Abstract
Regular exercise is promoted as a therapeutic strategy for age-associated endothelial dysfunction. Improvements in endothelial function are observed with endurance exercise in older men, but are diminished or absent in older women. This review examines the hypothesis that sex hormones modulate vascular adaptations to exercise training by influencing antioxidant defense systems, mitochondrial function, oxidative stress, and intracellular signaling.
Keywords: exercise, vascular function, oxidative stress, mitochondria, estrogen receptor, sex hormones, women
INTRODUCTION
Cardiovascular disease (CVD) has been the leading cause of death in the United States for the past century (26). Although CVD mortality has declined significantly over the previous three decades, the mortality trend differs greatly by sex, with steeper rates of decline in men compared to women (26). In fact, CVD mortality has increased in women 35-54 years old, and compared to men, more women aged 45 and older die within a year after suffering a myocardial infarction (26). The reasons for this sex disparity are unclear, but demonstrates the urgent need to understand the sex-specific pathophysiology of CVD for the development of therapeutic strategies for CVD prevention in women and men.
For the greater part of the century, women were mostly excluded from research studies because of the potential influence of varying sex hormone levels across the menstrual cycle and the menopause transition. As such, little was known about the etiology of CVD in women. Today, women comprise ~50% of research study participants, thanks in large part to the establishment of the National Institutes of Health (NIH) Office of Women’s Health Research in 1990 and the passage of the NIH Revitalization Act in 1993, which mandated the inclusion of women in NIH-funded research. Since then, we have learned much more about the manifestation of CVD in women and that some treatments that are effective for CVD prevention in men such as aspirin, are not as effective for CVD prevention in women. Despite these advances, many research studies still fail to consider potentially important sex differences when designing clinical investigations. This is especially critical when designing clinical trials aimed at prevention or treatment of disease as the response to drugs and interventions may be sex dependent. In a recent report, the US Food and Drug Administration (FDA) retrospectively found that among medications withdrawn by the FDA from 1997-2000, more harms were incurred by women than by men. This underscores the importance of sex differences, and the need to implement effective sex-specific strategies for the prevention and treatment of CVD.
One of the salient sex differences in the risk for CVD is vascular aging, a major risk factor for age-associated CVD. Endothelial dysfunction, characterized by impaired endothelial-dependent dilation, is a phenotypic feature of vascular aging, and is the key antecedent in the initiation and progression of atherosclerotic CVD. Vascular aging in women is unique, in that their arteries are also exposed to adverse changes in CVD risk factors (e.g., blood pressure, adiposity changes) during the menopause transition where profound changes in sex hormone levels are occurring. Indeed, the menopause transition appears to be a triggering event that leads to accelerated age-associated endothelial dysfunction (24, 39). Notably, premenopausal women have a lower incidence of CVD compared with age-matched men (26). This apparent female protection has long been attributed to the potent vasodilatory, antioxidant, anti-inflammatory and anti-proliferative effects of estrogen on the endothelium. As such, the accelerated decline in endothelial function around the menopause transition has been attributed to in a decline in ovarian function and the loss of endothelial protection by circulating estrogens (24, 39).
The Women’s Health Initiative finding that estrogen therapy was not effective for the prevention of CVD when initiated 10-20 years after menopause cast doubt on the cardioprotective effects of estrogen (34). Thus, CVD prevention strategies have shifted to encourage lifestyle recommendations such as physical activity and exercise programs in older women. Although regular exercise is promoted as a therapeutic strategy for delaying and improving vascular aging, there is growing awareness of potential sex differences in the beneficial effects of exercise, with lesser benefit on vascular health in women (25, 28, 30, 37). The reasons for this are unclear, but may be related to sex differences in the plasticity of the aging vasculature in response to exercise (28). We (25) and others (4, 30, 37) have shown that the improvements in endothelial function with endurance exercise training observed in older men are diminished or absent in older women. Because sex hormones have a modulatory influence on vascular aging, sex-related differences in vascular adaptations to exercise may be related to the marked, relatively abrupt reduction in circulating estrogen with menopause in women. In this regard, we recently demonstrated that endothelial function is improved with endurance exercise training in estrogen treated postmenopausal women, but not estrogen-deficient postmenopausal women, suggesting an essential role of estrogen in endurance exercise training effects on the vasculature in women (25). The reasons for the diminished vascular benefits to endurance exercise training in estrogen-deficient women are not completely understood. Our overarching hypothesis is that sex hormones modulate vascular adaptations to exercise training, particularly in women, and that sex hormone deficiency impairs intracellular signaling and antioxidant defense systems, decreasing the resistance of aged arteries to oxidative damage, consequently preventing improvements in endothelial function with exercise training (Figure 1). Thus, current exercise recommendations may need to be refined to consider the hormonal state of the woman in order to optimize exercise training benefits to her vascular health. This review article will focus on the hypothesized mechanisms for the modulatory influence of sex hormones in exercise training benefits on vascular endothelial function in women.
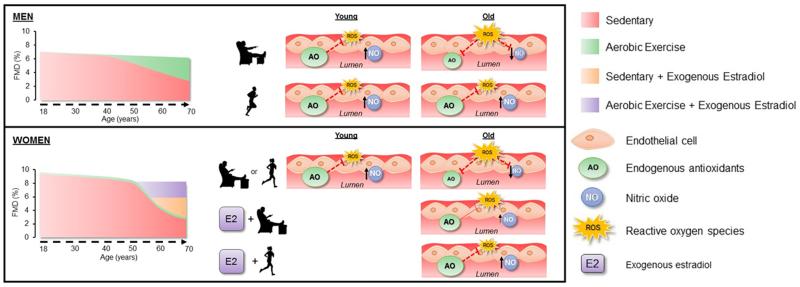
Figure 1.
Working Hypothesis by which estrogen-deficiency limits endothelial adaptations to exercise in healthy older women. In men, aging is associated with a gradual decline in brachial artery flow mediated dilation (FMD) up until age 40, with a faster rate of decline thereafter. Chronic exercise training preserves FMD with aging, partly due to reductions in reactive oxygen species (ROS), greater production of endogenous antioxidants (AO), resulting in increased nitric oxide (NO) production. Conversely, in women regardless of training status, the decline in FMD is attenuated up to the age of 50, after which there is an acceleration in the decline in FMD likely due to the menopause transition and decreased estrogen concentrations. The loss of estrogen in postmenopausal women likely impairs the antioxidant defense system, despite chronic aerobic exercise training, thereby increasing ROS levels that reduce NO concentrations. However, estradiol treatment in sedentary older women attenuates the age-related decline in endothelial function, whereas duel aerobic exercise training and estradiol treatment restores endogenous antioxidant defense systems and increases the resistance to oxidative damage, thus, restoring brachial artery endothelial function close to youthful levels.
SEX DIFFERENCES IN VASCULAR AGING
The vascular endothelium plays a key role in the maintenance of vascular health, and thus the loss of normal endothelial function is believed to be a critical step in the initiation and progression of atherosclerosis. Aging is associated with a progressive decline in endothelial function, however, the effects of aging on the vascular endothelium appear to be sex-specific. In general, premenopausal women have a healthier vascular endothelium compared to age-matched men (24, 30). Endothelial function gradually declines in men around the 4th decade of life, whereas in women, the decline begins about a decade later, then accelerates to where sex differences are no longer observed by the 6th decade of life (39). The sex-related differences in the decline in endothelial dysfunction have been attributed to changes in gonadal function with aging, particularly in women (39). Consistent with this, we recently demonstrated that ovarian function and circulating estrogen levels appear to be strong modulators of the vascular aging process in women (24). Although endothelial function was impaired in women who were categorized as early perimenopause (i.e., those demonstrating menstrual cycle length changes ≥7 days) compared to premenopausal women, these women still had robust endothelial function. In contrast, women who were of the same age as the early perimenopausal women, but categorized as late perimenopausal (i.e., missing 2 or more consecutive menstrual cycles but < 12 months of amenorrhea), had double the rate of decline from premenopausal women. Endothelial function was further reduced in postmenopausal women. Additionally, we and others demonstrate that endothelial function can be improved with estrogen treatment (25, 41). Collectively, these data support the idea that gonadal aging and declines in estrogen levels contribute to age-associated endothelial dysfunction in women.
SEX DIFFERENCES IN ENDOTHELIAL ADAPTATIONS TO EXERCISE TRAINING IN OLDER ADULTS
Historically, endurance exercise training has been shown to attenuate or ameliorate the age-related decline in macrovascular and microvascular endothelial function in older men (4, 30). However, surprisingly, this is not consistently observed in older (postmenopausal) women (25, 30), suggesting that the adaptive responses to endurance exercise training in older adults may be sex-specific. Pierce et al., demonstrated that macrovascular endothelial function measured via brachial artery flow-mediated dilation (FMD) increased ~50% in response to an 8 week moderate-intensity exercise training program (i.e., walking) in previously sedentary middle-aged and older men, but did not change in age-matched postmenopausal women (30). The authors corroborated the intervention findings with results of a very large cross-sectional analysis of FMD measured in endurance-trained and sedentary older men and women (30). Differences in training volume did not appear to explain the sex-specific endothelial adaptations to exercise training. These findings were consistent with previous cross-sectional studies in sedentary versus endurance-trained older men (4, 7), and in endurance exercise training intervention studies in postmenopausal women (25, 37). Although selective studies using small sample sizes and/or different measurements of endothelial function have reported inconsistent results (1), the majority of the literature suggests that the response of endothelial function to endurance exercise training in healthy postmenopausal women is diminished compared to middle-aged and older men.
SEX HORMONE MODULATION OF ENDOTHELIAL FUNCTION TO EXERCISE TRAINING
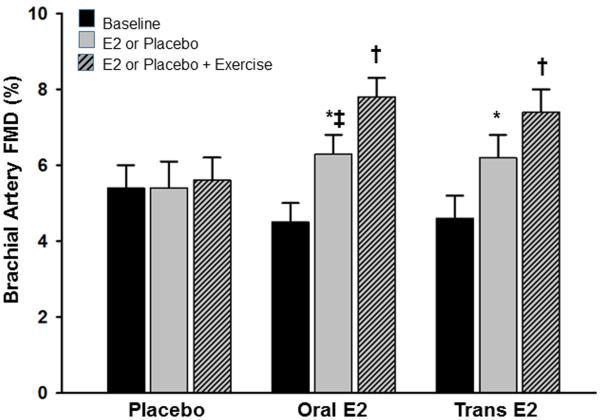
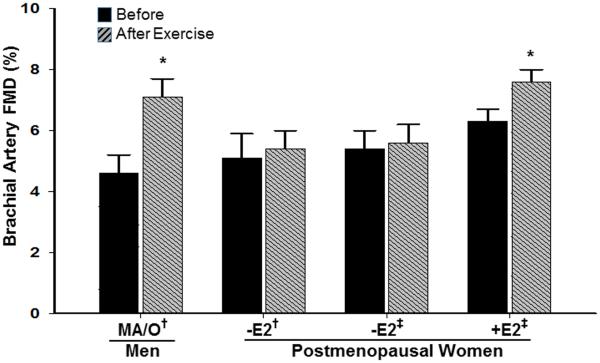
As mentioned above, sex hormones appear to have a modulatory influence on vascular aging in women and men, and as such, it has been suggested that sex-specific endothelial adaptations to exercise training could be related to differential exposure to sex hormones (28, 30). In women, endogenous sex hormone concentrations undergo an abrupt decrease with menopause, however, in men, a parallel change is not observed (11). Only 10-15% of 60 yr-old, 30% of 70 yr-old and 50% of 80 yr-old men have total serum testosterone levels below the normal range for young men (11). Thus, the lack of exercise training benefit on endothelial function in postmenopausal women could be related, in part, to a decline in sex hormones, particularly estrogen. In support of this, we recently reported that brachial artery FMD increased following a 12 week moderate intensity endurance exercise training program in previously sedentary postmenopausal women who were treated with either oral or transdermal estrogen (Figure 2) (25). However, postmenopausal women who were treated with placebo did not show FMD improvements with exercise training, consistent with previous observations in estrogen-deficient postmenopausal women (Figure 3) (29). These data provided the first direct evidence that estrogen is necessary to induce beneficial effects of endurance exercise training on endothelial function in postmenopausal women. Our hypothesis that estrogen plays a permissive role in endothelial adaptations to endurance exercise training in women is supported by endothelial function studies conducted in amenorrheic athletes, and leg exercise blood flow studies in perimenopausal and postmenopausal women. First, highly trained amenorrheic premenopausal athletes have reduced brachial artery FMD compared with eumenorrheic athletes and sedentary controls (27, 32, 44). Additionally, brachial artery FMD is improved in amenorrheic premenopausal athletes to levels observed in eumenorrheic athletes and sedentary controls with recovery of menses (44), and with oral contraceptives (33). Second, leg blood flow and vascular conductance during single-leg knee extensions was attenuated in late perimenopausal and postmenopausal compared to early perimenopausal women, suggesting that leg vasodilation and exercise hyperemia are impaired in states of reduced estrogen concentrations (22).
Figure 2.
Endothelial function measured via brachial artery flow-mediated dilation (FMD) before and after 12 weeks of placebo, or oral or transdermal estradiol treatment, and an additional 12 weeks of placebo or estradiol treatment plus aerobic exercise training. *P<0.01 vs baseline; † P<0.01 vs. 12 weeks; ‡P<0.01 vs placebo 12 weeks. (Reprinted from (25). Copyright 2013 Endocrine Society. Used with permission.)
Figure 3.
Sex differences in the improvement of brachial artery flow-mediated dilation (FMD) to endurance-exercise training in middle-aged/older (MA/O) men, and estrogen-deficient (E2) and estrogen-replete (E2) postmenopausal women. *, P<0.05 vs before; †, adapted from Pierce et al. (30); ‡, adapted from Moreau et al (25). (Reprinted from (25). Copyright 2013 Endocrine Society. Used with permission.)
POTENTIAL MECHANISMS UNDERYLING DIMINSHED VASCULAR EXERCISE TRAINING BENEFITS IN OLDER WOMEN
The reasons for the apparent sex-specificity in exercise training effects on the vascular endothelium, specifically diminished responses in estrogen-deficient postmenopausal women compared to age-matched men, are unknown. To identify potential reasons for this phenomenon, it is helpful to understand the mechanisms underlying age-associated endothelial dysfunction and the reported anti-aging effects of endurance exercise training on endothelial function.
Oxidative Stress
Age-associated endothelial dysfunction is attributed to reduced NO bioavailability, secondary to vascular oxidative stress (7, 25, 40). Oxidative stress represents the imbalance between the production and destruction of reactive oxygen species (ROS) by antioxidant defense systems. Aging is associated with excessive ROS generation within multiple cellular compartments including the plasma membrane (e.g., nicotinamide adenine dinucleotide phosphate [NADPH] oxidase), peroxisomes (e.g., lipid oxidation), mitochondria (e.g., oxidative phosphorylation) and cytoplasm (e.g., xanthine oxidase), in the absence of compensatory increases in antioxidant defense systems (e.g., superoxide dismutase [SOD], catalase, glutathione). Excessive ROS production impairs endothelial function by scavenging NO and by decreasing NO biosynthesis (17). The latter can occur by altering the metabolism of arginine, the substrate for NO production, and/or by oxidizing the enzyme that produces NO, endothelial nitric oxide synthase (eNOS) and its co-factors (i.e., tetrahydrobioptern [BH4]). Both processes would cause eNOS to uncouple, resulting in a greater production of superoxide and less NO, further contributing to vascular oxidative stress and reduced NO.
Mitochondrial Dysfunction
Mitochondrial dysfunction is a primary source of oxidative stress and plays a central role in vascular aging (3). Mitochondria are the power plants of cells and are important for maintaining cellular bioenergetics via oxidative phosphorylation. In the vasculature, mitochondria are important for many functions beyond ATP generation. Mitochondrial networks intersect with the nucleus and endoplasmic reticulum and serve as oxygen, nutrient and calcium sensors, playing critical roles for cell signaling, cellular redox homeostasis and regulation of programmed cell death. Mitochondria are both a source and target of ROS. As mentioned above, ROS (superoxide and hydrogen peroxide [H2O2]) are generated by the mitochondria via the electron transport chain (ETC) and oxidative phosphorylation (3). Even though mitochondria are equipped with antioxidant systems (i.e., manganese-dependent [Mn] SOD) that detoxify ROS, with aging mitochondria membranes, proteins and DNA become damaged from ROS and reactive nitrogen species (RNS) and produce excessive amounts of ROS which overwhelms antioxidant defenses (3). Damaged mitochondria can lead to alterations in cell bioenergetics, redox signaling and cell function. Excess mitochondrial-mediated oxidative damage has been shown to cause age-related endothelial dysfunction (3, 10).
Estrogen may benefit endothelial function through effects on mitochondrial function. Mitochondrial DNA (mtDNA) contains estrogen response elements and is elevated in cerebral blood vessels of ovariectomized mice treated with estrogen compared to placebo (14). Moreover, proteins involved in mitochondrial energy production and biogenesis (i.e., Nuclear respiratory factor [NRF]-1, peroxisome proliferator-activated receptor-γ coactivator-1 alpha [PGC-1α]) are elevated and mitochondrial ROS (i.e., H2O2) is reduced in the cerebral vasculature of ovariectomized rats treated with estrogen compared to placebo (14). Thus, estrogen appears to protect the vasculature in part by modulating mitochondrial function, increasing the efficiency of mitochondria and resulting in less mitochondrial ROS production.
Exercise Training Effects on Oxidative Stress and Mitochondrial Function
It is well established that acute exercise promotes increased ROS production from exercise-induced aerobic bioenergetic reactions in the mitochondria and cytosol (19). These exercise-induced ROS are crucial signaling molecules that promote adaptive mechanisms to endurance exercise training including upregulation of antioxidant defense systems (e.g., SOD, catalase, GPX) and increased mitochondrial biogenesis (19). Newly formed mitochondria are more efficient, and produce less ROS for the same level of ATP produced (19). Chronic exercise training thus enhances antioxidant defense systems and mitochondrial function, effectively lowering the concentration of ROS and increasing resistance to oxidative damage (19).
The beneficial adaptations of mitochondrial function and antioxidant defense systems to exercise training appear to be sustained with aging, at least in men. Animal and human studies have shown that habitual exercise attenuates or reverses endothelial dysfunction in older males by preserving mitochondrial function and mitigating age-associated oxidative stress (6, 7, 10, 29). Aortic mitochondrial ROS, RNS and mitochondrial swelling were reduced, mitochondrial ATP and DNA content were elevated, and endothelial function was preserved in aged exercise trained rats compared to aged sedentary controls (10). Infusion of the antioxidant ascorbic acid (i.e., vitamin C), a common experimental model that temporarily and reversibly reduces ROS and removes the tonic oxidative stress-related suppression of endothelial function, restored brachial artery FMD in older sedentary men but had no effect on young or older endurance-trained men (7). In contrast to older men, we reported that ascorbic acid infusion increased brachial artery FMD in both sedentary and endurance-trained estrogen-deficient postmenopausal women, as well as in placebo-treated (i.e., estrogen-deficient) postmenopausal women following 12 weeks of endurance exercise training (25). Collectively, these observations suggest that the enhanced endothelial function in older men is associated with an increased resistance of older arteries to oxidative stress, whereas, the lack of endothelial improvements in response to exercise training in estrogen-deficient postmenopausal women is related to decreased resistance to oxidative damage. Notably, amenorrheic female athletes have elevated plasma markers of oxidative stress, which is suspected to play a role in the endothelial dysfunction observed in this population (27).
The inability of endurance exercise training to mitigate the effects of oxidative stress on the vascular endothelium in estrogen-deficient postmenopausal women is puzzling, but may be related to sex differences in the versatility and adaptability of antioxidant defense systems to oxidant exposure. Consistent with this broad concept, older male mice engaging in voluntary wheel running for 10-14 weeks had increased aortic total, mitochondrial (manganese [Mn]) and cytosolic (copper-zinc [CuZn]) SOD activity compared to older caged controls (6). Additionally, these mice had reduced aortic nitrotyrosine levels and NADPH oxidase expression and activity, and preserved endothelial function (6). Similar findings have been reported in clinical studies in humans. Older endurance-trained men were found to have greater endothelial MnSOD, lower nitrotyrosine and NADPH oxidase p47 protein expression in harvested brachial artery endothelial cells, and reduced circulating endothelium-derived extracellular (ec) SOD activity compared to age-matched sedentary men (29). These data suggest that endurance exercise training preserves vascular endothelial function with aging in men by enhancing antioxidant defenses and protecting the vasculature from oxidant stress.
To our knowledge, there is no direct evidence of the effect of endurance exercise training on enzymatic and non-enzymatic antioxidants or ROS in arteries of postmenopausal women or female animals. However, one study examined the effects of 90 days of 1 hour daily swim training on antioxidant activity and ROS in female ovariectomized and intact (control) Wistar rats (18). Swim training increased the endogenous antioxidants SOD, glutathione peroxidase activity and glutathione content, and reduced lipoperoxidation in erythrocytes and liver tissue in control animals (18). However, there was no improvement in antioxidant enzymatic activities with swim training in ovariectomized rats, and lipoperoxidation oxidant damage was increased (18). These data indicate that in females, estrogen-deficiency prevents the upregulation in antioxidant defenses commonly observed with exercise training. Thus, we believe that the inability of endurance exercise training to preserve or restore endothelial function in estrogen-deficient postmenopausal women may be attributed to a failure of antioxidant defense systems to adapt to endurance exercise training and mitigate oxidant damage, presumably due to the lack of estrogen. Estrogen has a phenol-hydroxyl ring that donates hydrogen, enabling estrogen to scavenge major sources of vascular ROS. Estrogen also increases mitochondrial antioxidant defenses (i.e., MnSOD) and other intracellular antioxidants (36). In this regard, ovariectomized animals have elevated ROS compared to intact animals, and treating ovariectomized animals with estrogen prevents the development of ROS and preserves endothelial function (13). In humans, a local infusion of ascorbic acid restored NO bioavailability and microvascular endothelial function following oophorectomy in premenopausal women (41). However, there was no effect of the ascorbic acid infusion on microvascular endothelial function in the women before oophorectomy, in healthy controls, or in oophorectomized women treated with estrogen for 3 months (41). Importantly, in our previously-mentioned exercise training study, there was no effect of ascorbic acid infusion on brachial artery FMD beyond the improvement observed following 12 weeks of endurance exercise training in estrogen-treated postmenopausal women (25). Collectively, these observations support the idea that estrogen plays a permissive role in endothelial adaptations to endurance exercise training by enhancing antioxidant defenses and increasing the resistance to oxidative damage.
Impaired Estrogen Receptor/eNOS Signaling
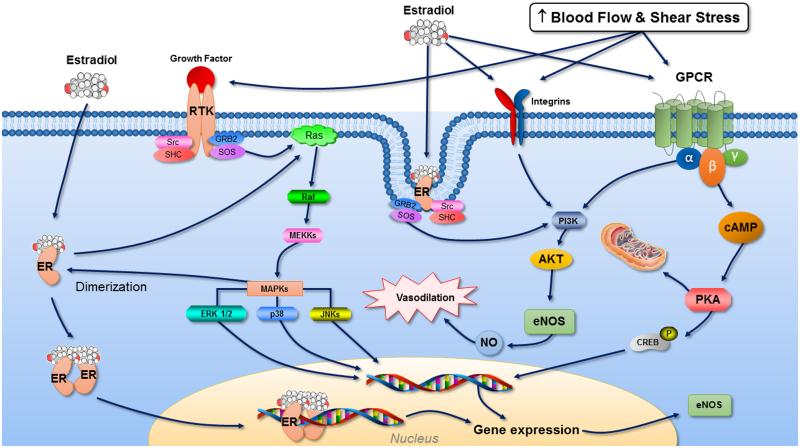
Both estrogen and exercise maintain the integrity of the endothelium by increasing NO bioavailability through common signaling pathways. Exercising blood flow increases the frictional forces (i.e., shear stress) along the surface of the endothelium stimulating mechanosensors including integrins, caveolae, ion channels and G-coupled protein receptors (GPCRs) that transduce mechanical forces into biochemical signals to phosphorylate and activate eNOS and increase NO-dependent vasodilation (16) (Figure 4). Similarly, estrogen causes NO to be released through activation of eNOS via estrogen receptor (ER)a-mediated non-genomic signaling pathways involving caveloae, integrins, ion channels and GPCRs (20). Additionally, both exercise and estrogen increase eNOS protein via transcriptional regulation of the eNOS gene (16, 20). Because estrogen and exercise share common intracellular signaling pathways to mediate NO release, it is plausible that they play synergistic roles in modulating intracellular signaling and gene expression in endothelial cells. In this regard, in vitro studies demonstrate that arterioles exposed to estrogen exhibit upregulated eNOS and augmented NO-mediated vasodilation in response to increased flow and shear stress (12). The increased NO-mediated vasodilation was prevented with ER antagonism, indicating that the increased dilation was mediated via ER upregulation of eNOS (12). Because prolonged estrogen deficiency and oxidative stress decrease ERα expression (2, 31), resulting in impaired ERα/eNOS signaling (31), it is plausible that the lack of exercise benefit in estrogen-deficient postmenopausal women may be related to reduced ERα and eNOS activation. We have previously demonstrated that in vivo endothelial cell ERα is reduced in estrogen-deficient postmenopausal compared with premenopausal women (8). There was a strong positive relation between ERα and brachial artery FMD, and endothelial eNOS and phosphorylated (p)-eNOSSer1177proteins, supporting the idea that ERα is important for normal eNOS activation and endothelial function in women. Taken together, these data suggest that a functioning ERα/eNOS signaling molecule may have relevance for vascular adaptations to exercise training in postmenopausal women.
Figure 4.
Proposed synergistic role between estrogen and exercise in modulating intracellular signaling and gene expression in endothelial cells. Estrogen and exercise share common intracellular signaling pathways to mediate NO release, and thus, estrogen may enhance exercise-induced increases in shear stress-associated signal transduction to increase NO production and vasodilation. Estrogen receptors (ER) found on the cell membrane and cytoplasm, particularly ERα, are involved with the transduction of the nongenomic effects of estrogen through second messengers such as nitric oxide (NO), receptor tyrosine kinases (RTKs), integrins, G-protein–coupled receptors (GPCRs), as well as protein kinases including phosphatidylInosiol-3-kinase (PI3K), serine-threonine kinase Akt, mitogen-activated protein kinase (MAPK), and protein kinases (PKA and PKC). Similarly, exercise increases blood flow and shear stress along the surface of endothelial cells, stimulating mechanosensors including GPCRs, integrins, cavoeloae and RTKs and activating protein kinases to phosphorylate and activate eNOS and release NO. Both estrogen and exercise also increase transcriptional regulation of the eNOS gene in the nucleus. Prolonged estrogen deficiency decreases ERα expression due to aging and oxidative stress, resulting in impaired ERα/eNOS signaling, and thus, we postulate that the lack of exercise benefit in estrogen-deficient women may be related to reduced ERα and eNOS activation.
ESTROGEN MIMETICS AND EXERCISE TRAINING ADAPTATIONS
If ERs are important for vascular adaptations to exercise training in women it is plausible that prescribing endurance-exercise training with ER agonists could enhance shear stress signal transduction and improve endothelial function with endurance-exercise training in estrogen-deficient postmenopausal women. One such non-pharmacological therapy is resveratrol, a polyphenol (3,5,4’-trihydroxystilbene) present in many plant species that has been thought to contribute to the cardiovascular benefits of drinking red wine (i.e., “French Paradox”). Resveratrol has been identified as a vasoactive nutraceutical and has been shown to benefit vascular endothelial function in preclinical and human studies (43). Acute (60 min) and chronic (6 wk) resveratrol treatment (dosed 30-270mg/d) increased brachial artery FMD in healthy obese adults with and without mild hypertension, with no differences between acute and chronic treatment (43). Similar to estrogen and exercise, resveratrol increases NO release through eNOS activation (15). Importantly, the increase in eNOS activity with resveratrol is attenuated with the ER blocker ICI 182,780, indicating that the effects of resveratrol on endothelial function are mediated in part, through ER activation of eNOS.
In addition to possibly enhancing ER/shear stress signaling, resveratrol could also permit exercise training adaptations in estrogen-deficient postmenopausal women by mitigating oxidative stress. In preclinical studies, resveratrol supplementation prevented the increase in ROS (xanthine oxidase and H2O2) and increased the activity of the antioxidants catalase and MnSOD in skeletal muscle following isometric contractions in aged mice (35).
Preclinical studies have demonstrated that resveratrol enhances exercise training effects on cardiovascular function and exercise performance (5). However, in a recent exercise training study conducted in older men, 8 weeks of exercise training combined with oral resveratrol (250 mg/d) was reported to have negative effects on CVD risk factors and cardiovascular fitness (9). In this study exercise training improved mean arterial blood pressure (MAP), LDL-cholesterol and maximal oxygen uptake (VO2max) with placebo treatment, but not with resveratrol treatment, suggesting a potentially adverse effect of resveratrol on exercise training adaptations (9). It is possible that the antioxidant effects of resveratrol blunted exercise generated free radicals and impaired the ROS -induced adaptations to exercise training, including mitochondrial biogenesis and upregulated antioxidant defenses. Additionally, this study was conducted in older men whose gonadal state was not reported (9), and thus, it is plausible that resveratrol combined with exercise training in estrogen-deficient postmenopausal women could elicit different effects.
Thoughts and Considerations
The focus of this review is on sex-specific endothelial adaptations to chronic exercise training in older adults. We did not discuss potential sex differences in endothelial adaptations in young adults.In general, there does not appear to be differences in endothelial function between sedentary and endurance-trained young adults. This is likely due to a ceiling effect because endothelial function is near optimal, and may not be amenable to improvements in vascular function, whereas in older adults there would be “room” for improvement with exercise training because of age-associated impairments (21).We have also not discussed sex-specific effects of resistance exercise training or acute exercise on endothelial function.
We focused this review primarily on the modulatory role of estrogen in endurance exercise training adaptations on endothelial function in women; we did not discuss the possible important modulatory role of testosterone (and/or estrogen) on vascular adaptations to exercise training in older men. We also did not discuss whether endothelial adaptations to exercise training is altered in young women with other conditions associated with estrogen deficiency (e.g., premature ovarian failure) or in perimenopausal women. To our knowledge no study has examined the effects of endurance exercise training on endothelial function in hypogonadal older men, young women with premature ovarian failure or in perimenopausal women. We also recognize that other mechanisms not mentioned in this review could explain the diminished endothelial adaptation to exercise training in older women. For example, vascular inflammation has been linked to vascular endothelial dysfunction in older men and in estrogen-deficient postmenopausal women, and habitual exercise attenuates endothelial dysfunction in older men by inhibiting the development of chronic low grade vascular inflammation (23, 29, 42). Finally, our review focused on exercise training adaptations in apparently healthy older adults; we did not discuss sex differences in exercise training adaptations in clinical populations such as diabetes, hypertension, CVD, etc., nor did we discuss the inter-individual variability in exercise training responses. Exercise training may benefit endothelial function in clinical populations of women, and there may be healthy estrogen-deficient postmenopausal women who do show benefit with exercise training (30, 38). All of these topics warrant further research and discussion.
SUMMARY
Vascular aging, featuring endothelial dysfunction, is the major risk factor for CVD. As the population ages, increasing numbers of adults will be living with CVD. Thus, effective prevention strategies are needed to mitigate the negative impact on quality of life, and the substantial societal and economic burdens of CVD. We acknowledge that regular exercise is the first-line strategy for primary prevention of CVD and should be promoted as a therapeutic strategy to target vascular aging. However, we believe that we have presented a compelling argument that gonadal hormones modulate vascular adaptations to exercise training, and thus, the gonadal state of older women (and possibly men) needs to be considered when prescribing exercise interventions. Our hypothesis is that estrogen deficiency prevents exercise training adaptive responses to mitochondrial function and antioxidant defense systems, promoting greater ROS production and decreasing the resistance of older arteries to oxidative damage. Moreover, oxidative stress decreases ERs, impairing critical vascular signaling cascades shared with exercise training mechanotransduction. Future research should investigate whether gonadal hormones modulate the response of the aging vasculature to exercise training and the underlying mechanisms (i.e., oxidative stress, ER signaling) for the failure of endothelial function to adapt to exercise in older estrogen-deficient women. Additionally, understanding the sex specific inter-individual variability to exercise training could provide information to possible mechanisms of endothelial “trainability”. Future investigations should also examine whether targeting mitochondrial ROS and/or other sources of ROS with pharmacological (e.g., selective estrogen receptor modulators) /non-pharmacological (e.g., neutraceuticals) therapies concurrent with exercise training could present a therapeutic strategy to permit vascular adaptations to exercise training in estrogen-deficient postmenopausal women.
Key Points.
Cardiovascular disease is the leading cause of death in men and women, with rates increasing in women 35-54 years old.
The menopause transition and fluctuations in sex hormone levels may accelerate age-associated endothelial dysfunction.
Adaptions to endurance exercise training on endothelial function are diminished in healthy postmenopausal women compared to middle-aged and older men.
Gonadal hormones modulate exercise training vascular endothelial responses, likely by increasing the resistance of aged arteries to oxidative damage.
Acknowledgments
Funding. The authors’ work was supported by the following National Institutes of Health awards R01AG027678, R56HL114073, R01AG22241, K01AG20683, T3263009794, Colorado Nutrition and Obesity Research Center P30 DK048520 and Colorado Clinical Translational Sciences Institute (CCTSI) RR-025780, and University of Colorado Denver (UCD) Center for Women’s Health Research, and UCD Women’s Health Research.
Footnotes
Conflict(s) of Interest/Disclosures: None
Summary for TOC: Exercise is promoted for cardiovascular disease prevention. However, sex and aging may modulate the cardioprotective effects of exercise.
References
- 1.Black MA, Cable NT, Thijssen DHJ, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. American Journal of Physiology - Heart and Circulatory Physiology. 2009;297(3):H1109–H16. doi: 10.1152/ajpheart.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakrabarti S, Davidge ST. High glucose-induced oxidative stress alters estrogen effects on ER[alpha] and ER[beta] in human endothelial cells: Reversal by AMPK activator. The Journal of Steroid Biochemistry and Molecular Biology. 2009;117(4-5):99–106. doi: 10.1016/j.jsbmb.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Dai D-F, Rabinovitch PS, Ungvari Z. Mitochondria and Cardiovascular Aging. Circulation Research. 2012;110(8):1109–24. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeSouza CA, Shapiro LF, Clevenger CM, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102(12):1351–7. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 5.Dolinsky VW, Jones KE, Sidhu RS, et al. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. The Journal of Physiology. 2012;590(11):2783–99. doi: 10.1113/jphysiol.2012.230490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durrant JR, Seals DR, Connell ML, et al. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–85. doi: 10.1113/jphysiol.2009.169771. Pt 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–24. doi: 10.1113/jphysiol.2003.057042. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular endothelial estrogen receptor alpha is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab. 2009;94(9):3513–20. doi: 10.1210/jc.2009-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gliemann L, Schmidt JF, Olesen J, et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. The Journal of Physiology. 2013;591(20):5047–59. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu Q, Wang B, Zhang XF, Ma YP, Liu JD, Wang XZ. Chronic aerobic exercise training attenuates aortic stiffening and endothelial dysfunction through preserving aortic mitochondrial function in aged rats. Exp Gerontol. 2014;56:37–44. doi: 10.1016/j.exger.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal Effects of Aging on Serum Total and Free Testosterone Levels in Healthy Men. J Clin Endocrinol Metab. 2001;86(2):724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 12.Huang A, Sun D, Koller A, Kaley G. 17β-Estradiol Restores Endothelial Nitric Oxide Release to Shear Stress in Arterioles of Male Hypertensive Rats. Circulation. 2000;101(1):94–100. doi: 10.1161/01.cir.101.1.94. [DOI] [PubMed] [Google Scholar]
- 13.Keaney JF, Jr., Shwaery GT, Xu A, et al. 17 beta-estradiol preserves endothelial vasodilator function and limits low-density lipoprotein oxidation in hypercholesterolemic swine. Circulation. 1994;89(5):2251–9. doi: 10.1161/01.cir.89.5.2251. [DOI] [PubMed] [Google Scholar]
- 14.Kemper MF, Stirone C, Krause DN, Duckles SP, Procaccio V. Genomic and non-genomic regulation of PGC1 isoforms by estrogen to increase cerebral vascular mitochondrial biogenesis and reactive oxygen species protection. European Journal of Pharmacology. 2014;723(0):322–9. doi: 10.1016/j.ejphar.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klinge CM, Blankenship KA, Risinger KE, et al. Resveratrol and Estradiol Rapidly Activate MAPK Signaling through Estrogen Receptors {alpha} and {beta} in Endothelial Cells. J. Biol. Chem. 2005;280(9):7460–8. doi: 10.1074/jbc.M411565200. [DOI] [PubMed] [Google Scholar]
- 16.Kojda G, Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovascular Research. 2005;67(2):187–97. doi: 10.1016/j.cardiores.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 17.Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43(3):562–71. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 18.Macedo UBdO, Martins RR, Freire Neto FP, et al. Oophorectomy hinders antioxidant adaptation promoted by swimming in Wistar rats. Applied Physiology, Nutrition, and Metabolism. 2013;38(2):148–53. doi: 10.1139/apnm-2012-0121. [DOI] [PubMed] [Google Scholar]
- 19.Mankowski RT, Anton SD, Buford TW, Leeuwenburgh C. Dietary Antioxidants as Modifiers of Physiologic Adaptations to Exercise. Med Sci Sports Exerc. 2015;47(9):1857–68. doi: 10.1249/MSS.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendelsohn ME. Mechanisms of estrogen action in the cardiovascular system. J Steroid Biochem Mol Biol. 2000;74(5):337–43. doi: 10.1016/s0960-0760(00)00110-2. [DOI] [PubMed] [Google Scholar]
- 21.Montero D, Padilla J, Diaz-Canestro C, et al. Flow-mediated dilation in athletes: influence of aging. Med Sci Sports Exerc. 2014;46(11):2148–58. doi: 10.1249/MSS.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 22.Moore DJ, Gonzales JU, Tucker SH, Elavsky S, Proctor DN. Exercise-induced vasodilation is associated with menopause stage in healthy middle-aged women. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2012;37(3):418–24. doi: 10.1139/h2012-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreau KL, Deane KD, Meditz AL, Kohrt WM. Tumor necrosis factor-α inhibition improves endothelial function and decreases arterial stiffness in estrogen-deficient postmenopausal women. Atherosclerosis. 2013;230(2):390–6. doi: 10.1016/j.atherosclerosis.2013.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial Function Is Impaired across the Stages of the Menopause Transition in Healthy Women. J Clin Endocrinol Metab. 2012;97(12):4692–700. doi: 10.1210/jc.2012-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential Role of Estrogen for Improvements in Vascular Endothelial Function With Endurance Exercise in Postmenopausal Women. Journal of Clinical Endocrinology & Metabolism. 2013;98(11):4507–15. doi: 10.1210/jc.2013-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics—2016 Update. A Report From the American Heart Association. 2015 doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 27.O'Donnell E, Goodman JM, Harvey PJ. Clinical review: Cardiovascular consequences of ovarian disruption: a focus on functional hypothalamic amenorrhea in physically active women. J Clin Endocrinol Metab. 2011;96(12):3638–48. doi: 10.1210/jc.2011-1223. [DOI] [PubMed] [Google Scholar]
- 28.Parker BA, Kalasky MJ, Proctor DN. Evidence for sex differences in cardiovascular aging and adaptive responses to physical activity. Eur. J. Appl. Physiol. 2010;110(2):235–46. doi: 10.1007/s00421-010-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell. 2011;10(6):1032–7. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond) 2011;120(1):13–23. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinna C, Cignarella A, Sanvito P, Pelosi V, Bolego C. Prolonged ovarian hormone deprivation impairs the protective vascular actions of estrogen receptor alpha agonists. Hypertension. 2008;51(4):1210–7. doi: 10.1161/HYPERTENSIONAHA.107.106807. [DOI] [PubMed] [Google Scholar]
- 32.Rickenlund A, Eriksson MJ, Schenck-Gustafsson K, Hirschberg AL. Amenorrhea in Female Athletes Is Associated with Endothelial Dysfunction and Unfavorable Lipid Profile. The Journal of Clinical Endocrinology & Metabolism. 2005;90(3):1354–9. doi: 10.1210/jc.2004-1286. [DOI] [PubMed] [Google Scholar]
- 33.Rickenlund A, Eriksson MJ, Schenck-Gustafsson K, Hirschberg AL. Oral Contraceptives Improve Endothelial Function in Amenorrheic Athletes. Journal of Clinical Endocrinology & Metabolism. 2005;90(6):3162–7. doi: 10.1210/jc.2004-1964. [DOI] [PubMed] [Google Scholar]
- 34.Rossouw JE, Manson JE, Kaunitz AM, Anderson GL. Lessons learned from the Women's Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121(1):172–6. doi: 10.1097/aog.0b013e31827a08c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan MJ, Jackson JR, Hao Y, et al. Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. J Gerontol A Biol Sci Med Sci. 2010;65(8):815–31. doi: 10.1093/gerona/glq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen Increases Mitochondrial Efficiency and Reduces Oxidative Stress in Cerebral Blood Vessels. Molecular Pharmacology. 2005;68(4):959–65. doi: 10.1124/mol.105.014662. [DOI] [PubMed] [Google Scholar]
- 37.Swift DL, Earnest CP, Blair SN, Church TS. The effect of different doses of aerobic exercise training on endothelial function in postmenopausal women with elevated blood pressure: results from the DREW study. British Journal of Sports Medicine. 2011 doi: 10.1136/bjsports-2011-090025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swift DL, Weltman JY, Patrie JT, et al. Predictors of Improvement in Endothelial Function after Exercise Training in a Diverse Sample of Postmenopausal Women. J Womens Health (Larchmt) 2013 doi: 10.1089/jwh.2013.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taddei S, Virdis A, Ghiadoni L, et al. Menopause is associated with endothelial dysfunction in women. Hypertension. 1996;28(4):576–82. doi: 10.1161/01.hyp.28.4.576. [DOI] [PubMed] [Google Scholar]
- 40.Taddei S, Virdis A, Ghiadoni L, et al. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38(2):274–9. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 41.Virdis A, Ghiadoni L, Pinto S, et al. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation. 2000;101(19):2258–63. doi: 10.1161/01.cir.101.19.2258. [DOI] [PubMed] [Google Scholar]
- 42.Walker AE, Kaplon RE, Pierce GL, Nowlan MJ, Seals DR. Prevention of age-related endothelial dysfunction by habitual aerobic exercise in healthy humans: possible role of nuclear factor kappaB. Clin Sci (Lond) 2014;127(11):645–54. doi: 10.1042/CS20140030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong RH, Coates AM, Buckley JD, Howe PR. Evidence for circulatory benefits of resveratrol in humans. Ann N Y Acad Sci. 2013;1290:52–8. doi: 10.1111/nyas.12155. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida N, Ikeda H, Sugi K, Imaizumi T. Impaired Endothelium-Dependent and -Independent Vasodilation in Young Female Athletes With Exercise-Associated Amenorrhea. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26(1):231–2. doi: 10.1161/01.ATV.0000199102.60747.18. [DOI] [PubMed] [Google Scholar]