Abstract
Opioids are routinely used analgesics in patients with chronic wounds; however the impact of opioid exposure on wound healing is poorly understood. The purpose of this study was to investigate the association between opioid exposure and wound outcome in the WE-HEAL study. This longitudinal observational study was conducted on 450 subjects enrolled in the WE-HEAL biorepository. Data were collected prospectively including baseline characteristics, pain score, longitudinal opioid exposure, and total wound surface area (tWSA). Data were analyzed using static multivariate models, fixed-effects mixed models, and time to event analysis. Using fixed-effects models, opioid dose was significantly associated with tWSA after accounting for the effects of pain score and baseline co-variates (p<0.0001). For each 1-unit increase in ln(opioid dose+1) the ln(tWSA+1) increased by 0.16 units (95% CI 0.13–0.19, p<0.0001). Visits where opioids were present had ln(tWSA+1) 0.48 units larger (95% CI 0.38–0.58, p<0.0001) than visits with no opioid exposure. Using time-to-event analysis, patients who never received opioids healed faster than those who received opioids (log-rank Chi-square 11.00, p 0.0009). Using Cox regression analysis, patients with mean opioid dose ≥10mg were significantly less likely to heal than those with no opioid (HR 0.67 [0.49–0.91], p 0.011) after adjusting for wound size. Patients with opioid dose >0 to <10mg had a similar hazard of not healing as those with no opioid exposure (HR 0.88 [0.65–1.19], p=0.40). In conclusion, opioid analgesics are commonly prescribed to patients with chronic wounds; however, the data presented suggest that opioid exposure is associated with reduced likelihood of healing in patients with chronic wounds. Whether this is a causal relationship will require further study.
Keywords: Chronic Wound, Opioid, Healing, Keratinocyte, Wound Healing, Pain score
INTRODUCTION
Chronic wounds that have failed to heal after 3 months of appropriate wound care affect approximately 6.5 million people in the US with a prevalence of 1% and costs estimated at $25 billion per year (1). In addition to the financial costs, these wounds significantly impact mortality (2) and cause considerable pain, affecting patient reported psychosocial well-being and quality of life (3, 4).
Recalcitrant wounds are often arrested in the inflammatory phase (1, 5–9). Transition of wounds to the healing phase is marked clinically by a dramatic improvement in wound-related pain; however, the molecular mechanisms driving this are not well understood, and it is not known whether more aggressive analgesic management could improve healing outcomes. The pain associated with chronic wounds has been postulated to be related to the stimulation of nociceptors by the complex inflammatory milieu in the wound microenvironment(10). Molecular studies investigating keratinocyte biology and wound healing have shown that functionally active μ-opiate receptors are present on human keratinocytes(11). Activation of these receptors by the μ-opiate receptor agonist β-endorphin in cultured keratinocytes results in upregulation of the type II transforming growth factor-β (TGF-β) receptor and cytokeratin 16 (CK16)(12). The type II TGF-β receptor plays an important role in wound healing; it is expressed in regenerating epithelial cells in acute wounds and in epithelial cells at the margin of chronic wounds. CK16 is a filament protein that is not expressed in normal skin, but appears in the suprabasal compartment of the epidermis during wound healing and in hyperproliferative skin disorders such as psoriasis(13). Additional experiments using cultured keratinocytes show that the CK16 response can be blocked by incubation together with the μ-opiate receptor antagonist naltrexone(11, 12, 14). This finding suggests that hypothetically the clinical use of opioids for patients with chronic wounds might be beneficial in upregulating molecular pathways contributing to wound healing and may be associated with improved clinical outcomes.
Other studies have suggested that opioid use may negatively impact wound healing, by reducing immune activation, impacting tissue oxygenation and angiogenesis(15, 16) and altering myofibroblast recruitment as well as impacting keratinocyte cytokine production, endothelial proliferation and angiogenesis(15–17). In contrast however, untreated pain can also impact wound healing since it potentially impacts tissue perfusion and oxygenation(18, 19) and may interfere with proper wound care, debridement and dressing changes(20).
The purpose of this project was to use data collected through the Wound Etiology and Healing (WE-HEAL) study to investigate the relationship between patient reported pain, opioid exposure and wound outcome in the clinical care of a longitudinal cohort of patients with chronic wounds.
METHODS
Setting and Population
The Wound Healing and Etiology (WE-HEAL) Study (IRB 041408, NCT 01352078) is a prospective biospecimen and data repository that recruits subjects with chronic wounds followed at tertiary referral centers focused on the management of patients with chronic wounds.
Cohort selection
The present analysis was conducted utilizing data from the WE-HEAL subjects who had an open wound at the initial visit, and had more than 1 follow-up visit available for analysis at the time of data abstraction.
Data management for WE-HEAL study
Longitudinal clinical and wound outcome data for the WE-HEAL study were abstracted from the clinical medical record and stored using REDCap(21).
Demographic data, baseline medical comorbidities (including diabetes, autoimmune disease, cardiovascular and renal disease, and smoking exposure), and laboratory data were abstracted from the EHR into the WE-HEAL database at enrollment. Clinical follow-up data were collected at each clinic visit, including wound surface area, wound specific interventions and medication exposures.
Wound Surface Area Assessment
Wound surface area (WSA) was calculated at every visit for each wound using the formula: maximum width × maximum length (cm2). For the purposes of the present analysis, if multiple wounds were present in a single patient on any given clinical visit, the total surface area (tWSA) of wounds was computed by summing the WSA for all wounds.
Pain Score Evaluation
Numerical pain score based on a verbal scale of pain (0–10 with 0 being no pain and 10 being worst pain ever) was collected from every patient, prior to removing dressings, during every clinical interaction. This is a valid and reproducible score that is in routine clinical use (22), and thus is a clinically relevant measure in this analysis. Baseline pain score was used as a covariate in the static multivariate models and time-to-event analyses. Pain was a time-varying covariate in the fixed-effects mixed models. This means that for each time point where wound size was measured, pain was also measured, and was controlled for in the analysis.
Opiate Exposure Calculation
Following each clinic visit, after completion of the medication reconciliation process by the clinical team, data on all prescribed opioids (including oral, topical and intravenous agents) was abstracted into the WE-HEAL Study database. Since medication reconciliation is a metric which is tracked and audited through the EHR, we are confident that it is completed at every clinical interaction. Any discrepancies were resolved by adjudication by two investigators (SM and VKS).
Each individual’s daily morphine-equivalent exposure was determined by first calculating the quantity of opioid medication dispensed by the milligram strength per dosage unit dispensed, then multiplying by the published opioid-specific morphine-equivalent conversion factor (23–25). Total daily opioid dose was calculated by dividing the morphine-equivalent dose per dispensing by the number of days supply dispensed. If opioids from multiple dispensings were ordered on the same day, morphine-equivalent doses were summed. Analysis was conducted using this opioid-equivalent dose per 24 hours, hereafter referred to as opioid dose.
Statistical Analysis
Data distributions were examined for outliers or non-normality. Since wound size and opioid dose were both strongly positively skewed, both were log-transformed prior to analysis, using Ln(x+1) to normalize the distribution. Parametric statistics could then be used to assess their association with other variables.
Opioid treatment, degree of pain, and wound size were expected to be correlated. Patients with larger wounds are expected to have more pain and to be more likely to receive opioids at higher doses, but those with larger wounds may also be less likely to heal. Thus, teasing out the causal effects of opioid treatment on healing was expected to be challenging. We used several statistical methods to try to address this issue. Data analytic methods used in this paper include a) Univariate association of healing status with opioid treatment and other baseline differences, b) Static multivariate logistic regression models which adjust the opioid-healing association for baseline differences (i.e. for possible confounds), c) Time-to-event analyses, including Kaplan-Meier analysis which accounts for time at risk, and Cox regression which adjusts the time-to-event results for baseline differences, and finally e) Dynamic fixed-effects mixed model regression. This last method, which may be unfamiliar to readers, goes beyond static multiple regression approaches, by using each subject’s changing wound size and pain as time-varying predictors, examining differences in wound size over time within patients, as a function of previous wound size, current opioid treatment and pain, at each time point, using each subject as his or her own control. This method addresses the question of whether change in opioid treatment over time within subjects, is associated with change in wound size, while controlling for each subject’s time-varying wound size at the previous visit, and current pain, and for all the relevant static baseline covariates.
Univariate analysis
Baseline differences between patients who had ever, versus never received opioids, as well as between final healing status groups were examined using chi-square or Fisher’s exact test for categorical variables and between-group t-tests for continuous variables. Healing groups were defined as follows:
Never-Healed: Patients who never attainted tWSA=0
Once-Healed: Patients who attained tWSA=0 on at least one measurement period, but not at their final measurement period
Final-Healed: Patients with tWSA=0 at their final measurement period
Ever-Healed: Combined group of both the final- and once-healed groups
Static multivariate models were used to examine predictors of healing status using logistic regression with the following binary dependent variables: a) Final-healed vs. once + never-healed; and b) Never-healed vs. ever-healed.
Time-to-event analysis
Kaplan-Meier analysis was used to examine time to healing (first visit when total wound size=0), stratified by a) whether or not the patient ever received opioids and b) opioid dose based on 3-level ordinal variable outlined above. For this analysis, patients who never achieved total wound size of zero were censored on their last date observed. Finally, Cox regression was used to analyze time to first healing adjusting for baseline differences between groups, using the same stratified opioid variables as well as a continuous log-transformed opioid dose as predictors. In order to test whether the association of opioid dose with time to healing differed at different levels of baseline pain, an opioid by pain interaction term was added to these models.
Dynamic models
Fixed-effects mixed model regression was used to test several models predicting variation in tWSA over time, including time-varying covariates for wound size, pain and opioid dose, as well as static covariates (baseline variables that were associated with opioid use with p<0.10). Each subject in this analysis is used as his or her own control, and the model accounts for within-subject auto-correlation of wound size by nesting observations within subjects. Fixed-effects mixed models were tested for the time-varying outcome variables tWSA and % change in wound size per week. Measures of opioid dose used in these models included a binary indicator variable (ever vs. never exposed), and a 3-level ordinal variable (0mg, >0 to <10mg, >10mg mean daily opioid equivalent dose). The dose effect in these analyses is interpreted as the impact of morphine dose change within subjects, adjusted for previous wound size, pain, and baseline covariates.
Power
We conducted post-hoc power analysis using simulations in order to determine the effect size (i.e, the association between opioid exposure and wound size). We generated 1000 simulated data sets in which we preserved the multivariate associations between the covariates and the auto-correlation within subjects, by using the actual data, but created increasing levels of randomness in the opioid exposure variable, so that we could test our power as the effect size dropped. The strength of the observed univariate association between the exposure variable (3-level ordinal opioid dose) and the outcome (natural log of wound size), across all subjects and time points, was approximated using the R2 as an approximation of the effect size. A fixed-effects mixed model was run on each simulated data set (adjusted for time, pain, last wound size, race, lymphedema, illicit drug use, pulmonary hypertension, sickle cell disease, and alcohol use), the p-value for the randomized opioid exposure variable predicting wound size was saved, and the distribution of these p-values across the 1000 simulations was used to determine power.
Statistical analysis was conducted using SAS software, version 9.3 (SAS Institute Inc., Cary, NC). A two-sided P value of less than 0.05 was considered to indicate statistical significance.
Ethical Considerations
The WE-HEAL study was approved by the institutional review boards at the participating institutions.
Role of the Funding Source
The funding source had no role in the design or conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript.
RESULTS
Characteristics of the patients
Of the 450 patients enrolled in the WE-HEAL study at the time of data export, 445 had at least one follow-up visit and were eligible for inclusion in the current analysis (Figure 1). Mean age was 61.0 ± 14.6, 49% were female, 34% were African American and 65% Caucasian. 329 patients (73.9%) received opioids at some point during follow-up (Table 1). Patients receiving opioids were more likely to be African American, to have used illicit drugs currently or in the past, and to have lymphedema, compared to those who never received opioids. Of the 445 patients, 115 were never-healed, 76 were once-healed, and 254 were final-healed. The ever-healed group combined the once-healed and final healed group (these patients had achieved tWSA=0 at least temporarily) and thus included 330 patients (74.2%).
FIGURE 1. Study enrollment.
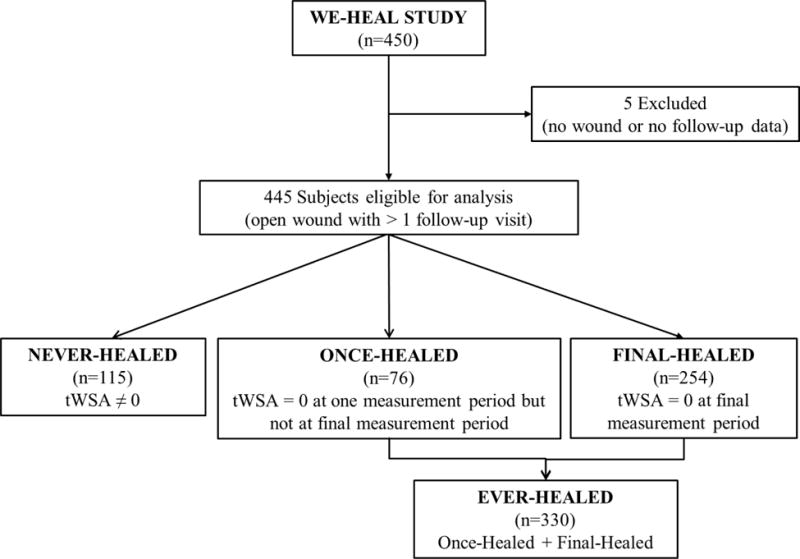
As 450 patients were enrolled in the WE-HEAL study at the time of data analysis. The present analysis was conducted utilizing data from the 445 WE-HEAL subjects who had an open wound at the initial visit, and had more than 1 follow-up visit available for analysis.
Table 1. Baseline patient characteristics by opioid status.
There were no significant differences in baseline characteristics between the group who were exposed to opioids and the group who was never exposed to opioids.
| Variable | Received Opioids (n=329) | Never Received Opioids (n=116) | p |
|---|---|---|---|
|
| |||
| Age mean (sd) | 60.4 (15.0) | 62.7 (13.4) | .14 |
|
| |||
| Sex female | 161 (48.9%) | 55 (47.4%) | .78 |
|
| |||
| Race | .032 | ||
| African American | 115 (35.0%) | 37 (31.9%) | |
| Asian | 3 (0.9%) | 1 (0.9%) | |
| Caucasian | 211 (64.1%) | 78 (67.2%) | |
|
| |||
| Hispanic ethnicity | 11 (3.3%) | 4 (3.5%) | .23 |
|
| |||
| Illicit drug use | .0015 | ||
| Current | 7 (2.1%) | 0 (0%) | |
| Past | 14 (4.3%) | 0 (0%) | |
| Never | 308 (93.6%) | 116 (100%) | |
|
| |||
| Smoking | .14 | ||
| Current | 56 (17.0%) | 11 (9.5%) | |
| Past | 116 (35.3%) | 43 (37.1%) | |
| Never | 157 (47.7%) | 62 (53.5%) | |
|
| |||
| Alcoholic drinks/week | .07 | ||
| 0 | 249 (75.7%) | 75 (64.7%) | |
| >0 to 3 | 37 (11.3%) | 24 (20.7%) | |
| >3 to 7 | 25 (7.6%) | 10 (8.6%) | |
| >7 | 18 (5.5%) | 7 (6.0%) | |
|
| |||
| Any renal disease | 63 (19.2%) | 18 (15.5%) | .38 |
|
| |||
| Renal transplant | 7 (2.1%) | 3 (2.6%) | .26 |
|
| |||
| Dialysis | 13 (4.0%) | 3 (2.6%) | .20 |
|
| |||
| Diabetes mellitus | 140 (42.6%) | 43 (37.1%) | .30 |
|
| |||
| Hepatitis C | 17 (5.2%) | 3 (2.6%) | .25 |
|
| |||
| Hepatitis B | 1 (0.3%) | 2 (1.7%) | .15 |
|
| |||
| HIV positive | 5 (1.5%) | 1 (0.9%) | .35 |
|
| |||
| Venous disease | 104 (31.6%) | 33 (28.5%) | .53 |
|
| |||
| Arterial disease | 87 (26.4%) | 27 (23.3%) | .50 |
|
| |||
| Lymphedema | 32 (9.7%) | 3 (2.6%) | .014 |
|
| |||
| Prothrombotic | 43 (13.1%) | 10 (8.6%) | .20 |
|
| |||
| Prior thrombosis | 58 (17.6%) | 19 (16.4%) | .76 |
|
| |||
| Hypertension | 213 (64.7%) | 74 (63.8%) | .85 |
|
| |||
| Pulmonary hypertension | 12 (3.7%) | 1 (0.9%) | .09 |
|
| |||
| Coronary artery disease | 53 (16.1%) | 20 (17.2%) | .78 |
|
| |||
| Sickle cell disease | 9 (2.7%) | 0 (0%) | .064 |
|
| |||
| Polycythemia Vera | 8 (2.4%) | 1 (0.9%) | .21 |
|
| |||
| Cancer diagnosis | 50 (15.2%) | 14 (12.1%) | .41 |
|
| |||
| Neurologic disorder | 94 (28.6%) | 27 (23.3%) | .27 |
|
| |||
| Hidradenitis suppurativa | 25 (7.6%) | 5 (4.3%) | .22 |
|
| |||
| Connective tissue disease | 89 (27.1%) | 25 (21.6%) | .24 |
N (column %) are shown for categorical variables. Mean (sd) are shown for continuous variables.
Univariate association with healed status
Univariate predictors of the final-healed group included Caucasian race, not having illicit drug use and moderate alcohol use (Table 2A). Final-healed status was negatively associated with venous disease, lymphedema, sickle cell disease, neurologic disorder (Table 2A). Never-healed was associated with African American race, prior or current illicit drug use, lymphedema, prothrombotic states, and sickle cell disease in the univariate analysis (Table 2B). In patients who ever had opioids, 19% were once-healed, 56% were final-healed, and 25% were never-healed. In patients who never had opioids, 10% were once-healed, 61% were final-healed and 28% were never-healed. In this univariate analysis there was no significant difference between the never and ever opioid users (p=0.08).
Table 2. Association of patient variables with healing status.
(A) association with final healing and (B) association with ever healing compared to never healing.
| A. Associations of patient variables with final healing | |||
|---|---|---|---|
|
| |||
| Variable | Final-healed (n=254) |
Never- or once-healed (n=191) |
p |
|
| |||
| Age, years | 60.2 ± 14.7 | 62.0 ± 14.4 | .21 |
|
| |||
| Sex female | 127 (50.0%) | 89 (46.6%) | .48 |
|
| |||
| Race | .006 | ||
| African American | 82 (32.3%) | 70 (36.7%) | |
| Asian | 4 (1.6%) | 0 (0%) | |
| Caucasian | 168 (66.1%) | 121 (63.4%) | |
|
| |||
| Hispanic ethnicity | 9 (3.5%) | 6 (3.1%) | .82 |
|
| |||
| Illicit drug use | .035 | ||
| Current | 4 (1.6%) | 3 (1.6%) | |
| Past | 6 (2.4%) | 8 (4.2%) | |
| Never | 244 (96.1%) | 180 (94.2%) | |
|
| |||
| Smoking | .98 | ||
| Current | 38 (15.0%) | 29 (15.2%) | |
| Past | 90 (35.4%) | 69 (36.1%) | |
| Never | 126 (49.6%) | 93 (48.7%) | |
|
| |||
| Alcoholic drinks/week | .0023 | ||
| 0 | 185 (72.8%) | 139 (72.8%) | |
| >0 to 3 | 36 (14.2%) | 25 (13.1%) | |
| >3 to 7 | 19 (7.5%) | 16 (8.4%) | |
| >7 | 14 (5.5%) | 11 (5.8%) | |
|
| |||
| Any renal disease | 43 (16.9%) | 38 (19.9%) | .42 |
|
| |||
| Renal transplant | 4 (1.6%) | 6 (3.1%) | .14 |
|
| |||
| Dialysis | 11 (4.3%) | 5 (2.6%) | .34 |
|
| |||
| Diabetes Mellitus | 99 (39.0%) | 84 (44.0%) | .29 |
|
| |||
| Hepatitis C | 9 (3.5%) | 11 (5.8%) | .26 |
|
| |||
| Hepatitis B | 1 (0.4%) | 2 (1.1%) | .32 |
|
| |||
| HIV positive | 3 (1.2%) | 3 (1.6%) | .30 |
|
| |||
| Venous disease | 68 (26.8%) | 69 (36.1%) | .034 |
|
| |||
| Arterial disease | 57 (22.4%) | 57 (29.8%) | .08 |
|
| |||
| Lymphedema | 10 (3.9%) | 25 (13.1%) | .0004 |
|
| |||
| Prothrombotic | 26 (10.2%) | 27 (14.1%) | .21 |
|
| |||
| Prior thrombosis | 39 (15.4%) | 38 (19.9%) | .21 |
|
| |||
| HTN | 161 (63.4%) | 126 (66.0%) | .57 |
|
| |||
| Pulmonary hypertension | 4 (1.6%) | 9 (4.7%) | .052 |
|
| |||
| Coronary Artery Disease | 40 (15.8%) | 33 (17.3%) | .67 |
|
| |||
| Sickle cell disease | 2 (0.8%) | 7 (3.7%) | .03 |
|
| |||
| Polycythemia | 4 (1.6%) | 5 (2.6%) | .20 |
|
| |||
| Cancer diagnosis | 31 (12.2%) | 33 (17.3%) | .13 |
|
| |||
| Neurologic disorder | 57 (22.4%) | 64 (33.5%) | .0094 |
|
| |||
| Hidradenitis suppurativa | 24 (9.5%) | 6 (3.1%) | .0086 |
|
| |||
| Connective tissue disease | 59 (23.2%) | 55 (28.8%) | .18 |
| B. Associations of patient variables with ever vs. never healing | |||
|---|---|---|---|
|
| |||
| Variable | Ever-healed (n=330) |
Never-healed (n=115) |
p |
|
| |||
| Age, years | 60.8 ± 14.9 | 61.7 ± 13.8 | .57 |
|
| |||
| Sex female | 159 (48.2%) | 57 (49.6%) | .80 |
|
| |||
| Race | .017 | ||
| African American | 108 (32.7%) | 44 (38.3%) | |
| Asian | 4 (1.2%) | 0 (0%) | |
| Caucasian | 218 (66.1%) | 71 (61.7%) | |
|
| |||
| Hispanic ethnicity | 13 (3.9%) | 2 (1.7%) | .14 |
|
| |||
| Illicit drug use | .01 | ||
| Current | 5 (1.5%) | 2 (1.7%) | |
| Past | 7 (2.1%) | 7 (6.1%) | |
| Never | 318 (96.4%) | 106 (92.2%) | |
|
| |||
| Smoking | .94 | ||
| Current | 49 (14.9%) | 18 (15.7%) | |
| Past | 117 (35.5%) | 42 (36.5%) | |
| Never | 164 (49.7%) | 55 (47.8%) | |
|
| |||
| Alcoholic drinks/week | .64 | ||
| 0 | 244 (73.9%) | 80 (69.6%) | |
| >0 to 3 | 44 (13.3%) | 17 (14.8%) | |
| >3 to 7 | 26 (7.9%) | 9 (7.8%) | |
| >7 | 16 (4.9%) | 9 (7.8%) | |
|
| |||
| Any renal disease | 60 (18.2%) | 21 (18.3%) | .98 |
|
| |||
| Renal transplant | 7 (2.1%) | 3 (2.6%) | .26 |
|
| |||
| Dialysis | 12 (3.6%) | 4 (3.5%) | .23 |
|
| |||
| Diabetes mellitus | 140 (42.4%) | 43 (37.4%) | .34 |
|
| |||
| Hepatitis C | 12 (3.6%) | 8 (7.0%) | .14 |
|
| |||
| Hepatitis B | 2 (0.6%) | 1 (0.9%) | .43 |
|
| |||
| HIV positive | 3 (0.9%) | 3 (2.6%) | .14 |
|
| |||
| Venous disease | 94 (28.5%) | 43 (37.4%) | .075 |
|
| |||
| Arterial disease | 83 (25.2%) | 31 (27.0%) | .70 |
|
| |||
| Lymphedema | 18 (5.5%) | 17 (14.8%) | .0014 |
|
| |||
| Prothrombotic | 32 (9.7%) | 21 (18.3%) | .015 |
|
| |||
| Prior thrombosis | 51 (15.5%) | 26 (22.6%) | .081 |
|
| |||
| Hypertension | 211 (63.9%) | 76 (66.1%) | .68 |
|
| |||
| Pulmonary hypertension | 8 (2.4%) | 5 (4.4%) | .14 |
|
| |||
| Coronary Artery Disease | 53 (16.1%) | 20 (17.4%) | .74 |
|
| |||
| Sickle cell disease | 3 (0.9%) | 6 (5.2%) | .01 |
|
| |||
| Polycythemia | 8 (2.4%) | 1 (0.9%) | .21 |
|
| |||
| Cancer diagnosis | 45 (13.6%) | 19 (16.5%) | .45 |
|
| |||
| Neurologic disorder | 89 (27.0%) | 32 (27.8%) | .86 |
|
| |||
| Hidradenitis suppurativa | 30 (9.1%) | 0 (0%) | .0008 |
|
| |||
| Connective tissue disease | 77 (23.3%) | 37 (32.2%) | .061 |
STATIC MULTIVARIATE MODELS
Predicting never-healed status (versus ever-healed)
The association of log-transformed mean opioid dose with never-healed status was not significant (OR 1.05 [95% CI 0.92–1.20, p=0.46). The only predictor included in the final model predicting never-healed status was log of baseline tWSA. Each 1 unit increase in ln(baseline tWSA+1) was associated with a 38% increase in the odds of never-healing (OR 1.39 [1.22–1.59], p<0.0001). Translating into the units of cm2, a 1-unit increase in ln(baseline tWSA+1) would be equivalent to the difference between 1 and 4.44cm2 or 50 and 137.7cm2.
Predicting final-healed status
Mean opioid dose was not significantly associated with final-healed status (OR 0.99 [0.88–1.12], p=0.90). Predictors that were independently associated with final-healed status included Hidradenitis Suppurativa (OR 2.63 [1.04–6.65], p=0.04) and lymphedema (OR 0.32 [0.15–0.69], p=0.0036). Sickle cell disease (OR 0.22 [0.05–1.11, p=0.067) and first visit tWSA (OR 0.90 [0.79–1.01], p=0.071) did not reach statistical significance.
TIME-TO-EVENT ANALYSIS
Kaplan-Meier
In this analysis the outcome of interest was time-to-healing defined by first occurrence of tWSA= 0. Stratified by the binary variable of opioid exposure, patients who never received opioids healed faster than those who received opioids (log rank chi-square 11.00, p=0.0009, Figure 2). Separation in the survival curves is clear at 26 weeks, with 36.2% patients not healed in the non-opioid exposed group (95% CI 26.2–46.3%) and 49.4% not healed in the opioid-exposed group (95% CI 43.4–55.4%, p=0.006).
Figure 2. Kaplan-Meier survival estimates for non-healing stratified by opioid exposure.
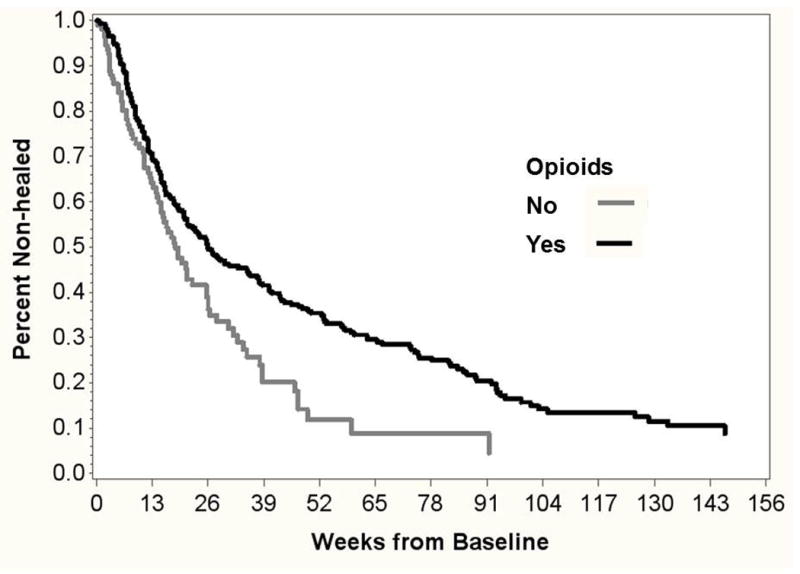
N=401 cases with initial tWSA>0. Time to first occurrence of tWSA= 0 is modeled for those who ever (n=292) vs. never (n=109) received opioids.
Stratifying by opioid dose into 0mg, <10mg and >10mg groups the log-rank chi-square was 19.77 (p<0.0001). Patients who were never exposed to opioids healed faster than those who received mean daily opioid equivalent doses of <10mg per day. Patients who received ≥10mg equivalent dosing per day healed slowest. The estimated percent of patients non-healed by 27 weeks was 36.2% (26.2–46.3%) in the 0mg dose group, 42.9% (34.0–51.8%) in the <10mg daily opioid equivalent dose group and 54.0% (45.9–62.0%) in the patients with >10mg daily opioid equivalent dose (p=0.01). However, baseline opioid dose was positively associated with both baseline wound size and pain level, so it was necessary to adjust for these, and for other relevant covariates in order to obtain an estimate of the independent effect of opioids on healing.
Cox regression
After adjusting for baseline tWSA (log-transformed), pain level, lymphedema, illicit drug use, and sickle cell disease, opioid dose was significantly associated with reduced likelihood of healing (HR 0.71 [0.53–0.94], p=0.019). Using the categorical opioid indicator, patients with mean opioid dose ≥10mg were significantly less likely to heal than those with no opioid (HR 0.60 [0.43–0.83], p=0.0024). Patients with opioid dose >0 to <10mg equivalent did not have a different hazard of not healing than those with no opioid (HR 0.82 [0.60–1.13], p=0.23) (Figure 3). The pain by opioid interaction terms were not significant, either in the model using the binary opioid indicator variable (p=.19) or the 3-level ordinal opioid dose variable (p=.32), indicating that the opioid association with time to first healing did not differ by baseline pain level.
Figure 3. Cox regression survival estimates for non-healing stratified by opioid dose.
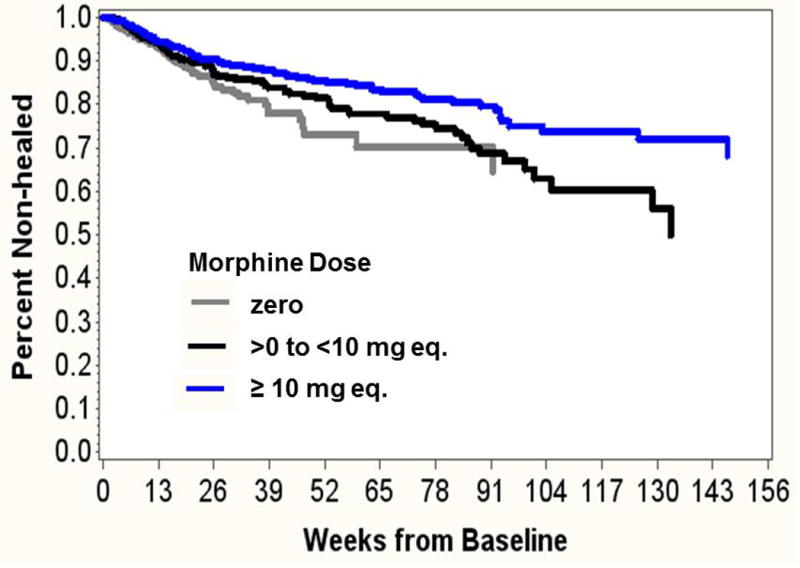
N=401 cases with initial tWSA >0. Time to first occurrence of tWSA= 0 is modeled, stratified by average opioid dose. There were n=109 with mean dose 0; n=127 with mean dose >0 to < 10 mg; and n=165 with mean dose ≥ 10 mg. Adjusted for baseline total wound size, pain level, lymphedema, illicit drug use, sickle cell disease.
DYNAMIC MULTIVARIATE MODELS
The first set of fixed-effects models used pain score and opioid dose as time-varying predictors of wound size. We used three different ways of coding the current opioid dose: a log-transformed continuous variable, a binary indicator, and a 3-level ordinal variable. In these models, the effects of the time-varying predictors are adjusted for the static (baseline) covariates: race, lymphedema, illicit drug use, pulmonary hypertension, sickle cell disease, alcohol use and baseline wound size.
Prediction of tWSA using time-varying opioid dose
After adjusting for the static variables, effects were estimated for time-varying predictors: pain score and log of visit opioid dose. The overall model was significant (F=14.44, p<0.0001) with 59% of the wound size variance coming from between-patient differences and the rest from within-patient differences. Opioid dose was significantly associated with wound size after accounting for the effects of pain score and baseline covariates (p<0.0001). For each 1-unit increase in Ln(opioid dose+1) between visits, Ln (tWSA+1) increased by 0.16 units (95% CI 0.13–0.19, p<0.0001). Translating this back into the original units of measurement, a 1-unit increase in Ln(opioid dose+1) would be equivalent to an opioid dose increase of 0 to 1.72mg or 10 to 28.8mg. A Ln(tWSA+1) increase of 0.16 units would be equivalent to a wound size increase of 0 to 0.175 cm2 or 10 to 11.9 cm2. Pain was also significantly associated with wound size: each 1-unit increase in the pain score was associated with a 0.09 log-unit increase in wound size (95% CI 0.07 to 0.10, p<0.0001), which is equivalent to a change from 0 to 0.094cm2 or 10 to 11cm2.
Prediction of tWSA Using Time-Varying Binary Opioid Indicator
The overall model was significant (F = 14.39, p<0.0001) with 58% of total wound size variance between-subjects and the rest within-subjects. Visits where opioids were present had Ln(tWSA + 1) 0.48 units larger (95% CI 0.38–0.58; p<0.0001) than visits when there was no opioid present. This is equivalent to an increase in tWSA from 0–0.62 cm2 or from 10 to 16.8 cm2. Pain remained significantly associated with wound size. Each 1-unit increase in pain score was associated with an increase in wound size of 0.09 log-units (95% CI 0.08–0.11, p<0.0001). This is equivalent to a change from 0 to 0.094 cm2 or from 10 to 11 cm2.
Prediction of tWSA using time-varying ordinal opioid dose variable
With current opioid dose coded as a 3-level ordinal variable (0, >0 to <10mg, or >10mg), the fixed effects model was significant (F=14.39, p<0.0001) with 59% of the wound size variance between-subjects. Visits where opioid equivalent dose was >0 to <10mg had wound size 0.35 log units larger (95% CI 0.22 to 0.49, p<0.0001) than those with no opioid present. Visits with opioid equivalent dose ≥10mg had wound size 0.56 log units larger than visits with no opioid present (95% CI 0.45 to 0.68, p<0.0001). A 0.35 log unit increase is equivalent to a change from 0 to 0.42 cm2 or 10 to 14.6 cm2. A 0.56 log unit increase is equivalent to a change from 0 to 0.75 cm2 or 10 to 18.3 cm2. For each 1-unit increase in the last visit’s pain score, wound size increased by 0.09 log units (95% CI 0.08 to 0.10, p<0.0001), which is equivalent to an increase from 0 to 0.094 cm2 or 10 to 11cm2.
Since the three methods for coding opioid equivalent dose produced similar results, only the 3-level ordinal coding results are presented for the remaining fixed-effects models.
Prediction of percent-change in tWSA
The model was significant (F=1.56, p<0.0001) with 16% of the variance between-subjects. Opioid dose was not significantly associated with percent-change in tWSA (p=0.90 for >0 to <10 mg, and p=0.13 for ≥10mg, versus no opioid). Pain score was not associated with percent-change in tWSA (p=0.07). Each 1-point increase in pain was associated with 14% increase in tWSA (95% CI −1% to +29%).
Prediction of current tWSA controlling for previous visit tWSA
In this model log-transformed tWSA from the previous visit is used as a time-varying covariate, rather than using a static baseline tWSA adjustment variable. The model was significant (F=18.60, p<0.0001) with 71% of the variance between-subjects. Visits where opioid dose was >0 to <10mg had wound size 0.18 log units larger (95% CI 0.06 to 0.30 units, p=0.0025) than if opioid was absent. Visits where opioid equivalent dose was >10mg had wound size 0.32 log units larger than if opioids were absent (95% CI, 0.22 to 0.42 units, p<0.0001). An increase of 0.18 log units is equivalent to an increase from 0 to 0.2 cm2 or 10 to 12.2 cm2. An increase of 0.32 log units is equivalent to an increase from 0 to 0.38 cm2 or 10 to 14.2 cm2. Each 1-point increase in pain score was associated with an increase of 0.06 log units in current wound size (95% CI 0.05 to 0.08 units, p<0.0001), which is equivalent to an increase from 0 cm2 to 0.065 cm2 or 10 to 10.7 cm2. Each 1 log unit increase in the last visit tWSA was associated with a 0.51 log unit increase in current wound size (95% CI 0.49 to 0.54, p<0.0001).
POST-HOC EMPIRICAL POWER ANALYSIS
The R2 was .081 in the actual data set. In our first set of 1000 simulated data sets the mean R2 across simulations was .062 (95% confidence interval.0617–.0621). Even when we required alpha=.01 for significance, we had power = .994 (exact 95% confidence limits .987 – .998) to detect this effect. We then reduced the effect size further, by allowing for more random variation in the ordinal morphine variable in another set of 1000 simulated data sets, such that the mean R2 across the second set of 1000 simulations was .053 (95% ci .0528–.0533). Again, using alpha=.01, in this second set of simulations, we still had power of .91 (exact 95% confidence limits .894–.930) to detect an effect of opioid dose on wound size across time, after adjusting for wound size at the previous time point, current level of pain, and baseline covariates in a fixed effects mixed model.
DISCUSSION
In patients with chronic wounds, after controlling for confounders including baseline tWSA, medical comorbidities and pain score, opioid exposure was associated with delayed healing. These findings corroborate observations at a cellular and molecular level that opioid exposure may impact keratinocyte biology and wound healing. This data also adds weight to the observation in a large retrospective study investigating risk factors for post-operative wound dehiscence, that opioid exposure in the postoperative period was associated with post-operative wound dehiscence(26).
Other smaller animal based studies have suggested the opioids may enhance collagen deposition and thus tensile strength in incisional wounds(27). However acute incisional wounds studied in that experiment are biologically distinct from the chronic wounds being investigated in the WE-HEAL study and these differences likely account for the absence of beneficial effect seen with opioid exposure in the WE-HEAL cohort.
Findings of this study have important implications for clinical care of patients with chronic wounds, since patients with substantial wound-related pain are frequently prescribed opioids. The data presented here suggest that opioid exposure may adversely impact ultimate wound healing and increase time to healing. There is a continued clinical need to better understand the impact of opioid exposure on wound healing at a cellular and molecular level.
This study does have some limitations which merit discussion. Firstly, higher pain likely contributed to higher opioid exposure in patients with refractory wounds. Conditions that were associated with poor outcomes in this study such as lymphedema, sickle cell disease and hidradenitis are known to also be associated with painful wounds which may have been a confounder. There may have been other confounds that we did not measure or control for. While our multivariate models controlled for confounds such as wound size and pain, and our fixed effects model further reduced the possible effects of reverse-causation, the only way to truly test the causal effect of opiate use or dosage on wound healing would be to randomly vary these interventions in a prospective experiment. Such an experiment would be ethically impossible to undertake in human patients; therefore retrospective data may be the only type of data we will ever have in a clinical sample. The impact of opioids on keratinocyte biology can be studied using in vitro systems and experiments to further investigate this effect at a cellular level are planned.
Investigations of chronic and acute wound specimens show significantly decreased expression of μ-opiate receptors on keratinocytes at the margin of chronic compared to acute wounds(28), suggesting that differential expression of opiate receptors may be important in molecular mechanisms that may contribute to delayed wound healing. Cutaneous activation of the opioid system especially in inflamed and damaged skin can influence cell differentiation and apoptosis, and thus may be important in the mechanisms of skin repair(29). To better understand the molecular mechanisms driving the observed association of opioid exposure and delayed wound healing, we intend to correlate the opiate exposure and clinical outcome data with tissue mRNA expression array data which should allow correlation of clinical data with molecular pathways of opiate action.
A second important limitation is that wound etiologies differed across patients in this study, and it is possible that wound size and level of pain as well as the effect of opioids on healing might vary with wound etiology. This possibility should be further investigated.
A third limitation is that medication records rather than patient diaries were used to assess medication exposure. Since clinicians are required to reconcile the medication list at every clinical interaction, and internal audits show good compliance with this process we believe that the medication records are an accurate representation of opioid prescribing in this study. Alternative methods to confirm compliance, such as pill diaries or therapeutic levels, would not be feasible in this clinical population, but might be appropriate if a prospective clinical trial investigating opiate use in wound healing was planned. It is possible those patients in the study did not comply with all prescribed opioid treatment, or that they had additional opioids from outside the study. However, reduced compliance or outside medications would tend to reduce the observed correlation between opioid treatment and healing, rather than increase it, so, to the extent that poor compliance or outside medication use occurred, the relationship between opioid use and reduced healing would actually be stronger than that observed in this study.
CONCLUSIONS
In conclusion, we found that patients in the WE-HEAL study who never received opioids healed faster than those who received opioids. Opioid exposure was a strong predictor of wound size and patients who received opioids at doses above 10mg per day had slower rates of healing than those with no exposure or dosing less than 10mg per day.
Table 3. Logistic regression models.
Predictors included log of mean opioid dose along with those baseline patient variables that had p<.10 with final healing: race, alcohol, illicit drug use, venous disease, lymphedema, sickle cell disease, neurological disorder, pulmonary hypertension, hidradenitis supportiva. Stepwise inclusion was used with p<.10 required for model entry and exit (i.e. variables not listed in the table were not associated with final healing, p>.10), except that opioid dose was retained in the model regardless of its p-value.
| Effect | Odds Ratio | 95% Wald Confidence Limits | P | |
|---|---|---|---|---|
| A. Predict Final-Healed (vs. Once + Never-Healed) | ||||
| Opioid Dose | 0.993 | 0.881 | 1.119 | 0.90 |
| Hidradenitis Suppurativa | 2.633 | 1.043 | 6.648 | 0.04 |
| Lymphedema | 0.315 | 0.145 | 0.684 | 0.0036 |
| Sickle Cell | 0.224 | 0.045 | 1.109 | 0.067 |
| Baseline log tWSA | 0.895 | 0.794 | 1.010 | 0.071 |
| B. Predict Never-Healed (vs. Ever-Healed) | ||||
| Opioid dose | 1.051 | 0.922 | 1.199 | 0.46 |
| Baseline log tWSA | 1.390 | 1.219 | 1.585 | <0.0001 |
Acknowledgments
This work was supported by award R01NR013888 from the National Institute of Nursing Research and by award number UL1 TR000075 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through the Clinical and Translational Science Awards Program (CTSA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Shanmugam had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Shanmugam, Amdur
Acquisition of data. Shanmugam, McNish,
Analysis and interpretation of data. Shanmugam, McNish, Couch, Amdur
References
- 1.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair and Regeneration. 2009;17(6):763–71. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Escandon J, Vivas AC, Tang J, Rowland KJ, Kirsner RS. High mortality in patients with chronic wounds. Wound Repair and Regeneration. 2011;19(4):526–8. doi: 10.1111/j.1524-475X.2011.00699.x. [DOI] [PubMed] [Google Scholar]
- 3.Price P, Harding K. The impact of foot complications on health-related quality of life in patients with diabetes. J Cutan Med Surg. 2000;4(1):45–50. doi: 10.1177/120347540000400112. [DOI] [PubMed] [Google Scholar]
- 4.Price P, Harding K. Cardiff Wound Impact Schedule: the development of a condition-specific questionnaire to assess health-related quality of life in patients with chronic wounds of the lower limb. International Wound Journal. 2004;1(1):10–7. doi: 10.1111/j.1742-481x.2004.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–21. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 6.Shanmugam V, DeMaria D, Attinger C. Lower extremity ulcers in rheumatoid arthritis: features and response to immunosuppression. Clinical Rheumatology. 2011;30(6):849–53. doi: 10.1007/s10067-011-1710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanmugam V, Price P, Attinger C, Steen V. Lower Extremity Ulcers in Systemic Sclerosis: Features and Response to Therapy. Int J Rheumatol. 2010 doi: 10.1155/2010/747946. pii:747946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shanmugam VK, McNish S, Shara N, Hubley KJ, Kallakury B, Dunning DM, et al. Chronic Leg Ulceration Associated with Polycythemia Vera Responding to Ruxolitinib (Jakafi®) The Journal of Foot and Ankle Surgery(0) doi: 10.1053/j.jfas.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shanmugam VK, Schilling A, Germinario A, Mete M, Kim P, Steinberg J, et al. Prevalence of immune disease in patients with wounds presenting to a tertiary wound healing centre. International Wound Journal. 2011:1–9. doi: 10.1111/j.1742-481X.2011.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein C, Küchler S. Targeting inflammation and wound healing by opioids. Trends in pharmacological sciences. 2013;34(6):303–12. doi: 10.1016/j.tips.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Bigliardi-Qi M, Bigliardi PL, BÜChner S, Rufli T. Characterization of μ-Opiate Receptor in Human Epidermis and Keratinocytes. Annals of the New York Academy of Sciences. 1999;885(1):368–71. doi: 10.1111/j.1749-6632.1999.tb08692.x. [DOI] [PubMed] [Google Scholar]
- 12.Bigliardi-Qi M, Bigliardi P, Eberle A, Buchner S, Rufli T. [beta]-Endorphin Stimulates Cytokeratin 16 Expression and Downregulates [mu]-Opiate Receptor Expression in Human Epidermis. J Investig Dermatol. 2000;114(3):527–32. doi: 10.1046/j.1523-1747.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- 13.Gerritsen MJP, Elbers ME, de Jong EMGJ, van de Kerkhof PCM. Recruitment of cycling epidermal cells and expression of filaggrin, involucrin and tenascin in the margin of the active psoriatic plaque, in the uninvolved skin of psoriatic patients and in the normal healthy skin. Journal of Dermatological Science. 1997;14(3):179–88. doi: 10.1016/s0923-1811(96)00570-1. [DOI] [PubMed] [Google Scholar]
- 14.Bigliardi-Qi M, Sumanovski LT, Büchner S, Rufli T, Bigliardi PL. Mu-Opiate Receptor and Beta-Endorphin Expression in Nerve Endings and Keratinocytes in Human Skin. Dermatology. 2004;209(3):183–9. doi: 10.1159/000079887. [DOI] [PubMed] [Google Scholar]
- 15.Martin JL, Charboneau R, Barke RA, Roy S. Chronic morphine treatment inhibits LPS-induced angiogenesis: Implications in wound healing. Cellular Immunology. 2010;265(2):139–45. doi: 10.1016/j.cellimm.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin JL, Koodie L, Krishnan AG, Charboneau R, Barke RA, Roy S. Chronic Morphine Administration Delays Wound Healing by Inhibiting Immune Cell Recruitment to the Wound Site. The American Journal of Pathology. 2010;176(2):786–99. doi: 10.2353/ajpath.2010.090457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rook JM, Hasan W, McCarson KE. Morphine-induced early delays in wound closure: Involvement of sensory neuropeptides and modification of neurokinin receptor expression. Biochemical Pharmacology. 2009;77(11):1747–55. doi: 10.1016/j.bcp.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akca O. Pain and Tissue Oxygenation. Crit Care Med. 2015;43(10):e462–3. doi: 10.1097/CCM.0000000000001126. [DOI] [PubMed] [Google Scholar]
- 19.Hoiseth LO, Hisdal J, Hoff IE, Hagen OA, Landsverk SA, Kirkeboen KA. Tissue oxygen saturation and finger perfusion index in central hypovolemia: influence of pain. Crit Care Med. 2015;43(4):747–56. doi: 10.1097/CCM.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 20.Woo KY, Abbott LK, Librach L. Evidence-based approach to manage persistent wound-related pain. Curr Opin Support Palliat Care. 2013;7(1):86–94. doi: 10.1097/SPC.0b013e32835d7ed2. [DOI] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gulur P, Soldinger SM, Acquadro MA. Concepts in Pain Management. Clinics in podiatric medicine and surgery. 2007;24(2):333–51. doi: 10.1016/j.cpm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Boudreau D, Von Korff M, Rutter CM, Saunders K, Ray GT, Sullivan MD, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiology and Drug Safety. 2009;18(12):1166–75. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raebel MA, Newcomer SR, Reifler LM, et al. CHronic use of opioid medications before and after bariatric surgery. JAMA. 2013;310(13):1369–76. doi: 10.1001/jama.2013.278344. [DOI] [PubMed] [Google Scholar]
- 25.Korff MV, Saunders K, Thomas Ray G, Boudreau D, Campbell C, Merrill J, et al. De Facto Long-term Opioid Therapy for Noncancer Pain. The Clinical Journal of Pain. 2008;24(6):521–7. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shanmugam VK, Fernandez S, Evans KK, McNish S, Banerjee A, Couch K, et al. Postoperative wound dehiscence: predictors and associations. Wound Repair and Regeneration. 2015 doi: 10.1111/wrr.12268. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang PJ, Chen MY, Huang YS, Lee CH, Huang CC, Lam CF, et al. Morphine enhances tissue content of collagen and increases wound tensile strength. J Anesth. 2010;24(2):240–6. doi: 10.1007/s00540-009-0845-1. [DOI] [PubMed] [Google Scholar]
- 28.Bigliardi PL, Sumanovski LT, Buchner S, Rufli T, Bigliardi-Qi M. Different Expression of [mu]-Opiate Receptor in Chronic and Acute Wounds and the Effect of [beta]-Endorphin on Transforming Growth Factor [beta] Type II Receptor and Cytokeratin 16 Expression. J Invest Dermatol. 2003;120(1):145–52. doi: 10.1046/j.1523-1747.2003.12018.x. [DOI] [PubMed] [Google Scholar]
- 29.Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, Bigliardi-Qi M. Opioids and the skin – where do we stand? Experimental Dermatology. 2009;18(5):424–30. doi: 10.1111/j.1600-0625.2009.00844.x. [DOI] [PubMed] [Google Scholar]


