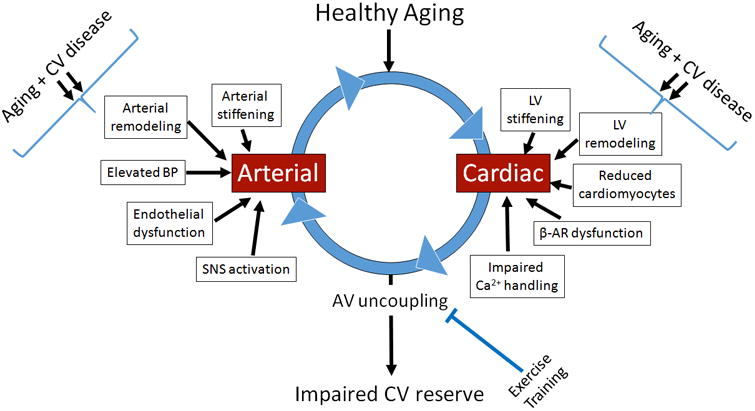
Arterial and cardiac adaptations with healthy aging and the relationship with arterial-ventricular coupling. With healthy aging the arterial and cardiac systems undergo considerable remodeling (change in collagen, reduced elastin, accumulation of advanced glycation end products, loss in cardiomyocytes, and altered autonomic tone), which uncouple the heart and arterial system at rest and during exercise. This uncoupled arterial and cardiac interaction (AV uncoupling) is further exacerbated (double arrows) in the presence of cardiovascular (CV) diseases (such as hypertension and metabolic syndrome) especially during exercise. However, evidence suggests that exercise training can re-couple the interaction between the heart and arterial systems. SNS, sympathetic nervous system; BP, blood pressure; Ca+, calcium; LV, left ventricle; Beta-adrenergic receptors.
Important future contributions to this topic should include the following. The type, frequency, intensity, and duration of exercise training required to preserve/improve EA/ELV with advancing age is incompletely understood. It remains unknown how other exercise modalities, such as resistance training or high intensity interval training affects EA/ELV. Given the reported sex differences in CV function with exercise training, future studies should ensure sufficiently numbers of males and females to adequately compare sex-specific EA/ELV responses.
Key Points.
It is known that healthy aging impairs the coupling between the heart and arterial system.
The age-associated impairment in arterial-ventricular coupling is further increased, especially during exercise, in the presence of cardiovascular diseases.
Exercise training is known to have beneficial effects on the heart and blood vessels.
It is relatively unknown to what extent exercise training can improve the coupling between the heart and blood vessels at rest and during peak performance.
This review article examines how aging and cardiovascular disease alters resting and peak arterial-ventricular coupling and provides evidence indicating that exercise training can recouple this interaction.
Acknowledgments
The author would like his research lab, and the support provided through Center for Cardiovascular and Respiratory Sciences and the Clinical and Translational Sciences Institute at the West Virginia University Health Sciences Center. This work was supported by the American Heart Association (11CRP7370056), the National Institutes of Health (5P20 GM109098), and the National Institute of General Medical Sciences of the National Institutes of Health under Award Number U54GM104942. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure of conflicts of interest: I have no conflicts of interest
References
- 1.Antonini-Canterin F, Enache R, Popescu BA, et al. Prognostic value of ventricular-arterial coupling and B-type natriuretic peptide in patients after myocardial infarction: a five-year follow-up study. J Am Soc Echocardiogr. 2009;22(11):1239–45. doi: 10.1016/j.echo.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Aslanger E, Assous B, Bihry N, Beauvais F, Logeart D, Cohen-Solal A. Effects of Cardiopulmonary Exercise Rehabilitation on Left Ventricular Mechanical Efficiency and Ventricular-Arterial Coupling in Patients With Systolic Heart Failure. J Am Heart Assoc. 2015;4(10):e002084. doi: 10.1161/JAHA.115.002084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggish AL, Wang F, Weiner RB, et al. Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. Journal of Applied Physiology. 2008;104(4):1121–8. doi: 10.1152/japplphysiol.01170.2007. [DOI] [PubMed] [Google Scholar]
- 4.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic Exercise Training Can Reverse Age-Related Peripheral Circulatory Changes in Healthy Older Men. Circulation. 1999;100(10):1085–94. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- 5.Bombardini T, Costantino MF, Sicari R, Ciampi Q, Pratali L, Picano E. End-systolic elastance and ventricular-arterial coupling reserve predict cardiac events in patients with negative stress echocardiography. Biomed Res Int. 2013;2013:235194. doi: 10.1155/2013/235194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borlaug BA, Olson TP, Lam CSP, et al. Global Cardiovascular Reserve Dysfunction in Heart Failure With Preserved Ejection Fraction. Journal of the American College of Cardiology. 2010;56(11):845–54. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borlaug BA, Redfield MM, Melenovsky V, et al. Longitudinal changes in left ventricular stiffness: a community-based study. Circ Heart Fail. 2013;6(5):944–52. doi: 10.1161/CIRCHEARTFAILURE.113.000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. Journal of Applied Physiology. 2008;105(4):1342–51. doi: 10.1152/japplphysiol.90600.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chantler PD, Melenovsky V, Schulman SP, et al. The sex-specific impact of systolic hypertension and systolic blood pressure on arterial-ventricular coupling at rest and during exercise. American Journal of Physiology-Heart and Circulatory Physiology. 2008;295(1):H145–H53. doi: 10.1152/ajpheart.01179.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chantler PD, Melenovsky V, Schulman SP, et al. Use of the Frank-Starling Mechanism during Exercise is Linked to Exercise-Induced Changes in Arterial Load. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.00147.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chantler PD, Melenovsky V, Schulman SP, et al. Use of the Frank-Starling mechanism during exercise is linked to exercise-induced changes in arterial load. American Journal of Physiology - Heart and Circulatory Physiology. 2012;302(1):H349–H58. doi: 10.1152/ajpheart.00147.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chantler PD, Nussbacher A, Gerstenblith G, et al. Abnormalities in arterial-ventricular coupling in older healthy persons are attenuated by sodium nitroprusside. American Journal of Physiology - Heart and Circulatory Physiology. 2011;300(5):H1914–H22. doi: 10.1152/ajpheart.01048.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chemla D, Antony I, Lecarpentier Y, Nitenberg A. Contribution of systemic vascular resistance and total arterial compliance to effective arterial elastance in humans. Am J Physiol Heart Circ Physiol. 2003;285(2):H614–20. doi: 10.1152/ajpheart.00823.2002. [DOI] [PubMed] [Google Scholar]
- 14.Chen CH, Fetics B, Nevo E, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38(7):2028–34. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 15.Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32(5):1221–7. doi: 10.1016/s0735-1097(98)00374-x. [DOI] [PubMed] [Google Scholar]
- 16.Chirinos JA, Rietzschel ER, De Buyzere ML, et al. Arterial load and ventricular-arterial coupling: physiologic relations with body size and effect of obesity. Hypertension. 2009;54(3):558–66. doi: 10.1161/HYPERTENSIONAHA.109.131870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chirinos JA, Rietzschel ER, Shiva-Kumar P, et al. Effective arterial elastance is insensitive to pulsatile arterial load. Hypertension. 2014;64(5):1022–31. doi: 10.1161/HYPERTENSIONAHA.114.03696. [DOI] [PubMed] [Google Scholar]
- 18.Cohen-Solal A, Caviezel B, Himbert D, Gourgon R. Left ventricular-arterial coupling in systemic hypertension: analysis by means of arterial effective and left ventricular elastances. J Hypertens. 1994;12(5):591–600. [PubMed] [Google Scholar]
- 19.Cohen-Solal A, Caviezel B, Laperche T, Gourgon R. Effects of aging on left ventricular-arterial coupling in man: assessment by means of arterial effective and left ventricular elastances. J Hum Hypertens. 1996;10(2):111–6. [PubMed] [Google Scholar]
- 20.De Tombe PP, Jones S, Burkhoff D, Hunter WC, Kass DA. Ventricular stroke work and efficiency both remain nearly optimal despite altered vascular loading. Am J Physiol. 1993;264(6 Pt 2):H1817–24. doi: 10.1152/ajpheart.1993.264.6.H1817. [DOI] [PubMed] [Google Scholar]
- 21.DeSouza CA, Shapiro LF, Clevenger CM, et al. Regular Aerobic Exercise Prevents and Restores Age-Related Declines in Endothelium-Dependent Vasodilation in Healthy Men. Circulation. 2000;102(12):1351–7. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 22.DeVallance E, Fournier SB, Donley DA, et al. Is obesity predictive of cardiovascular dysfunction independent of cardiovascular risk factors? Int J Obes (Lond) 2014 doi: 10.1038/ijo.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donley DA, Fournier SB, Reger BL, et al. Aerobic exercise training reduces arterial stiffness in metabolic syndrome. J Appl Physiol (1985) 2014;116(11):1396–404. doi: 10.1152/japplphysiol.00151.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehsani A, Ogawa T, Miller T, Spina R, Jilka S. Exercise training improves left ventricular systolic function in older men. Circulation. 1991;83(1):96–103. doi: 10.1161/01.cir.83.1.96. [DOI] [PubMed] [Google Scholar]
- 25.Fahs CA, Rossow LM, Yan H, et al. Resting and post exercise arterial-ventricular coupling in endurance-trained men and women. J Hum Hypertens. 2013;27(9):552–6. doi: 10.1038/jhh.2013.7. [DOI] [PubMed] [Google Scholar]
- 26.Fleg JL, Tzankoff SP, Lakatta EG. Age-related augmentation of plasma catecholamines during dynamic exercise in healthy males. J Appl Physiol. 1985;59(4):1033–9. doi: 10.1152/jappl.1985.59.4.1033. [DOI] [PubMed] [Google Scholar]
- 27.Fournier SB, Donley DA, Bonner DE, Devallance E, Olfert IM, Chantler PD. Improved arterial-ventricular coupling in metabolic syndrome after exercise training: a pilot study. Med Sci Sports Exerc. 2015;47(1):2–11. doi: 10.1249/MSS.0000000000000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fournier SB, Reger BL, Donley DA, et al. Exercise reveals impairments in left ventricular systolic function in patients with metabolic syndrome. Exp Physiol. 2014;99(1):149–63. doi: 10.1113/expphysiol.2013.075796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujimoto N, Hastings JL, Carrick-Ranson G, et al. Cardiovascular Effects of One year of Alagebrium and Endurance Exercise Training in Healthy Older Individuals. Circulation: Heart Failure. 2013 doi: 10.1161/CIRCHEARTFAILURE.113.000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kappus RM, Ranadive SM, Yan H, et al. Validity of predicting left ventricular end systolic pressure changes following an acute bout of exercise. J Sci Med Sport. 2013;16(1):71–5. doi: 10.1016/j.jsams.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kass DA. Ventricular Arterial Stiffening: Integrating the Pathophysiology. Hypertension. 2005;46(1):185–93. doi: 10.1161/01.HYP.0000168053.34306.d4. [DOI] [PubMed] [Google Scholar]
- 32.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107(5):714–20. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 33.Ky B, French B, May Khan A, et al. Ventricular-arterial coupling, remodeling, and prognosis in chronic heart failure. J Am Coll Cardiol. 2013;62(13):1165–72. doi: 10.1016/j.jacc.2013.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107(3):490–7. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 35.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346–54. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 36.Lam CS, Roger VL, Rodeheffer RJ, et al. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115(15):1982–90. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lane AD, Yan H, Ranadive SM, et al. Sex differences in ventricular-vascular coupling following endurance training. Eur J Appl Physiol. 2014;114(12):2597–606. doi: 10.1007/s00421-014-2981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milewska A, Minczykowski A, Krauze T, et al. Prognosis after acute coronary syndrome in relation with ventricular-arterial coupling and left ventricular strain. Int J Cardiol. 2016;220:343–8. doi: 10.1016/j.ijcard.2016.06.173. [DOI] [PubMed] [Google Scholar]
- 39.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise Capacity and Mortality among Men Referred for Exercise Testing. New England Journal of Medicine. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 40.Najjar SS, Schulman SP, Gerstenblith G, et al. Age and gender affect ventricular-vascular coupling during aerobic exercise. J Am Coll Cardiol. 2004;44(3):611–7. doi: 10.1016/j.jacc.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 41.Nualnim N, Barnes JN, Tarumi T, Renzi CP, Tanaka H. Comparison of central artery elasticity in swimmers, runners, and the sedentary. Am J Cardiol. 2011;107(5):783–7. doi: 10.1016/j.amjcard.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 42.Nussbacher A, Gerstenblith G, O'Connor FC, et al. Hemodynamic effects of unloading the old heart. Am J Physiol. 1999;277(5 Pt 2):H1863–71. doi: 10.1152/ajpheart.1999.277.5.H1863. [DOI] [PubMed] [Google Scholar]
- 43.Ogawa T, Spina RJ, Martin WH, 3rd, et al. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86(2):494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- 44.Otsuki T, Maeda S, Iemitsu M, et al. Contribution of systemic arterial compliance and systemic vascular resistance to effective arterial elastance changes during exercise in humans. Acta Physiol. 2006;188(1):15–20. doi: 10.1111/j.1748-1716.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- 45.Otsuki T, Maeda S, Iemitsu M, et al. Systemic arterial compliance, systemic vascular resistance, and effective arterial elastance during exercise in endurance-trained men. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2008;295(1):R228–R35. doi: 10.1152/ajpregu.00009.2008. [DOI] [PubMed] [Google Scholar]
- 46.Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond) 2010;120(1):13–23. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and Gender-Related Ventricular-Vascular Stiffening: A Community-Based Study. Circulation. 2005;112(15):2254–62. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 48.Rinder MR, Miller TR, Ehsani AA. Effects of endurance exercise training on left ventricular systolic performance and ventriculoarterial coupling in patients with coronary artery disease. American Heart Journal. 1999;138(1):169–74. doi: 10.1016/s0002-8703(99)70264-4. [DOI] [PubMed] [Google Scholar]
- 49.Saba PS, Ganau A, Devereux RB, Pini R, Pickering TG, Roman MJ. Impact of arterial elastance as a measure of vascular load on left ventricular geometry in hypertension. J Hypertens. 1999;17(7):1007–15. doi: 10.1097/00004872-199917070-00018. [DOI] [PubMed] [Google Scholar]
- 50.Schulman SP, Fleg JL, Goldberg AP, et al. Continuum of Cardiovascular Performance Across a Broad Range of Fitness Levels in Healthy Older Men. Circulation. 1996;94(3):359–67. doi: 10.1161/01.cir.94.3.359. [DOI] [PubMed] [Google Scholar]
- 51.Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol. 1983;245(5 Pt 1):H773–80. doi: 10.1152/ajpheart.1983.245.5.H773. [DOI] [PubMed] [Google Scholar]
- 52.Sunagawa K, Maughan WL, Sagawa K. Optimal arterial resistance for the maximal stroke work studied in isolated canine left ventricle. Circ Res. 1985;56(4):586–95. doi: 10.1161/01.res.56.4.586. [DOI] [PubMed] [Google Scholar]
- 53.Yoshizawa M, Maeda S, Miyaki A, et al. Additive beneficial effects of lactotripeptides intake with regular exercise on endothelium-dependent dilatation in postmenopausal women. Am J Hypertens. 2010;23(4):368–72. doi: 10.1038/ajh.2009.270. [DOI] [PubMed] [Google Scholar]


